Abstract
Objectives:
Coated-platelets are a subset of platelets produced by dual-agonist activation with collagen and thrombin. These platelets retain full-length amyloid precursor protein on their surface, are elevated in patients with amnestic as compared to nonamnestic mild cognitive impairment (MCI), and correlate with disease progression in Alzheimer disease (AD). Prompted by these findings, we investigated the association between coated-platelet production in amnestic MCI and rate of progression to AD.
Methods:
Coated-platelet levels were assayed in 74 patients with amnestic MCI who were subsequently followed longitudinally for up to 36 months in an outpatient dementia clinic. Levels are reported as percent of cells converted into coated-platelets. Subjects were categorized into tertiles of coated-platelet levels. The distributions of time to progression to AD were estimated for each tertile using cumulative incidence curves and compared statistically using a log-rank test. Cox proportional hazards regression was used to adjust for potential confounders.
Results:
The 24-month cumulative incidence of progression to AD was different among tertiles: 4% for the first tertile (lowest coated-platelet levels), 13% for the second tertile, and 37% for the third tertile (overall log-rank test, p = 0.02). The hazard rate of progression to AD for patients in the highest coated-platelet tertile was 5.1 times that for patients in the lowest tertile (p = 0.04), whereas the hazard rate for the middle tertile was similar to that for the lowest tertile (hazard rate ratio = 1.5, p = 0.7).
Conclusions:
Elevated coated-platelet levels in patients with amnestic MCI are associated with increased risk for progression to AD.
Coated-platelets are a subset of activated platelets observed upon dual-agonist stimulation with collagen and thrombin.1–3 In contrast to platelets activated with a single agonist, coated-platelets retain full-length amyloid precursor protein (APP) on their surface upon activation.4 Prompted by this abnormality in APP metabolism, we started investigating coated-platelet production in patients with Alzheimer disease (AD). We have shown previously in cross-sectional studies that coated-platelet synthesis correlates with the severity of AD, with elevated levels noted in the early stage as compared to more advanced stages of the disease.4,5 In a longitudinal study of disease progression in individuals with AD, we reported that initial coated-platelet levels correlate with the rate of disease advancement, such that higher initial coated-platelet levels were associated with a more rapid progression of dementia.6 In addition, our group has determined that coated-platelet levels are elevated in patients with amnestic compared to nonamnestic mild cognitive impairment (MCI).7
Because patients with amnestic MCI represent the group at highest risk for developing AD8,9 and also have elevated coated-platelet levels similar to patients with early-stage AD,4–7 we decided to investigate whether there is an association between coated-platelet production in patients with amnestic MCI and the rate of progression to AD. We hypothesized that patients with amnestic MCI with higher coated-platelet levels would progress more rapidly to AD than patients with amnestic MCI with lower coated-platelet levels.
METHODS
Patients.
Seventy-four consecutive patients with amnestic MCI were recruited through the Center for Alzheimer's and Neurodegenerative Disorders (CANDO) at the Oklahoma City Veterans Affairs Medical Center (VAMC) between September 1, 2005, and May 31, 2008. CANDO is an outpatient referral-based general dementia clinic that actively manages the longitudinal care of approximately 1,000 patients.10 All 74 patients fulfilled accepted clinical criteria for amnestic MCI and had complete diagnostic evaluations, including brain MRI scans, or CT scans when a medical contraindication for MRI was present.8,11 A neuroradiologist provided a definitive reading for every CT and MRI scan obtained. Standard laboratory tests performed at the time of diagnosis included complete blood count, serum electrolytes, serum glucose, blood urea nitrogen, B12, folate, thyroid function tests, and serology for syphilis. Mini-Mental State Examination (MMSE)12 and Clinical Dementia Rating Scale (CDR)13 assessments were obtained for all subjects prior to enrollment. All individuals in the study were men as a result of the composition of the United States armed forces during the time these veterans served.
