Abstract
Objective:
Niemann-Pick disease type C (NPC) is an inherited disorder characterized by intracellular accumulation of lipids such as cholesterol and glycosphingolipids in endosomes and lysosomes. This accumulation induces progressive degeneration of the nervous system. NPC shows some intriguing similarities with Alzheimer disease (AD), including neurofibrillary tangles, but patients with NPC generally lack amyloid-β (Aβ) plaques. Lipids affect γ-secretase-dependent amyloid precursor protein (APP) metabolism that generates Aβ in vitro, but this has been difficult to prove in vivo. Our aim was to assess the effect of altered lipid constituents in neuronal membranes on amyloidogenic APP processing in humans.
Methods:
We examined Aβ in CSF from patients with NPC (n = 38) and controls (n = 14). CSF was analyzed for Aβ38, Aβ40, Aβ42, α-cleaved soluble APP, β-cleaved soluble APP, total-tau, and phospho-tau.
Results:
Aβ release was markedly increased in NPC, with a shift toward the Aβ42 isoform. Levels of α- and β-cleaved soluble APP were similar in patients and controls. Patients with NPC had increased total-tau. Patients on treatment with miglustat (n = 18), a glucosylceramide synthase blocker, had lower Aβ42 and total-tau than untreated patients.
Conclusion:
Increased CSF levels of Aβ38, Aβ40, and Aβ42 and unaltered levels of β-cleaved soluble APP are consistent with increased γ-secretase-dependent Aβ release in the brains of patients with NPC. These results provide the first in vivo evidence that neuronal lipid accumulation facilitates γ-secretase-dependent Aβ production in humans and may be of relevance to AD pathogenesis.
Abnormal amyloid-β (Aβ) metabolism is a core pathologic event in Alzheimer disease (AD).1 Aβ is released from the transmembrane protein Aβ precursor protein (APP) through cleavages by the enzymes β-secretase and γ-secretase. Aβ metabolism has been linked to lipid homeostasis2,3 and several studies suggest that γ-secretase efficiency is affected by membrane lipid raft topography.4–7 Evidence from humans of the effects of cellular cholesterol homeostasis on amyloid metabolism is lacking. To evaluate the effects of altered lipid constituents in neuronal membranes on APP processing in vivo, we examined Aβ in CSF from patients with Niemann-Pick type C disease (NPC).
NPC is a lysosomal storage disorder resulting from mutations in the genes encoding for the NPC1 and NPC2 proteins.8 The clinical spectrum is broad, but progressive neurologic impairment is the major clinical problem.9,10 NPC is characterized by altered neuronal membrane lipid topography and intracellular accumulation of cholesterol and glycosphingolipids.11,12
We compared a group of well-characterized patients with NPC against age- and sex-matched controls in a cross-sectional design to 1) assess the effects of altered lipid topography of neuronal membranes on Aβ metabolism in vivo in humans and 2) examine CSF biomarkers for Aβ metabolism and axonal degeneration in NPC. We hypothesized that patients with NPC would have increased γ-secretase-dependent Aβ production and signs of axonal degeneration. The study was designed in accordance with the STROBE statement.13
METHODS
Standard protocol approvals and patient consent.
All subjects or guardians of subjects provided written informed consent, and when appropriate assent. The study was approved by the National Institute of Child Health and Development (NICHD) Institutional Review Board.
Subjects.
