Abstract
Objective:
To reassess the role of plasmapheresis in the treatment of neurologic disorders.
Methods:
We evaluated the available evidence based on a structured literature review for relevant articles from 1995 through September 2009. In addition, due to revision of the definitions of classification of evidence since the publication of the previous American Academy of Neurology assessment in 1996, the evidence cited in that manuscript was reviewed and reclassified.
Results and Recommendations:
Plasmapheresis is established as effective and should be offered in severe acute inflammatory demyelinating polyneuropathy (AIDP)/Guillain-Barré syndrome (GBS) and in the short-term management of chronic inflammatory demyelinating polyneuropathy (Class I studies, Level A). Plasmapheresis is established as ineffective and should not be offered for chronic or secondary progressive multiple sclerosis (MS) (Class I studies, Level A). Plasmapheresis is probably effective and should be considered for mild AIDP/GBS, as second-line treatment of steroid-resistant exacerbations in relapsing forms of MS, and for neuropathy associated with immunoglobulin A or immunoglobulin G gammopathy, based on at least one Class I or 2 Class II studies (Level B). Plasmapheresis is probably not effective and should not be considered for neuropathy associated with immunoglobulin M gammopathy, based on one Class I study (Level B). Plasmapheresis is possibly effective and may be considered for acute fulminant demyelinating CNS disease (Level C). There is insufficient evidence to support or refute the use of plasmapheresis for myasthenia gravis, pediatric autoimmune neuropsychiatric disorders associated with streptococcus infection, and Sydenham chorea (Class III evidence, Level U).
Plasmapheresis, also known as therapeutic plasma exchange, is a procedure that involves separating the blood, exchanging the plasma (typically with donor plasma or albumin solution), and returning the other components, primarily red blood cells, to the patient. The mechanics of plasmapheresis have not changed since the introduction of continuous flow machines. This guideline summarizes evidence for the usefulness of plasmapheresis in the treatment of neurologic disorders and updates the previous American Academy of Neurology (AAN) assessment published in 1996,1 employing updated methodology for the development of clinical practice guidelines.
DESCRIPTION OF THE ANALYTIC PROCESS
The Therapeutics and Technology Assessment (TTA) subcommittee of the AAN appointed panel members for this assessment based on their expertise in the neurologic disorders under discussion, their familiarity with the guideline process, or both.
The MEDLINE, Cochrane Library, Web of Science, and EMBASE databases were searched from 1995 to September 2009 using the terms “plasmapheresis” and “neurologic disease (exploded)” and key text words and index words for plasmapheresis, plasma exchange, immunoadsorption, and double filtration plasmapheresis. The search was limited to reports in humans and abstracts available in English. Standard search procedures were used, and subheadings were applied as appropriate.
The initial search yielded 2,263 articles. This list was refined by reviewing the abstracts and including only articles reporting results from controlled clinical trials in humans. Fifty-nine articles considered relevant to the guideline were reviewed in their entirety (table e-1 on the Neurology® Web site at www.neurology.org). The evidence was rated according to the AAN criteria for the classification of therapeutic articles (appendix e-1), and recommendations were linked to the strength of the evidence (appendix e-2). A summary of the conclusions and strength of the evidence is provided in table 1. In addition, due to revision of the definitions of classification of evidence since 1996, the evidence cited in the previous AAN assessment1 was reviewed and reclassified accordingly.
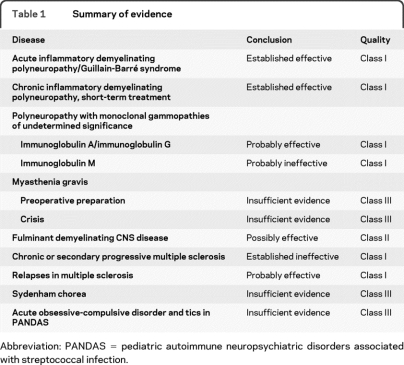
Table 1.
Summary of evidence
Abbreviation: PANDAS = pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection.
ANALYSIS OF EVIDENCE
Acute inflammatory demyelinating polyneuropathy/Guillain-Barré syndrome.