These patients were followed clinically every 6 months in CANDO and at each visit a complete neurologic examination was performed that included MMSE and CDR assessments. Telephone contact with the patients was maintained between clinic visits and upcoming clinic encounter reminders were delivered through telephone and mail in order to minimize loss to follow-up.
In cases where progression to AD was detected, the repeat diagnostic evaluation included neuropsychological testing, brain MRI scans, or CT scans when a medical contraindication for MRI was present, and standard laboratory tests. All diagnoses of AD in MCI that progressed were based on accepted clinical criteria14 after multidisciplinary consensus meetings in CANDO.
Exclusion criteria included current cigarette smoking or history of stroke,15,16 traumatic brain injury, or acute coronary syndrome. We did not exclude individuals reporting use of medications that may influence coated-platelet levels,17 such as selective serotonin reuptake inhibitors (SSRIs), HMG-CoA reductase inhibitors (statins), and antiplatelet agents,17,18 at the time of enrollment because of the relatively high percentage of individuals in this age group taking one or more of these drugs. Instead, any potential modifying or confounding effects of these medications were explored statistically.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board of the University of Oklahoma and the VAMC Research and Development Committee (IRB no. 14178) in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed consent was obtained for all participants prior to inclusion in the study.
Coated-platelet assay.
After obtaining informed consent, 5 mL of blood was drawn into a plastic syringe containing 0.5 mL of acid citrate dextrose (ACD), and platelet-rich plasma (PRP) was prepared as described.16 Coated-platelets were assayed as previously described16 with 1 μL of PRP in a 100-μL assay with the following reagents (final concentrations): 1.0 μg/mL biotin-fibrinogen, 0.4 mM gly-pro-arg-pro, 500 ng/mL convulxin, 0.5 U/mL bovine thrombin, 2 mM CaCl2, 1 mM MgCl2, 150 mM NaCl, and 10 mM N-(2-hydroxyethyl)-piperazine-N′-(4-butanesulfonic acid) (HEPES), pH 7.5. After 5 minutes at 37°C, 0.8 μg of phycoerythrin-streptavidin and 0.5 μg of FITC-abciximab were added. After an additional 5 minutes at 37°C, the reaction was stopped with 0.2 mL of 1.5% (w/v) formalin in 150 mM NaCl, 10 mM HEPES, pH 7.5. The percentage of abciximab-positive events (platelets) with bound biotin-fibrinogen was quantitated by flow cytometry.3,7,16 Results are reported as percent of cells converted to coated-platelets.16,19 Individuals performing the coated-platelet assay were not aware of the clinical diagnosis corresponding to the blood sample analyzed.
Statistical analysis.
Data were analyzed using SAS (SAS System for Windows, version 9.1, SAS Institute Inc., Cary, NC) and SPSS (SPSS for Windows, release 15.0, SPSS, Chicago, IL). Descriptive statistics were used to summarize the distribution of patients' baseline characteristics. The distribution of baseline characteristics was compared among patient groups defined by tertiles of the observed distribution of coated-platelets using the nonparametric Kruskal-Wallis test or Fisher exact test. The distributions of the time to progression to AD were estimated for each of the 3 coated-platelet groups using cumulative incidence curves and compared statistically using a log-rank test. Cox proportional hazards regression modeling was used to adjust for potential confounding factors and to identify effect modifiers. Given the limited number of deaths in this data sample (n = 2), standard time-to-event analyses were used. The time to AD was censored at the time of death for subjects who did not develop AD prior to the date of death. SDs are provided when means are presented both in the text and tables.