Patients with NPC1 were enrolled in an ongoing longitudinal observational trial at the NIH between August 2006 and April 2009 (figure 1). The presence of the study was made known to the NPC community and all patients or guardians of patients who expressed interest in participating were invited. The inclusion criterion was NPC diagnosis, established by biochemical testing and mutation analysis. Forty patients were eligible. One was excluded due to warfarin treatment, which was a contraindication to lumbar puncture. The remaining 39 underwent CSF sampling. One patient was under 1 year of age at sampling and therefore excluded from this particular study, due to strong postnatal effects on the CSF biomarkers for Aβ metabolism and axonal degeneration.14 The remaining 38 patients were included. Disease severity was scored as described by Yanjanin et al.15 This phenotyping index ascertains neurologic signs and symptoms in 9 major (ambulation, cognition, eye movement, fine motor, hearing, memory, seizures, speech, and swallowing) and 8 minor (auditory brainstem response, behavior, gelastic cataplexy, hyperreflexia, incontinence, narcolepsy, and psychiatric and respiratory problems) domains. The total possible score ranges from 0 to 61, with a higher score indicating more severe clinical impairment. APOE genotyping was performed in patients according to standard procedures. NPC may be treated with substrate reduction therapy using miglustat (N-butyldeoxynojirimycin, Zavesca®, Actelion Pharmaceuticals Ltd, Allschwil, Switzerland), an inhibitor of glucosylceramide synthase that produces glycosphingolipids. This treatment may improve neurologic symptoms.16 Eighteen (47%) patients were on off-label miglustat use (usage without indication approved by the United States Food and Drug Administration). This is representative of miglustat use in the United States during the study period, and miglustat use was primarily determined by availability of insurance coverage. This was not a clinical trial, and investigators with this study neither provided nor prescribed miglustat; however, the NICHD Institutional Review Board specifically approved following patients who were prescribed miglustat by other physicians in this observational trial. Patients on miglustat did not differ in age from patients without miglustat (7.9 [2.9–17.2] years vs 9.1 [1.9–51.3] years, p = 0.76). Nineteen patients who were undergoing CSF collection on other clinical indications were eligible as controls. One of these was excluded due to sample error (all CSF parameters below detection level), and 4 were excluded due to age under 1 year. The remaining 14 were included in the study as controls. The clinical indications for CSF collection were acute lymphatic leukemia (n = 12), pseudotumor (n = 1), and seizures (n = 1). No control had a fever above 38.5°C. For samples with available data, glucose was normal, protein was slightly elevated in one sample, and cultures were negative. White and red blood cell counts were normal in all samples. Demographic data are available in table 1. Data on subjects excluded due to young age are available in table e-1 on the Neurology® Web site at www.neurology.org.
Figure 1. Flow diagram for participating patients with Niemann-Pick type C (NPC).
Number of patients with NPC in the study. One patient did not undergo CSF tapping due to warfarin treatment. One patient was under 1 year of age and excluded from analysis due to known effects of young age on the CSF biomarkers under study.14
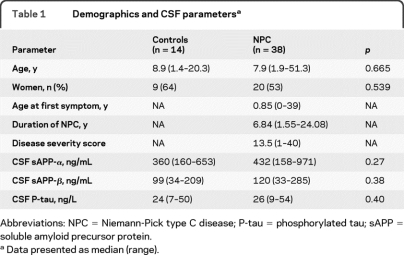
Table 1.
Demographics and CSF parametersa
Abbreviations: NPC = Niemann-Pick type C disease; P-tau = phosphorylated tau; sAPP = soluble amyloid precursor protein.
Data presented as median (range).
Variables.
The endpoints of the study were differences in CSF biomarker levels between groups. The main predictor was NPC diagnosis. Within the NPC group, we examined miglustat treatment, disease severity, and duration as predictors of biomarker levels. Potential confounders were age, sex, and APOE genotype.
CSF sampling.
All CSF samples were collected in the morning by lumbar puncture in the L4/L5 interspace, after an age-appropriate overnight fast. The lumbar puncture was done under anesthesia and concurrent with MRI and ABR testing. CSF was collected in a polystyrene tube, and immediately transported to a local laboratory where it was aliquoted into polypropylene tubes. Samples were frozen on dry ice and stored at −80°C prior to assay. Samples were coded prior to sending to the Clinical Neurochemistry Laboratory in Mölndal, Sweden.
CSF biomarkers of amyloid metabolism and neuronal cell damage.