As described in the original TTA assessment of plasmapheresis, 3 randomized controlled trials between 1985 and 1995 demonstrated improvement for patients with severe acute inflammatory demyelinating polyneuropathy (AIDP)/Guillain-Barré syndrome (GBS) treated with plasmapheresis.2–4 Since 1995, another randomized controlled trial was reported by the French Cooperative group.5 Although none of these studies employed masked outcome assessment by the principal investigators, 3 of the studies3–5 used a functional scale that assesses a patient's ability to walk. This outcome was considered objective and unlikely to be affected by expectation bias. Therefore, these studies were considered Class I.
The earlier Class I studies covered by the previous TTA assessment3,4 limited enrollment to patients with disease severe enough to prevent independent walking. Since then, the French Cooperative Group reported on 556 patients with AIDP/GBS stratified according to severity of disease.5 The mild group (n = 91) could stand unaided or walk 5 meters without assistance, the moderate group (n = 304) was unable to stand unaided, and the severe group (n = 161) was mechanically ventilated. The stratified groups were randomized as follows: observation vs 2 plasmapheresis (mild); 2 vs 4 plasmapheresis (moderate); and 4 vs 6 plasmapheresis (severe). The primary outcome measure for mild disease was time to motor recovery, defined as improvement on at least 2 items of a functional muscular score or improvement on one item and improvement in cranial nerve function or trunk or respiratory involvement. For moderate and severe disease, time to recover walking with assistance served as the primary outcome measure. Each procedure exchanged 1.5 plasma volumes for colloid return fluid. Plasmapheresis improved the outcome of all these groups. This study also addressed the optimal number of plasmapheresis sessions for each group. For the mild group, 2 sessions were better than none (p = 0.0002), with plasmapheresis leading to twice the chance of motor recovery compared to controls (95% confidence interval [CI] 1.4–3.7, p = 0.001). In the moderate group, 4 sessions were better than 2 (p = 0.04), with a relative chance of motor recovery of 1.2 (95% CI 0.95–1.6; p = 0.11). In the severe group, there was no benefit of 6 sessions over 4 for any endpoints. The relative chance of recovering the ability to walk with assistance was not different (95% CI 0.6–1.4, p = 0.89).5
Conclusions.
On the basis of consistent findings from Class I studies, plasmapheresis is established as effective for the treatment of AIDP/GBS severe enough to impair the ability to walk independently or severe enough to require mechanical ventilation. For milder AIDP/GBS, in which ambulation is preserved, plasmapheresis is probably effective, based on a single Class I study.
Recommendations.
Plasmapheresis should be offered in the treatment of AIDP/GBS severe enough to impair independent walking or to require mechanical ventilation (Level A). Plasmapheresis should be considered in the treatment of milder clinical presentations of AIDP/GBS (Level B).
Clinical context.
IV immunoglobulin (IVIg) is an alternative treatment used in patients with AIDP/GBS. There is insufficient evidence to demonstrate the superiority of one treatment over the other.6–9
Chronic inflammatory demyelinating neuropathy.
Prior to 1995, one Class I double-blind, randomized, placebo-controlled trial examined the efficacy of plasmapheresis in chronic inflammatory demyelinating neuropathy (CIDP).10 In this study, 34 patients with CIDP were randomized to receive either plasmapheresis or sham exchange; 29 patients completed the trial. The plasmapheresis group showed improvement in the Neuropathy Disability Scale (NDS) score (p = 0.025), but the improvement generally began to fade 10–14 days after treatment was stopped.
Since the original TTA publication on plasmapheresis, a second Class I randomized, placebo-controlled, double-blind, crossover study has been conducted. In this study, 18 patients with CIDP (equal numbers of patients with chronic progressive and relapsing CIDP) were randomized to receive either plasmapheresis or sham treatment.11 Primary outcome measures included the NDS, a clinical grade and grip strength measurement, and electrophysiologic measures. Three patients were excluded (1 failed venous access, 1 had a stroke, and 1 dropped out of sham treatment for unstated reasons). Twelve patients (80%) improved with plasmapheresis, with improvement in clinical and electrophysiologic outcome measures as compared with controls (NDS, p < 0.001; clinical grade, p < 0.001; grip strength, p < 0.003; proximal compound muscle action potential [CMAP] [mV], p < 0.01; distal CMAP [mV], p < 0.06; motor conduction velocity [m s−1], p < 0.006; distal motor latency [ms], p < 0.01). Rebound worsening of symptoms occurred in 8 of the 15 patients (66%). In 7 patients this occurred within 7–14 days of the last plasmapheresis treatment, while in one patient the worsening occurred during the 5 weeks following the last treatment. All patients improved with open-label plasmapheresis, although 5 patients required long-term immunosuppression with prednisone, cyclophosphamide, or both for 6 months or more (duration not further specified by authors).11
Conclusions.