The current sample size was calculated to achieve greater than 90% power to detect a hazard ratio for progression to AD of 2.0 or greater associated with a 1 SD increase in coated-platelet levels. This calculation assumes a SD of 14% points in coated-platelet levels, an average progression rate to AD of approximately 15% per year in the MCI population, a 2-sided 0.05 α level, and that 25% of variability in coated-platelet levels can be explained by other clinical and demographic factors. The effect size and SD estimates were based on our previously published research data on coated-platelet levels in AD and MCI.5,7,19 Sample size calculations were performed using PASS software.20
RESULTS
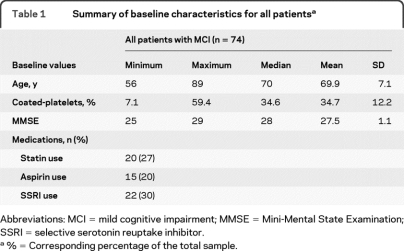
The study population consisted of 74 men. The mean age of the MCI population was 69.9 ± 7.1 years (mean ± SD) with a mean MMSE score of 27.5 ± 1.1, and mean coated-platelet levels of 34.7% ± 12.2%. The CDR score of all these patients was 0.5. The distributions of patient characteristics at baseline are summarized in table 1.
Table 1.
Summary of baseline characteristics for all patientsa
Abbreviations: MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; SSRI = selective serotonin reuptake inhibitor.
%= Corresponding percentage of the total sample.
Follow-up among patients who did not progress to AD ranged from 6 to 36 months, with a median follow-up of 24 months. Two of the patients (3%) died after 12 and 18 months of follow-up. Neither was diagnosed with AD prior to their deaths.
Patients were categorized into tertiles based on the observed distribution of coated-platelet levels at the time of entry into the study (lowest tertile: 7% to 26%, middle tertile: 26.1% to 40%, and upper tertile: 40.1% to 60%). The duration of follow-up among subjects who did not progress to AD was very similar among the 3 tertiles, with a median of 24 months and a range of 6 to 36 months for each tertile.
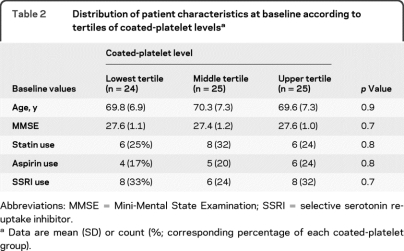
Table 2 summarizes the distribution of patient characteristics at baseline according to tertiles of coated-platelet levels. Data are summarized using the mean and SD (or count and corresponding percentage) of each coated-platelet group. No significant differences were found among the coated-platelet groups in terms of median age or the percentage of subjects using statins, aspirin, or SSRI.
Table 2.
Distribution of patient characteristics at baseline according to tertiles of coated-platelet levelsa
Abbreviations: MMSE = Mini-Mental State Examination; SSRI = selective serotonin reuptake inhibitor.
Data are mean (SD) or count (%; corresponding percentage of each coated-platelet group).
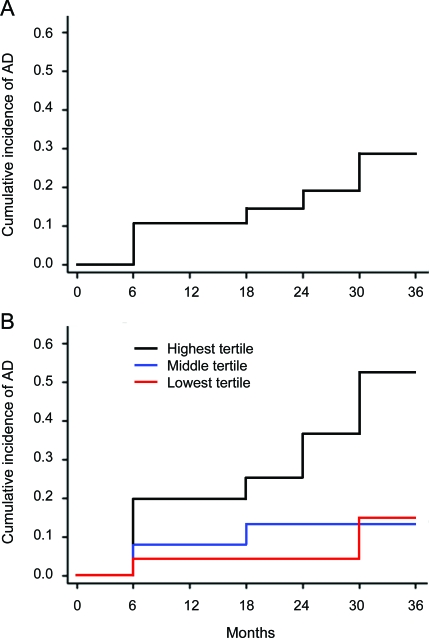
Among the entire cohort, 12 subjects progressed to AD by 24 months, resulting in a cumulative incidence of AD of 19% (95% CI confidence interval [CI] 11%–31%) at 24 months. By 36 months, 15 subjects progressed to AD, resulting in a cumulative incidence of 29% (95% CI 17%–45%) at 36 months, which corresponds to a progression risk for AD of approximately 10% per year (figure, A). Table 3 summarizes the distribution of patient characteristics at baseline according to progression to AD or lack of progression to AD.
Figure. Incidence of conversion to Alzheimer disease (AD).