CSF levels of Aβ1–42, the axonal damage marker T-tau, and tau phosphorylated at threonine 181 (P-tau) were determined using xMAP technology, as previously described.17 APP cleavage by β-secretase releases the extracellular sAPP-β fragment. APP may also be cut by α-secretase within the Aβ domain, precluding Aβ formation and releasing sAPP-α. CSF sAPP-α and sAPP-β levels were determined using the MSD® sAPPα/sAPPβ Multiplex Assay as described by the manufacturer (Meso Scale Discovery, Gaithersburg, MD). This assay employs the 6E10 antibody to capture sAPP-α and a neoepitope-specific antibody to capture sAPP-β. Both isoforms are detected by SULFO-TAG™-labeled anti-APP antibody p2–1. CSF Aβx-38, Aβx-40, and Aβx-42 were measured using the MSD® Human/Rodent (4G8) Abeta Triplex Assay as described by the manufacturer. This assay employs C-terminal specific antibodies to specifically capture Aβx-38, Aβx-40, and Aβx-42. All isoforms are detected by SULFO-TAG™-labeled 4G8 detection antibody. Intra-assay coefficients of variation (CVs) were <5% for all analyses, except for Aβ38 (11.7%), sAPP-β (10.9%), and 1 kit of P-tau (5.13%). Aβ42 measured by MSD correlated to Aβ1–42 measured by Luminex in the total study population (R = 0.938, p < 0.001) and in the subgroups of patients (R = 0.898, p < 0.001) and controls (R = 0.933, p < 0.001). When not otherwise stated, results for Aβ1–42 were similar to those for Aβ42. All biochemical analyses were performed at the Clinical Neurochemistry Laboratory in Mölndal, Sweden, by experienced and certified laboratory technicians who were blinded to diagnoses and clinical data. Two internal control samples (aliquots of pooled CSF) were run on each plate, and strict acceptance criteria were used for approval of each assay.
Statistics.
Statistical calculations were performed using SPSS 17.0 (SPSS Inc., Chicago, IL). As the distribution of quantitative measures was significantly skewed as determined by the Shapiro-Wilk test of normality, statistical tests involving these variables were conducted using the nonparametric Kruskal-Wallis test for comparisons of multiple groups and the Mann-Whitney U test for pairwise comparisons between groups. χ2 statistics with Fisher exact test were used for group comparisons of dichotomized data. The Spearman correlation coefficient was used for analyses of correlation between variables. Quantitative variables are presented as median (range). To control for potential confounding factors, correlations were examined between biomarkers and age, sex, and APOE genotypes. Subgroup analyses were done on patients with or without treatment, and patients with or without high disease severity score (above the median value). The significance level threshold was set to p < 0.05. Due to sample error, data were missing for all CSF parameters in one subject. This subject was excluded from the study.
RESULTS
CSF levels of Aβ.
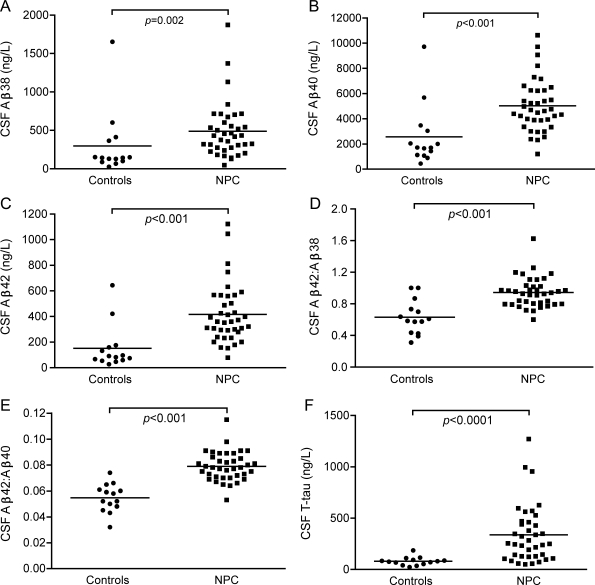
Patients with NPC had higher CSF levels of the Aβ isoforms Aβ38, Aβ40, and Aβ42 than controls (figure 2, A–C). Also, ratios of Aβ42:Aβ40 and Aβ42:Aβ38 were higher in NPC, indicating a shift in release toward the Aβ42 isoform (figure 2, D–E). sAPP-α and sAPP-β were not affected (table 1). Since several patients with NPC had normal Aβ levels, we sought to identify factors related to Aβ in NPC. No correlations were seen between Aβ and age, sex, or disease duration (p > 0.05), but there were correlations to disease severity. Patients with high disease severity score (above the median value 13.5, n = 19) had lower Aβ38, Aβ40, Aβ42, and sAPP-β (figure e-1). When subgrouping by miglustat treatment, these disease severity–dependent differences remained only in untreated patients (Aβ38, 338 [129–1,873] vs 573 [321–1,370] ng/L, p = 0.016; Aβ40, 4,105 [2,312–9,719] vs 6,256 [4,698–10,637] ng/L, p = 0.004; Aβ42, 314 [152–1,122] vs 535 [311–1,045] ng/L, p = 0.007). Correlations between Aβ38, Aβ40, Aβ42, sAPP-α, and sAPP-β are summarized in table e-2.