Based on 2 Class I studies, plasmapheresis is established as effective in the short-term treatment of CIDP; both studies showed the beneficial effect is not sustained, with worsening beginning 1–5 weeks after last plasmapheresis treatment.
Recommendation.
Plasmapheresis should be offered as a short-term treatment for patients with CIDP (Level A).
Clinical context.
Steroids, IVIg, and immunosuppressants have also been used in the treatment of CIDP.12,13
Dysimmune neuropathies.
As detailed in the previous assessment, one Class I study showed the efficacy of plasmapheresis in polyneuropathies associated with immunoglobulin A (IgA) and immunoglobulin G (IgG) monoclonal gammopathy of undetermined significance (MGUS), while the same study found no significant benefit in immunoglobulin M (IgM)-associated MGUS.14 Since 1995, one Class III open-label, randomized study of 44 patients with polyneuropathy associated with IgM MGUS compared plasmapheresis with chlorambucil to chlorambucil alone and did not show any benefit of plasmapheresis.15
Conclusions.
Plasmapheresis is probably effective in IgA- and IgG-MGUS–associated polyneuropathy, based on one Class I study. On the basis of one Class I and one Class III study, plasmapheresis is probably not effective in polyneuropathy associated with IgM MGUS.
Recommendations.
Plasmapheresis should be considered in polyneuropathy associated with IgA and IgG MGUS (Level B). Plasmapheresis should not be considered in the treatment of polyneuropathy associated with IgM MGUS (Level B).
Myasthenia gravis.
As reported in the original assessment, there are still no randomized placebo-controlled clinical trials of plasmapheresis in myasthenia gravis (MG). One nonrandomized Class III treatment trial compared treatment with pyridostigmine to plasmapheresis in 9 patients. This study found improvement in respiratory measures, including a decrease in functional residual capacity and residual volume and an increase in forced expiratory volume in 1 second, maximum inspiratory pressure, and maximum expiratory pressure (p < 0.05) in the plasmapheresis cohort.16
A retrospective Class III study compared 19 patients treated with a single session of plasmapheresis prior to thymectomy vs 32 patients treated with thymectomy alone. The patients treated with plasmapheresis had less occurrence of crisis in the following month (p = 0.0724) and year (p = 0.049) and a greater remission rate at 5–7 years postoperatively.17
Conclusions.
There are inadequate data to evaluate the use of plasmapheresis in the treatment of myasthenic crisis or in the treatment of MG prethymectomy.
Recommendation.
Because of the lack of randomized controlled studies with masked outcomes, there is insufficient evidence to support or refute the efficacy of plasmapheresis in the treatment of myasthenic crisis (Level U) or MG prethymectomy (Level U).
Clinical context.
Despite the fact that the use of plasmapheresis in myasthenic crisis and MG prethymectomy receives a Level U recommendation, plasmapheresis is used at many medical centers for these indications.
CNS demyelinating disease.
In the previous TTA report, one of the studies reviewed investigated the role of plasmapheresis in the treatment of exacerbations of demyelinating CNS disease. This Class I randomized, sham-controlled, double-blind study18 investigating the effectiveness of plasmapheresis as adjunctive therapy found no benefit in the treatment of multiple sclerosis (MS) exacerbations in the course of chronic progressive disease. However, in a subgroup analysis, exacerbations during the course of relapsing forms of MS did improve more quickly, and improvements were maintained at 1 month compared to controls (p < 0.04).