(A) Coated-platelet levels were measured in 74 patients with amnestic mild cognitive impairment (MCI) who were then monitored clinically for progression to AD. Data are plotted as the cumulative incidence of AD (vertical axis) vs follow-up time in months (horizontal axis). (B) Patients with amnestic MCI were categorized into tertiles of initial coated-platelet levels (lowest, middle, and highest, n = 24, 25, and 25, respectively) and cumulative incidence of progression to AD was calculated for each tertile (see text for additional details). Data are plotted as the cumulative incidence of progression for each tertile (vertical axis) vs follow-up time in months (horizontal axis).
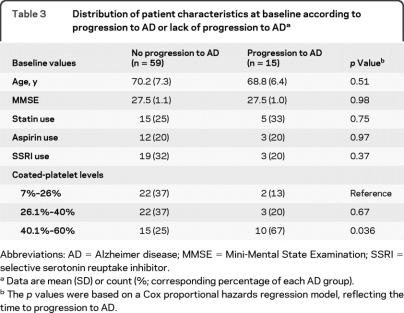
Table 3.
Distribution of patient characteristics at baseline according to progression to AD or lack of progression to ADa
Abbreviations: AD = Alzheimer disease; MMSE = Mini-Mental State Examination; SSRI = selective serotonin reuptake inhibitor.
Data are mean (SD) or count (%; corresponding percentage of each AD group).
The p values were based on a Cox proportional hazards regression model, reflecting the time to progression to AD.
Cumulative incidence of AD at 24 months was estimated for each tertile of coated-platelet levels. For patients in the highest tertile (n = 25), 8 progressed to AD by 24 months, resulting in a cumulative incidence of AD of 37% (95% CI 20%–61%), whereas for patients in the middle (n = 25) and lowest (n = 24) tertiles, 3 and 1 patients progressed to AD by 24 months, resulting in cumulative incidences of AD of 13% (95% CI 4%–36%) and 4% (95% CI 1%–26%) (figure, B). An overall log-rank test showed that the time to progression to AD differed among the coated-platelet tertiles (p = 0.02).
The hazard rates for progression to AD were calculated for the middle and highest tertile of coated-platelets and compared to the AD hazard rate in the lowest tertile. The hazard rate for patients in the highest coated-platelet tertile was 5.1 times the hazard rate for patients in the lowest tertile (95% CI 1.1–23.1, p = 0.04), whereas the hazard rates for the middle and lowest tertile patients were similar (hazard rate ratio = 1.5, 95% CI 0.2–8.9, p = 0.7).
To investigate the potential modifying effects of age and use of statins, antiplatelets, or SSRIs on the association of coated-platelets and progression of MCI to AD, differences in progression risk among the coated-platelet tertiles were examined separately in subgroups of subjects defined by each of these factors. No modifying effects for age or use of statin, antiplatelets, or SSRIs were detected. To investigate potential confounding effects of these variables, the association between each factor and the risk of progression of MCI to AD was first investigated. Age and the use of statins, antiplatelets, or SSRIs were not significantly associated with the risk of progression of MCI to AD. Specifically, the hazard ratio value associated with age, modeled as a continuous factor, was 0.97 (95% CI 0.90–1.06, p = 0.51) and the hazard ratio values associated with the use of the medications were use of statins (hazard rate ratio = 1.20, 95% CI 0.41–3.51, p = 0.75), use of antiplatelets (hazard rate ratio = 1.02, 95% CI 0.29–3.62, p = 0.97), and use of SSRIs (hazard rate ratio = 0.56, 95% CI 0.16–2.00, p = 0.37). Given that there is no clear association between the risk of progression of MCI to AD and age or medication use in the observed data, these factors were not acting as confounding variables of the association between risk of progression of MCI to AD and coated-platelet levels.