Figure 2. Elevated CSF levels of Aβ38, Aβ40, and Aβ42 in Niemann-Pick type C (NPC).
CSF levels of Aβ38, Aβ40, and Aβ42 (A–C), ratios of Aβ42 to Aβ40 and Aβ38 (D–E), and CSF levels of T-tau (F) in controls and patients with NPC.
CSF levels of T-tau and P-tau.
The axonal damage marker T-tau was higher in patients with NPC (figure 2F), but P-tau levels were normal (table 1). T-tau correlated to Aβ and sAPP levels in controls and patients, while P-tau only correlated with other biomarkers in patients (table e-2). In patients, T-tau and P-tau decreased with disease duration (R = −0.509, p = 0.001; R = −0.619, p < 0.001) and age (R = −0.461, p = 0.004; R = −0.540, p < 0.001). T-tau and P-tau were not related to sex or disease severity. The only exception was in the subgroup of untreated patients, where those with high disease score (above the median value 13.5) had lower P-tau levels (22 [10–30] vs 31 [21–54] ng/L, p = 0.004).
Influence of APOE genotype on CSF biomarkers.
No APOE-dependent differences were seen on any CSF biomarker in patients (APOE ϵ4/ϵ3, n = 5; APOE ϵ2/ϵ3, n = 2; APOE ϵ3/ϵ3, n = 26). APOE genotype was not available for controls.
Effects of miglustat treatment on Aβ and tau.
Although miglustat treatment did not seem to affect Aβ38, Aβ40, or Aβx-42 (p > 0.05), treated patients had lower levels of Aβ1–42 and T-tau than untreated patients (243 [106–351] vs 277 [172–373] ng/L, p = 0.048; 170 [51–627] vs 348 [59–1,271] ng/L, p = 0.033). sAPP-α and sAPP-β were also lower in the treated group (326 [164–801] vs 502 [158–971] ng/mL, p = 0.015; 81 [34–264] vs 140 [33–285] ng/mL, p = 0.028).
DISCUSSION
Using a well-characterized group of patients with NPC, a condition with altered neuronal lipid homeostasis, we found increased γ-secretase-dependent Aβ production in humans in vivo. The patients had increased levels of the γ-secretase-dependent APP metabolites Aβ38, Aβ40, and Aβ42 in parallel with unaltered sAPP-β levels. These findings were in accordance with our hypothesis, which was based on experimental studies of lipid effects on γ-secretase function. We also found that CSF Aβ and T-tau are promising biomarkers in NPC. This is the first systematic study of these parameters in patients with NPC. This study utilized patient CSF, generating data on in vivo human properties that are unobtainable from animal or cell model studies. The sample size was large considering the rarity of NPC, which favors generalization of the results to other patients with NPC. However, since most patients were children, generalization to adult patients is limited.
Due to its ability to cut APP at different positions, γ-secretase yields Aβ peptides of different length.18 The patients in this study had increased Aβ42:Aβ40 and Aβ42:Aβ38 ratios, demonstrating a shift of the cleavage site activity toward production of Aβ42. It has been proposed that the cleavage specificity might be influenced by membrane thickness19 and different lipid species have different effects on γ-secretase activity in vitro.20 In NPC, it is not clear which lipid species is the primary offending metabolite.21,22 Also, although NPC neurons accumulate cholesterol in late endosomes and lysosomes, the total cholesterol content in NPC brains is not increased.23,24 The altered Aβ production in this study may have been caused by changed γ-secretase activity due to changed lipid constituents of neuronal membranes. However, γ-secretase activity is located in late secretory, endosomal, and synaptic pathways.25,26 It is therefore possible that lysosomal impairment, as a result of lipid accumulation, led to decreased clearance of C-terminal APP fragments with more substrate available for γ-secretase.27 This would implicate alterations in lysosomes or endosomes rather than changed lipid constitution of the plasma membrane as key pathways of abnormal APP processing. Studies on APP processing in patients with other lysosomal storage diseases, both with and without neurologic manifestations, could elucidate this further.