Since the previous TTA report, there has been an additional Class II, randomized, double-blind, sham-controlled trial which included 22 patients with acute, severe attacks of CNS demyelination who had failed to improve after at least 5 days of high-dose parenteral steroids.19 Patients were included in the trial if they had clinically definite or laboratory-supported MS or if they had idiopathic inflammatory demyelinating CNS diseases (confirmed by biopsy when necessary) and acute neurologic deficit affecting consciousness, language, and brainstem function, or spinal cord function with impairment in one or more of the targeted neurologic deficits (coma, aphasia, acute severe cognitive dysfunction, hemiplegia, paraplegia, or quadriplegia). While the inclusion criteria are clearly defined, they are broad and encompass a heterogeneous group of inflammatory conditions with potentially diverse underlying pathogenic mechanisms. For this reason, this study is considered Class II rather than Class I. In all, the study included 12 patients with MS, 4 patients with transverse myelitis (TM), 1 patient with acute disseminated encephalomyelitis (ADEM), 1 patient with Marburg variant, 2 patients with neuromyelitis optica (NMO), 1 patient with recurrent myelitis, and 1 patient with focal cerebral demyelination. The primary outcome measures were evaluated by masked assessment by 2 neurologists (A and B) based on changes on standardized clinical scales for the targeted neurologic deficits. Treated patients showed a 42.1% response rate vs a 5.9% response rate in controls (p = 0.032 according to Neurologist A and p = 0.011 according to Neurologist B).
Prior to this TTA report, 3 Class I studies and one Class II study of plasmapheresis in chronic progressive MS have been published which did not provide evidence of benefit.18,20–22 Since the last TTA report, an additional Class II study of azathioprine and plasmapheresis in 11 patients with secondary progressive MS (8 patients completed the trial) concluded that plasmapheresis did not improve outcomes.23
Conclusions.
Plasmapheresis as adjunctive therapy is probably effective for management of exacerbations in relapsing forms of MS, based on a single Class I study. Based on a single Class II study, plasmapheresis is possibly effective for acute fulminant CNS demyelinating diseases (including MS, ADEM, NMO, and TM) that fail to respond to high-dose corticosteroid treatment. Because the study included subgroups of patients with demyelinating diseases, it is not possible to determine if plasmapheresis is more or less effective in patients with different demyelinating diseases. For chronic progressive or secondary progressive MS, plasmapheresis is established as ineffective based on consistent Class I evidence. (Note that the term chronic progressive MS is no longer used, but previously included patients are now described as having either primary progressive MS or secondary progressive MS.)
Recommendations.
Plasmapheresis should be considered for the adjunctive treatment of exacerbations in relapsing forms of MS (Level B). Plasmapheresis may be considered in the treatment of fulminant CNS demyelinating diseases that fail to respond to high-dose corticosteroid treatment (Level C). Plasmapheresis should not be offered for chronic progressive or secondary progressive MS (Level A).
Clinical context.
No studies on the efficacy of plasmapheresis compared to other treatment options in MS are available.
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection.
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) is defined as the abrupt onset or exacerbation of a tic or obsessive-compulsive disorder (OCD) in prepubertal children, considered to be triggered by a Group A β-hemolytic streptococcal infection, but there is controversy in the medical community regarding this syndrome as a disease entity.24,25 Thirty children were enrolled in a randomized, controlled study comparing the effectiveness of plasmapheresis, IVIg, or placebo in the treatment of severe, infection-triggered exacerbations of OCD or tic disorders (PANDAS). Investigators were not blinded with regard to which patients underwent plasmapheresis; therefore, this study is Class III. Results of this study showed that at 1 month, patients treated with plasmapheresis showed improvement in OCD symptoms (58%, p < 0.006), anxiety (47%, p < 0.001), overall functioning (35%, p < 0.0009), and tics (49%, p < 0.005) compared to placebo, and these gains were maintained at 1 year post-treatment.26
Conclusions.
There are inadequate data to determine the efficacy of plasmapheresis in the treatment of acute OCD and tic symptoms in the setting of PANDAS (one Class III study).
Recommendation.
There is insufficient evidence to support or refute the use of plasmapheresis in the treatment of acute OCD and tic symptoms in the setting of PANDAS (Level U).
Sydenham chorea.
In a randomized, controlled study, 18 children with Sydenham chorea received plasmapheresis, IVIg, or prednisone.27 Investigators were not blinded as to which patients underwent plasmapheresis; therefore, this is a Class III study. The primary outcome measures were chorea severity as measured by a 6-item chorea severity scale and the ability to perform selected activities of daily living. All groups responded to treatment, and at 1-month follow-up there was 48% improvement for all arms in the mean chorea severity scores, with no superiority of any treatment. Although this improvement was not significant, the study may not have been adequately powered to detect a meaningful difference between the treatment groups.