DISCUSSION
The data presented here show the existence of an association between coated-platelet production in patients with amnestic MCI and subsequent progression to AD. Individuals with the highest initial coated-platelet levels were significantly more likely to develop AD, and they did so earlier than individuals with the lowest coated-platelet levels (figure, B), even though the risk of progression to AD for the group as a whole was similar to that reported in the literature (figure, A). These findings support the existence of a link between coated-platelets and the chronic disease model proposed for AD21; however, the nature of that link is currently unknown.
Our initial work demonstrated that coated-platelet synthesis is associated with an altered metabolism of APP characterized by retention and derivatization of full-length APP on the surface of these activated cells.4 This alteration is not present in platelets activated with a single agonist. Although the exact mechanism for retention of APP remains unclear, a covalent alteration of APP appears to be involved with formation of coated-platelets.4 Prompted by our initial observation, we subsequently demonstrated that patients with early-stage AD produce coated-platelets at levels significantly above that of aged controls and patients with advanced stage AD.4 In a cross-sectional study, a robust correlation was observed between coated-platelet levels and AD severity, with higher levels correlating with higher MMSE scores.5 There is also a correlation between initial coated-platelet levels and the rate of AD progression, with the most severe decline noted in individuals with the highest initial coated-platelet production.6 More recently, we studied coated-platelet production in frontotemporal dementia (FTD),19 a neurodegenerative disease characterized by a variety of neuropathologic changes that do not involve an abnormality in the amyloid-β peptide pathway.22 Our results showed that the increased coated-platelet synthesis noted in patients with early-stage AD is not present in FTD and that the correlation with disease severity noted in patients with AD was not present in FTD.19
Previous investigators have examined a possible role of platelets as either a surrogate indicator of putative events involved in AD or as reservoirs of telltale amyloid markers. In the first instance, abnormal serotonin content,23 mitochondrial function,24 and nitric oxide abnormalities25 in platelets have been observed in platelets from patients with AD. In the second instance, several studies have examined the presence of abnormal amyloid isoforms in advanced AD.26,27 For a variety of reasons, no consensus has developed supporting further use of these observations.
Similar to the increased coated-platelet synthesis noted in early-stage AD, individuals with amnestic MCI have significantly increased coated-platelet levels compared to individuals with nonamnestic MCI.4,5,7 This observation is of particular clinical relevance as MCI is considered a nosologic entity situated at the interface between normal aging and very early dementia,8,11 amnestic MCI is widely considered a prodrome for AD,8,11,28 and biomarkers that identify AD and its precursor conditions at the earliest stages are highly sought.29–31
Our initial observations in AD, FTD, and MCI appear to support a relationship, direct or indirect, between coated-platelet synthesis and the APP metabolism alterations that occur in AD.32 In addition, recent studies showed abnormal coated-platelet synthesis in patients with cerebrovascular disease, with elevated coated-platelet levels in patients with nonlacunar ischemic stroke and decreased coated-platelet levels in patients with spontaneous intracerebral hemorrhage.15,16,33,34 Finally, limited studies have indicated that coated-platelets are potentiated by inflammation.35 Perhaps coated-platelet synthesis represents a sensitive biomarker of the disease process that includes APP abnormalities, cerebrovascular disease, and inflammatory components and may produce insight into the multifaceted pathways associated with AD.36
Limitations of the current study include a relatively small sample size and a patient population consisting exclusively of men. Future studies will need to address these limitations and examine the possibility of generalizing these findings to the entire MCI population. Further studies are also warranted to determine the potential clinical application of coated-platelet levels as a biomarker in the early diagnosis of AD and the pathologic significance of elevated coated-platelet levels in patients with amnestic MCI and incipient AD.
ACKNOWLEDGMENT
The authors thank Linda Hershey, Marilee Monnot, Bobbie Branscum-Parrish, Robert Cox, Erin Nagode, Carletta Rehbine, and Paul Friese for assistance.