A large body of evidence supports that BACE1 is the main β-secretase.28 BACE1 is a transmembrane protease with a low pH optimum, found in acidic intracellular endosomes and transgolgi. Upon maturation, BACE1 is S-palmitoylated on residues located at the junction of the transmembrane and cytosolic domains, facilitating targeting to lipid rafts.29,30 Although this might enhance BACE1-mediated processing of APP,31 nonpalmitoylated BACE1 seems equally efficient in APP processing.32 We found similar levels of sAPP-β in patients with NPC and controls. Since CSF sAPP-β reflects brain β-secretase activity in primates, this argues against increased activity of β-secretase.33 Likewise, decreased activity of α-secretase was unlikely, since sAPP-α was similar in patients and controls. It is unknown which enzyme exerts the major α-secretase activity in vivo in humans, but the putative α-secretase ADAM10 is absent from lipid rafts, linking low cholesterol content to nonamyloidogenic APP processing.34 The normal sAPP levels in this study argue against increased production or intracellular transport of APP in NPC, but protein expression studies on brain tissue are needed to verify this.
Previous findings on lipid homeostasis and amyloid metabolism are contradictory. Hypercholesterolemia is a risk factor for AD in epidemiologic studies35 and the major genetic risk factor for sporadic AD is the ϵ4 allele of APOE, the main cholesterol carrier in the CNS.36 However, although cholesterol-lowering agents reduce Aβ in experimental studies, results from clinical trials are ambiguous.5,37–40,e1 Clinical correlations between hypercholesterolemia and AD are difficult to interpret, since cholesterol does not cross the blood–brain barrier and nearly all brain cholesterol is synthesized in situ.e2
NPC has some intriguing similarities with AD, including intraneuronal tangles containing P-tau.e3 Despite this, CSF P-tau levels were normal in patients with NPC. This is actually not surprising, since several neurodegenerative conditions, including frontotemporal dementia, have neurofibrillary tangles despite normal CSF P-tau levels.e4 Increased CSF P-tau appears to be a rather AD-specific finding.e5 Increased CSF T-tau in patients with NPC is consistent with axonal degeneration, and CSF T-tau is increased in AD also. Miglustat-treated patients had lower CSF T-tau than untreated patients, which suggests that treatment might have reduced axonal degeneration. Similarly, CSF T-tau was reduced in antibody responders in the AN1792 AD trial with immunization against Aβ, interpreted as a possible reduction of cellular degeneration.e6 These findings allow us to propose that CSF T-tau is a biomarker for treatment effects on axonal degeneration. However, detailed follow-up studies are needed to validate that CSF T-tau was indeed reduced as a consequence of miglustat treatment. Such studies could also include measurements of CSF glycolipids, which would be expected to be lowered by the direct mechanism of action of the drug. Other similarities between AD and NPC include endosomal alterations,e7 lysosomal dysfunction,e8 and accelerated neurologic deterioration in the presence of APOE ϵ4.e9,e10 In autopsy studies, patients with NPC homozygous for APOE ϵ4 had amyloid plaques, but these were diffuse and AD-type dense core plaques were absent.e9,e11 This lack of dense core Aβ plaques is surprising considering the increased production of Aβ42 reported here. Patients with NPC in autopsy studies might be too young to present dense core plaque pathology,e11,e12 but patients up to 40 years of age have been examined, corresponding well to age at autopsy in familial AD, where dense core plaques are readily detected.e13-e15 In this study, APOE genotype did not affect biomarker levels, but only 5 patients carried the APOE ϵ4 allele and these patients were young (age 3.3–12.7 years). Amyloid markers did not correlate with disease duration or age, arguing against amyloid accumulation even in later stages. If patients with NPC indeed do not aggregate extracellular Aβ, increased Aβ42 production might be insufficient for extracellular dense core plaque formation.
The role of Aβ in NPC neurodegeneration is unclear. NPC endosomes accumulate Aβe16 and intracellular Aβ accumulation might be toxic.e17 Correlations between amyloid markers and the clinical disease severity score suggest that CSF Aβ may be used to evaluate disease activity. It is not clear why more severely affected patients had lower CSF Aβ, but advanced neurodegeneration could hypothetically compromise the ability to release Aβ, since synaptic activity is required for Aβ release.e18 Longitudinal studies will clarify if Aβ production changes over time in NPC, which will influence the possibility of using CSF Aβ measurements as disease biomarkers. Experimental studies with drugs targeting amyloid metabolism may give clues on the role of amyloid in NPC neurodegeneration. Studies including larger numbers of adult patients with NPC may show if these initial observations hold true also for older patients where the disease phenotype may be even more heterogeneous.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Åsa Källén, Monica Christiansson, Sara Hullberg, and Dzemila Secic for technical assistance; Shirley Fridlund for language review of the manuscript; Chris Hempel for support of this study; and the caretakers and patients for their participation.