Conclusions.
There are inadequate data to determine the efficacy of plasmapheresis in Sydenham chorea (one Class III study).
Recommendation.
There is insufficient evidence to support or refute the use of plasmapheresis in the treatment of Sydenham chorea (Level U).
RECOMMENDATIONS FOR FUTURE RESEARCH
For all indications, the optimal plasma exchange protocol (number of exchanges and volumes exchanged) remains to be established through future research.
The role of plasmapheresis in mild AIDP/GBS, in which ambulation is preserved, and the role of plasmapheresis in patients with AIDP/GBS who fail to respond or who relapse after an initial response remains to be defined.
The role of plasmapheresis in the long-term management of CIDP remains to be clarified.
Adequately powered studies that address the duration of benefit are needed to confirm the role of plasmapheresis in the treatment of neuropathies associated with IgA or IgG gammopathy, and to clarify its role in neuropathies associated with IgM gammopathy. Furthermore, differentiation between demyelinating and axonal neuropathies as well as between IgM neuropathies with and without anti-MAG will be needed in future studies.
The use of plasmapheresis in myasthenic crisis and MG prethymectomy requires further research.
The role of plasmapheresis in fulminant demyelinating CNS disease that has not responded to first-line treatment with corticosteroids will need to be confirmed. Individual demyelinating diseases (e.g., NMO, MS, TM) should be addressed separately in future studies to clarify the role of plasmapheresis in each.
Initial data suggest a role of plasmapheresis in accelerating the clearance of natalizumab and restoring leukocyte function.28 Whether this translates into a clinical benefit in the setting of infectious complications of treatment with natalizumab remains to be determined.
Supplementary Material
Supplemental data at www.neurology.org
Appendices e-1–e-4 and table e-1 are available on the Neurology® Web site at www.neurology.org.
Approved by the Therapeutics and Technology Assessment Subcommittee on February 6, 2010; by the Practice Committee on June 28, 2010; and by the AAN Board of Directors on October 18, 2010.
- AAN
- American Academy of Neurology
- ADEM
- acute disseminated encephalomyelitis
- AIDP
- acute inflammatory demyelinating polyneuropathy
- CI
- confidence interval
- CIDP
- chronic inflammatory demyelinating neuropathy
- CMAP
- compound muscle action potential
- GBS
- Guillain-Barré syndrome
- IgA
- immunoglobulin A
- IgG
- immunoglobulin G
- IgM
- immunoglobulin M
- IVIg
- IV immunoglobulin
- MG
- myasthenia gravis
- MGUS
- monoclonal gammopathy of undetermined significance
- MS
- multiple sclerosis
- NDS
- Neuropathy Disability Scale
- NMO
- neuromyelitis optica
- OCD
- obsessive-compulsive disorder
- PANDAS
- pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection
- TM
- transverse myelitis
- TTA
- Therapeutics and Technology Assessment.
DISCLOSURE
Dr. Cortese reports no disclosures. Dr. Chaudhry serves on the editorial board of Neurologist; is an inventor on patent(s) re: Total Neuropathy Score (TNS)—a score for evaluating peripheral neuropathies, for which he receives technology royalties from Abbott, Johnson & Johnson, and sanofi-aventis; receives publishing royalties for Harrison's Principles of Internal Medicine, 17th ed. (McGraw Hill Companies, Inc., 2008); estimates that 40% of his clinical effort is spent on nerve conduction studies; has given expert testimony for the Department of Health and Human Services Vaccine Injury Compensation program; and receives research support from the Neuropathy Association, Nutricia, and Insmed Inc. Dr. So receives publishing royalties for Occupational & Environmental Medicine (Appleton & Lange, 2007), Occupational & Environmental Medicine (Appleton & Lange, 2007), and contributions to UpToDate; receives research support from the NIH (NIEHS, NINDS) and holds stock in Sartoris, Inc. Dr. Cantor has received honoraria from Elsevier and research support from NINDS Intramural Research Funds. Dr. Cornblath has served on a scientific advisory board or as a consultant for Merck Serono, Sun Pharmaceutical Industries Ltd., DP Clinical, Inc., Geron Corporation, Schwarz Biosciences, Avigen, Inc., Pfizer Inc, Johnson & Johnson, GlaxoSmithKline, Abbott, Acorda Therapeutics Inc., Alexion Pharmaceuticals, Inc., Astellas Pharma Inc., Baxter International Inc., Bionevia Pharmaceuticals Inc., Bristol-Myers Squibb, Cebix Incorporated, CSL Behring, Eisai Inc., Exelixis Inc., FoldRx Pharmaceuticals, Genzyme Corporation, Neryx Biopharmaceuticals, Inc., Mitsubishi Tanabe Pharma Corporation, Octapharma AG, Sangamo BioSciences, sanofi-aventis, and Talecris Biotherapeutics; is an inventor on patent(s) re: Total Neuropathy Score (TNS)—a score for evaluating peripheral neuropathies, for which he receives technology royalties from Abbott, Johnson & Johnson, and sanofi-aventis; receives publishing royalties for Diagnosis and Management of Peripheral Nerve Disorders (Oxford University Press, 2001); and has given expert testimony, prepared affidavits, and acted as a witness or consult with regard to legal proceedings. Dr. Rae-Grant has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., and EMD Serono, Inc.; receives publishing royalties for Handbook of Multiple Sclerosis (Springer Healthcare, 2010); and has served on the speakers' bureau for Biogen Idec.