Footnotes
- ACD
- acid citrate dextrose
- AD
- Alzheimer disease
- APP
- amyloid precursor protein
- CANDO
- Center for Alzheimer's and Neurodegenerative Disorders
- CDR
- Clinical Dementia Rating Scale
- CI
- confidence interval
- FTD
- frontotemporal dementia
- MCI
- mild cognitive impairment
- MMSE
- Mini-Mental State Examination
- PRP
- platelet-rich plasma
- SSRI
- selective serotonin reuptake inhibitor
- VAMC
- Veterans Affairs Medical Center
DISCLOSURE
Dr. Prodan receives research support from the Alzheimer's Association and the Oklahoma Center for Advancement of Science and Technology. Dr. Ross serves on the editorial board of Cognitive and Behavioral Neurology. Dr. Stoner receives/has received research support from the NIH (NHLBI, NIDDK, and NIDCR). Dr. Cowan receives research support from the NIH and the World Health Organization. Dr. Vincent receives research support from the Alzheimer's Association, the Oklahoma Center for the Advancement of Science and Technology, and the US Department of Veterans Affairs. Dr. Dale receives research support from the American Heart Association.
REFERENCES
- 1. Dale GL, Friese P, Batar P, et al. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 2002; 415: 175–179 [DOI] [PubMed] [Google Scholar]
- 2. Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of alpha-granule factor V in human platelets: Effects of ionophore A23187, thrombin, collagen and convulxin. Blood 2000; 95: 1694–1702 [PubMed] [Google Scholar]
- 3. Dale GL. Coated-platelets: an emerging component of the procoagulant response. J Thromb Haemost 2005; 3: 2185–2192 [DOI] [PubMed] [Google Scholar]
- 4. Prodan CI, Szasz R, Vincent AS, Ross ED, Dale GL. Coated-platelets retain amyloid precursor protein on their surface. Platelets 2006; 17: 56–60 [DOI] [PubMed] [Google Scholar]
- 5. Prodan CI, Ross ED, Vincent AS, Dale GL. Coated-platelets correlate with disease progression in Alzheimer disease. J Neurol 2007; 254: 548–549 [DOI] [PubMed] [Google Scholar]
- 6. Prodan CI, Ross ED, Vincent AS, Dale GL. Rate of progression in Alzheimer's disease correlates with coated-platelet levels-a longitudinal study. Transl Res 2008; 152: 99–102 [DOI] [PubMed] [Google Scholar]
- 7. Prodan CI, Ross ED, Vincent AS, Dale GL. Coated-platelets are higher in amnestic versus non-amnestic patients with mild cognitive impairment. Alzheimer Dis Assoc Disord 2007; 21: 259–261 [DOI] [PubMed] [Google Scholar]
- 8. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256: 183–194 [DOI] [PubMed] [Google Scholar]
- 9. Petersen RC. Mild cognitive impairment: current research and clinical implications. Semin Neurol 2007; 27: 22–31 [DOI] [PubMed] [Google Scholar]
- 10. Ross ED, Shah SN, Prodan CI, Monnot M. Changing relative prevalence of Alzheimer disease versus non-Alzheimer disease dementias: have we underestimated the looming dementia epidemic? Dement Geriatr Cogn Disord 2006; 22: 273–277 [DOI] [PubMed] [Google Scholar]
- 11. Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure: report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry 2006; 77: 714–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198 [DOI] [PubMed] [Google Scholar]
- 13. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–2414 [DOI] [PubMed] [Google Scholar]
- 14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939–944 [DOI] [PubMed] [Google Scholar]
- 15. Prodan CI, Joseph PM, Vincent AS, Dale GL. Coated-platelets in ischemic stroke: Differences between lacunar and cortical stroke. J Thromb Haemost 2008; 6: 609–614 [DOI] [PubMed] [Google Scholar]
- 16. Prodan CI, Stoner JA, Cowan LD, Dale GL. Lower coated-platelet levels are associated with early hemorrhagic transformation in patients with non-lacunar brain infarction. J Thromb Haemost 2010; 8: 1185–1190 [DOI] [PubMed] [Google Scholar]