Editorial, page 316
Supplemental data at www.neurology.org
References e1–e18 are available on the Neurology® Web site at www.neurology.org.
- AD
- Alzheimer disease
- APP
- amyloid precursor protein
- CV
- coefficient of variation
- NICHD
- National Institute of Child Health and Development
- NPC
- Niemann-Pick disease type C
- P-tau
- phosphorylated tau
AUTHOR CONTRIBUTIONS
N.M., K.B., H.Z., and F.P. designed the study. S.B., N.Y., and F.P. established the clinical protocol, managed patients, and collected samples. R.F. performed genotyping. N.M. and K.B. analyzed the data and performed the statistical analysis. N.M., K.B., H.Z., and J.M. interpreted the data. N.M. wrote the manuscript. All authors revised the manuscript.
DISCLOSURE
Dr. Mattsson receives research support from Sahlgrenska University Hospital, Gamla Tjänarinnor, Pfannenstills stiftelse, Demensfonden, Thureus stiftelse, Anna-Lisa och Bror Björnssons stiftelse, Hjärnfonden, Svenska Läkaresällskapet, Neurologiskt handikappades riksförbund, Göteborgs läkaresällskap. Dr. Zetterberg has served on a scientific advisory board for GlaxoSmithKline; serves as an Associate Editor of the Journal of Alzheimer's Disease; and receives research support from the Swedish Research Council, the Alzheimer's Association, and the Royal Swedish Academy of Sciences. Dr. Bianconi receives intramural research support from the NIH/NICHD. Dr. Yanjanin receives salary support from the Ara Parseghian Medical Research Foundation and Dana's Angels Research Trust. Dr. Fu has received fellowship support from the NIH. Dr. Månsson has served on a scientific advisory board for Genzyme Corp. Dr. Porter receives intramural research support from the NIH/NICHD; receives research support from Ara Paseghian Medical Research Foundation and Autism Speaks; and holds/has held stock in Pfizer Inc, Curis, Human Genome Sciences, and Genzyme Corporation. Dr. Blennow has served on scientific advisory boards for Innogenetics, AdLyfe Corporation, Bayer Schering Pharma, Bristol-Myers Squibb, Merz Pharmaceuticals, LLC, AstraZeneca, and sanofi-aventis; has received speaker honoraria from Janssen Immunotherapy; serves as a consultant for AstraZeneca and Pfizer Inc; and receives research support from Innogenetics, Bristol-Myers Squibb, the Swedish Research Council, the Swedish Brain Power project, the Swedish Council for Working Life and Social Research, the Swedish Alzheimer Foundation, Stiftelsen för Gamla Tjänarinnor, and the King Gustaf V's and Queen Victoria's Foundation.