DISCLAIMER
This statement is provided as an educational service of the American Academy of Neurology. It is based on an assessment of current scientific and clinical information. It is not intended to include all possible proper methods of care for a particular neurologic problem or all legitimate criteria for choosing to use a specific procedure. Neither is it intended to exclude any reasonable alternative methodologies. The AAN recognizes that specific patient care decisions are the prerogative of the patient and the physician caring for the patient, based on all circumstances involved. The clinical context section is made available in order to place the evidence-based guideline(s) into perspective with current practice habits and challenges. No formal practice recommendations should be inferred. The views expressed here are those of the authors and do not represent those of the National Institutes of Health or any other part of the US Government. No official support or endorsement by the National Institutes of Health is intended or should be inferred.
CONFLICT OF INTEREST
The American Academy of Neurology is committed to producing independent, critical and truthful clinical practice guidelines (CPGs). Significant efforts are made to minimize the potential for conflicts of interest to influence the recommendations of this CPG. To the extent possible, the AAN keeps separate those who have a financial stake in the success or failure of the products appraised in the CPGs and the developers of the guidelines. Conflict of interest forms were obtained from all authors and reviewed by an oversight committee prior to project initiation. AAN limits the participation of authors with substantial conflicts of interest. The AAN forbids commercial participation in, or funding of, guideline projects. Drafts of the guideline have been reviewed by at least 3 AAN committees, a network of neurologists, Neurology® peer reviewers, and representatives from related fields. The AAN Guideline Author Conflict of Interest Policy can be viewed at www.aan.com.
REFERENCES
- 1. Assessment of plasmapheresis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 1996; 47: 840–843 [PubMed] [Google Scholar]
- 2. Farkkila M, Kinnunen E, Haapanen E, Iivanainen M. Guillain-Barré syndrome: quantitative measurement of plasma exchange therapy. Neurology 1987; 37: 837 [DOI] [PubMed] [Google Scholar]
- 3. Guillain-Barré Study Group. Plasmapheresis and acute Guillain-Barré syndrome. Neurology 1985; 35: 1096 [PubMed] [Google Scholar]
- 4. French Cooperative Group on Plasma Exchange in Guillain-Barré Syndrome. Efficacy of plasma exchange in Guillain-Barré syndrome: role of replacement fluids. Ann Neurol 1987; 22: 753–761 [DOI] [PubMed] [Google Scholar]
- 5. French Cooperative Group on Plasma Exchange in Guillain-Barré syndrome. Appropriate number of plasma exchanges in Guillain-Barré syndrome. Ann Neurol 1997; 41: 298–306 [DOI] [PubMed] [Google Scholar]
- 6. van der Meché FGA, Schmitz PIM. Dutch Guillain-Barré Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. N Engl J Med 1992; 326: 1123–1129 [DOI] [PubMed] [Google Scholar]
- 7. Bril V, Ilse WK, Pearce R, Dhanani A, Sutton D, Kong K. Pilot trial of immunoglobulin versus plasma exchange in patients with Guillain-Barré syndrome. Neurology 1996; 46: 100–103 [DOI] [PubMed] [Google Scholar]
- 8. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet 1997; 349: 225–230 [PubMed] [Google Scholar]
- 9. Nomura T, Hamaguchi K, Hosakawa T, et al. A randomized controlled trial comparing intravenous immunoglobulin and plasmapheresis in Guillain-Barré syndrome. Neurol Ther 2001; 18: 69–81 [Google Scholar]