- 17. Prodan CI, Joseph PM, Vincent AS, Dale GL. Coated-platelet levels are influenced by smoking, aspirin and selective serotonin reuptake inhibitors. J Thromb Haemost 2007; 5: 2149–2151 [DOI] [PubMed] [Google Scholar]
- 18. Norgard NB, Saya S, Hann CL, Hennebry TA, Schechter E, Dale GL. Clopidogrel attenuates coated-platelet production in patients undergoing elective coronary catheterization. J Cardiovasc Pharmacol 2008; 52: 536–539 [DOI] [PubMed] [Google Scholar]
- 19. Prodan CI, Ross ED, Vincent AS, Dale GL. Differences in coated-platelet production between frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord 2009; 23: 234–237 [DOI] [PubMed] [Google Scholar]
- 20. NCSS, LLC [computer program]. Kaysville, UT: PASS; 2008. [Google Scholar]
- 21. Katzman R. The prevalence and malignancy of Alzheimer disease: a major killer [editorial]. Arch Neurol 1976; 33: 217–218 [DOI] [PubMed] [Google Scholar]
- 22. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–1554 [DOI] [PubMed] [Google Scholar]
- 23. Muck-Seler D, Presecki P, Mimica N, et al. Platelet serotonin concentration and monoamine oxidase type B activity in female patients in early, middle and late phase of Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 1226–1231 [DOI] [PubMed] [Google Scholar]
- 24. Shi C, Guo K, Yew DT, et al. Effects of ageing and Alzheimer's disease on mitochondrial function of human platelets. Exp Gerontol 2008; 43: 589–594 [DOI] [PubMed] [Google Scholar]
- 25. Vignini A, Nanetti L, Moroni C, et al. Modifications of platelet from Alzheimer disease patients: a possible relation between membrane properties and NO metabolites. Neurobiol Aging 2007; 28: 987–994 [DOI] [PubMed] [Google Scholar]
- 26. Borroni B, Perani D, Broli M, et al. Pre-clinical diagnosis of Alzheimer disease combining platelet amyloid precursor protein ratio and rCBF SPECT analysis. J Neurol 2005; 252: 1359–1362 [DOI] [PubMed] [Google Scholar]
- 27. Tang K, Hynan LS, Baskin F, Rosenberg RN. Platelet amyloid precursor protein processing: A bio-marker for Alzheimer's disease. J Neurol Sci 2006; 240: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246 [DOI] [PubMed] [Google Scholar]
- 29. Borroni B, DiLuca M, Cattabeni F, Padovani A. Advance on the diagnostic potential of biological markers in the early detection of Alzheimer disease. Neurosci Res Commun 2004; 35: 232–245 [Google Scholar]
- 30. Sonnen JA, Montine KS, Quinn JF, Kaye JA, Breitner JC, Montine TJ. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol 2008; 7: 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mortimer JA, Petersen RC. Detection of prodromal Alzheimer's disease. Ann Neurol 2008; 64: 479–480 [DOI] [PubMed] [Google Scholar]
- 32. Masters CL, Beyreuther K. Alzheimer's centennial legacy: prospects for rational therapeutic intervention targeting the Abeta amyloid pathway. Brain 2006; 129: 2823–2839 [DOI] [PubMed] [Google Scholar]
- 33. Prodan CI, Vincent AS, Padmanabhan R, Dale GL. Coated-platelet levels are low in patients with spontaneous intracerebral hemorrhage. Stroke 2009; 40: 2578–2580 [DOI] [PubMed] [Google Scholar]
- 34. Prodan CI, Vincent AS, Dale GL. Coated platelet levels correlate with bleed volume in patients with spontaneous intracerebral hemorrhage. Stroke 2010; 41: 1301–1303 [DOI] [PubMed] [Google Scholar]
- 35. Valaydon ZS, Lee P, Dale GL, et al. Increased coated-platelet levels in chronic haemodialysis patients. Nephrology 2009; 14: 148–154 [DOI] [PubMed] [Google Scholar]
- 36. Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010; 362: 329–344 [DOI] [PubMed] [Google Scholar]