REFERENCES
- 1. Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329–344 [DOI] [PubMed] [Google Scholar]
- 2. Grimm MO, Grimm HS, Hartmann T. Amyloid beta as a regulator of lipid homeostasis. Trends Mol Med 2007;13:337–344 [DOI] [PubMed] [Google Scholar]
- 3. Hirsch-Reinshagen V, Burgess BL, Wellington CL. Why lipids are important for Alzheimer disease? Mol Cell Biochem 2009;326:121–129 [DOI] [PubMed] [Google Scholar]
- 4. Zha Q, Ruan Y, Hartmann T, Beyreuther K, Zhang D. GM1 ganglioside regulates the proteolysis of amyloid precursor protein. Mol Psychiatry 2004;9:946–952 [DOI] [PubMed] [Google Scholar]
- 5. Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA 2001;98:5856–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 1998;95:6460–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wahrle S, Das P, Nyborg AC, et al. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis 2002;9:11–23 [DOI] [PubMed] [Google Scholar]
- 8. Vanier MT, Millat G. Niemann-Pick disease type C. Clin Genet 2003;64:269–281 [DOI] [PubMed] [Google Scholar]
- 9. Imrie J, Dasgupta S, Besley GT, et al. The natural history of Niemann-Pick disease type C in the UK. J Inherit Metab Dis 2007;30:51–59 [DOI] [PubMed] [Google Scholar]
- 10. Sevin M, Lesca G, Baumann N, et al. The adult form of Niemann-Pick disease type C. Brain 2007;130:120–133 [DOI] [PubMed] [Google Scholar]
- 11. Zervas M, Dobrenis K, Walkley SU. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J Neuropathol Exp Neurol 2001;60:49–64 [DOI] [PubMed] [Google Scholar]
- 12. Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol 2001;11:1283–1287 [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 14. Mattsson N, Savman K, Osterlundh G, Blennow K, Zetterberg H. Converging molecular pathways in human neural development and degeneration. Neurosci Res 2010;66:330–332 [DOI] [PubMed] [Google Scholar]
- 15. Yanjanin NM, Velez JI, Gropman A, et al. Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet 2010;153B:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol 2007;6:765–772 [DOI] [PubMed] [Google Scholar]
- 17. Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem 2005;51:336–345 [DOI] [PubMed] [Google Scholar]
- 18. Portelius E, Price E, Brinkmalm G, et al. A novel pathway for amyloid precursor protein processing. Neurobiol Aging Epub 2009 [DOI] [PubMed] [Google Scholar]
- 19. Hartmann T, Bieger SC, Bruhl B, et al. Distinct sites of intracellular production for Alzheimer's disease A beta40/42 amyloid peptides. Nat Med 1997;3:1016–1020 [DOI] [PubMed] [Google Scholar]
- 20. Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem 2008;283:22529–22540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic 2010;11:419–428 [DOI] [PubMed] [Google Scholar]
- 22. Gondre-Lewis MC, McGlynn R, Walkley SU. Cholesterol accumulation in NPC1-deficient neurons is ganglioside dependent. Curr Biol 2003;13:1324–1329 [DOI] [PubMed] [Google Scholar]
- 23. Ory DS. The Niemann-Pick disease genes; regulators of cellular cholesterol homeostasis. Trends Cardiovasc Med 2004;14:66–72 [DOI] [PubMed] [Google Scholar]
- 24. Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta 2004;1685:48–62 [DOI] [PubMed] [Google Scholar]
- 25. Bergmans BA, De Strooper B. Gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol 2010;9:215–226 [DOI] [PubMed] [Google Scholar]
- 26. Frykman S, Hur JY, Franberg J, et al. Synaptic and endosomal localization of active gamma-secretase in rat brain. PLoS One 2010;5:e8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karten B, Peake KB, Vance JE. Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim Biophys Acta 2009;1791:659–670 [DOI] [PubMed] [Google Scholar]
- 28. Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J Neurosci 2009;29:12787–12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benjannet S, Elagoz A, Wickham L, et al. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding: the pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem 2001;276:10879–10887 [DOI] [PubMed] [Google Scholar]
- 30. Walter J, Fluhrer R, Hartung B, et al. Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem 2001;276:14634–14641 [DOI] [PubMed] [Google Scholar]
- 31. Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci USA 2003;100:11735–11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vetrivel KS, Meckler X, Chen Y, et al. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J Biol Chem 2009;284:3793–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sankaranarayanan S, Holahan MA, Colussi D, et al. First demonstration of cerebrospinal fluid and plasma A beta lowering with oral administration of a beta-site amyloid precursor protein-cleaving enzyme 1 inhibitor in nonhuman primates. J Pharmacol Exp Ther 2009;328:131–140 [DOI] [PubMed] [Google Scholar]
- 34. Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc Natl Acad Sci USA 2001;98:5815–5820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001;322:1447–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron 2009;63:287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Refolo LM, Pappolla MA, LaFrancois J, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis 2001;8:890–899 [DOI] [PubMed] [Google Scholar]
- 38. Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 2000;57:1439–1443 [DOI] [PubMed] [Google Scholar]
- 39. Simons M, Schwarzler F, Lutjohann D, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer's disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol 2002;52:346–350 [DOI] [PubMed] [Google Scholar]
- 40. Sparks DL, Sabbagh MN, Connor DJ, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol 2005;62:753–757 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.