- 10. Dyck PJ, Daube J, O'Brien P, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med 1986; 314: 461–465 [DOI] [PubMed] [Google Scholar]
- 11. Hahn AF, Bolton CF, Pillay N, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy: a double-blind, sham-controlled, cross-over study. Brain 1996; 119: 1055–1066 [DOI] [PubMed] [Google Scholar]
- 12. Dyck PJ, Litchy WJ, Kratz KM, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol 1994; 36: 838–845 [DOI] [PubMed] [Google Scholar]
- 13. Hughes R, Bensa S, Willison HJ, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol 2001; 50: 195–201 [DOI] [PubMed] [Google Scholar]
- 14. Dyck PJ, Low PA, Windebank AJ, et al. Plasma exchange in polyneuropathy associated with monoclonal gammopathy of undetermined significance. N Engl J Med 1991; 325: 1482–1486 [DOI] [PubMed] [Google Scholar]
- 15. Oksenhendler E, Chevret S, Leger JM, Louboutin JP, Bussel A, Brouet JC. Plasma exchange and chlorambucil in polyneuropathy associated with monoclonal IgM gammopathy. J Neurol Neurosurg Psychiatry 1995; 59: 243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goti P, Spinelli A, Marconi G, et al. Comparative effects of plasma exchange and pyridostigmine on respiratory muscle strength and breathing pattern in patients with myasthenia gravis. Thorax 1995; 50: 1080–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagayasu T, Yamayoshi T, Matsumoto K, et al. Beneficial effects of plasmapheresis before thymectomy on the outcome in myasthenia gravis. Jpn J Thorac Cardiovasc Surg 2005; 53: 2–7 [DOI] [PubMed] [Google Scholar]
- 18. Weiner HL, Dau PC, Khatri BO, et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression of acute attacks of multiple sclerosis. Neurology 1989; 39: 1143–1149 [DOI] [PubMed] [Google Scholar]
- 19. Weinshenker BG, O'Brien PC, Petterson TM, et al. A trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 1999; 46: 878–886 [DOI] [PubMed] [Google Scholar]
- 20. Khatri BO, McQuillen MP, Harrington GJ, Schmoll D, Hoffmann RG. Chronic progressive multiple sclerosis: double-blind controlled study of plasmapheresis in patients taking immunosuppressive drugs. Neurology 1985; 35: 312–319 [DOI] [PubMed] [Google Scholar]
- 21. The Canadian Cooperative Multiple Sclerosis Study Group. The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Lancet 1991; 337: 441–446 [PubMed] [Google Scholar]
- 22. Gordon PA, Carroll DJ, Etches WS, et al. A double-blind controlled pilot study of plasma exchange versus sham apheresis in chronic progressive multiple sclerosis. Can J Neurol Sci 1985; 12: 39–44 [DOI] [PubMed] [Google Scholar]
- 23. Sorensen PS, Wanscher B, Szpirt W, et al. Plasma exchange combined with azathioprine in multiple sclerosis using serial gadolinium-enhanced MRI to monitor disease activity: a randomized single-masked cross-over pilot study. Neurology 1996; 46: 1620–1625 [DOI] [PubMed] [Google Scholar]
- 24. Swedo S, Leonard HL, Rapoport JL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) subgroup: separating fact from fiction. Pediatrics 2004; 113: 907–911 [DOI] [PubMed] [Google Scholar]
- 25. Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics 2004; 113: 883–886 [DOI] [PubMed] [Google Scholar]
- 26. Perlmutter S, Leiman S, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999; 354: 1153–1158 [DOI] [PubMed] [Google Scholar]
- 27. Garvey MA, Snider LA, Leitman SF, Werden R, Swedo SE. Treatment of Sydenham's chorea with intravenous immunoglobulin, plasma exchange or prednisone. J Child Neurol 2005; 20: 424–429 [DOI] [PubMed] [Google Scholar]
- 28. Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009; 72: 402–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.