Abstract
Background:
Plectin crosslinks intermediate filaments to their targets in different tissues. Defects in plectin cause epidermolysis bullosa simplex (EBS), muscular dystrophy (MD), and sometimes pyloric atresia. Association of EBS with a myasthenic syndrome (MyS) was documented in a single patient in 1999.
Objectives:
To analyze the clinical, structural, and genetic aspects of a second and fatal case of EBS associated with a MyS and search for the genetic basis of the disease in a previously reported patient with EBS-MD-MyS.
Methods:
Clinical observations; histochemical, immunocytochemical, and electron microscopy studies of skeletal muscle and neuromuscular junction; and mutation analysis.
Results:
An African American man had EBS since early infancy, and progressive muscle weakness, hyperCKemia, and myasthenic symptoms refractory to therapy since age 3 years. Eventually he became motionless and died at age 42 years. At age 15 years, he had a marked EMG decrement, and a reduced miniature endplate potential amplitude. The myopathy was associated with dislocated muscle fiber organelles, structurally abnormal nuclei, focal plasmalemmal defects, and focal calcium ingress into muscle fibers. The neuromuscular junctions showed destruction of the junctional folds, and remodeling. Mutation analysis demonstrated a known p.Arg2319X and a novel c.12043dupG mutation in PLEC1. The EBS-MD-MyS patient reported in 1999 also carried c.12043dupG and a novel p.Gln2057X mutation. The novel mutations were absent in 200 Caucasian and 100 African American subjects.
Conclusions:
The MyS in plectinopathy is attributed to destruction of the junctional folds and the myopathy to defective anchoring of muscle fiber organelles and defects in sarcolemmal integrity.
Plectin is a ∼500 kDa dumbbell-shaped molecule with a central coiled-coil rod domain flanked by globular N- and C-terminal domains. Owing to tissue and organelle-specific transcript isoforms, plectin is a versatile linker of cytoskeletal components to target organelles in cells of different tissues.1–3 In skeletal muscle, multiple alternatively spliced transcripts of exon preceding common exon 2 link desmin intermediate filaments (IFs) to specific targets: the outer nuclear membrane (isoform 1), the outer mitochondrial membrane (isoform 1b), Z disks (isoform 1 d), and costameres in the sarcolemma (isoform 1f).3 Plectin is also highly expressed at the neuromuscular junction where it provides crucial structural support for the junctional folds.4 Plectin deficiency in muscle results in progressive muscular dystrophy (MD).4–19 Plectin is also highly expressed in intercalated disks in the heart but only a single patient with EBS/MD and cardiomyopathy was identified to date.18 Plectin deficiency in skin causes epidermolysis bullosa simplex (EBS).20 Some patients with EBS and MD (EBS-MD) also had symptoms suggesting a myasthenic disorder9,21–23 but this was not suspected or confirmed by specific studies. The association of EBS-MD with a myasthenic syndrome (MyS) was well-documented in a single patient (P1) in 1999.4 Although numerous autosomal recessive and one dominant mutation in PLEC have been detected,20 the genetic basis of EBS-MD-MyS in P1 was not identified. We describe our findings in a second patient with EBS-MD-MyS (P2), report additional observations in P1, and identify the genetic basis of the disease in both patients.
METHODS
All human studies described here were in accord with the guidelines of the Institutional Review Board of the Mayo Clinic.
Structural observations.
Routine histochemical studies on cryostat sections and electron microscopy studies were performed as previously described.24 Immunoglobulin G and the C3 and C9 complement components were immunolocalized as previously reported.25,26 We immunolocalized the last 50 C-terminal residues of plectin with 4 μg/mL goat polyclonal C-20 antibody (anti-C Ab), and the plectin rod domain with 4 μg/mL 10F6 mouse monoclonal antibody (anti-Rod Ab) (both from Santa Cruz Biotechnology), followed by 3 μg/mL biotinylated donkey antigoat or antimouse immunoglobulin G (IgG) (Jackson ImmunoResearch Laboratories) and the ABC peroxidase kit (Vector Laboratories). Intrafiber calcium excess was evaluated by the Alizarin red stain.27 Synaptic contact regions were visualized on fixed, teased muscle fibers by a cytochemical reaction for acetylcholinesterase.28 The acetylcholine receptor (AChR) and plectin were colocalized at endplates (EPs) with rhodamine-labeled α-bungarotoxin and the plectin anti-Rod Ab followed by fluorescent goat antimouse IgG. EPs were localized for electron microscopy24 and quantitatively analyzed29 by established methods. Peroxidase-labeled α-bungarotoxin was used for the ultrastructural localization of AChR.30
Molecular genetic studies.
Genomic DNA was isolated from blood of P1 and muscle of P2 and mRNA from intercostal muscles of both by standard methods. PLEC nucleotides were numbered according to the mRNA sequence (GenBank reference no: NM_000445). We used PCR primer pairs to amplify and directly sequence the 32 exons and flanking noncoding regions of PLEC isoform 1 and also first exons of isoforms 1b, 1d, and 1f. We screened for the identified novel mutations in 200 Caucasian and 100 African American control subjects using allele-specific PCR. To estimate expression of the rodless isoform of PLEC at the mRNA level, we used real-time PCR and SYBR green I (Roche) with 5′ GTGTCATCCAGGAGTACGTG 3′ as the forward primer in exon 30, 5′ AGCGACAGCAGAGTGACCAT 3′ as the forward primer in exon 31 that encodes the rod domain, 5′ GCCTTCTCCTGCTCGATGAA 3′ as the reverse primer in exon 32 for both forward primers, and GAPDH as the housekeeping gene. All experiments were done in triplicate.
RESULTS
Clinical observations.
P1 is an African American woman. Her case was reported in 1999 when she was 23 years of age.4 In brief, she was diagnosed with EBS as an infant and her myasthenic symptoms began around the age of 9 years. Since 1999, her weakness has worsened so she can now only take a few steps, has dysphagia, is dyspneic on slight exertion and at night, and is resistant to anticholinesterase drugs. However, her skin symptoms are mild, with new skin blisters appearing infrequently.
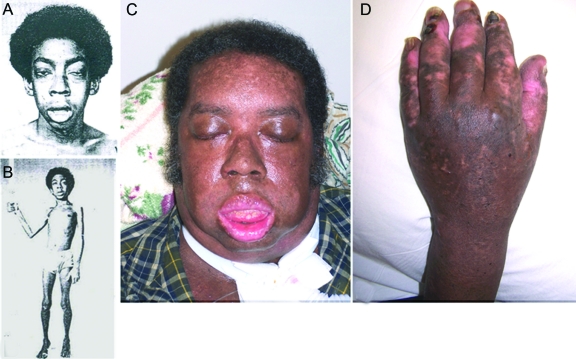
P2 is an African American man. He was a single child without similarly affected family members. He sucked poorly during infancy but this gradually improved. Since the age of 6 weeks, he had an intermittent vesicular eruption over his skin and oral mucosa and developed nail deformities. He attained his motor milestones on time, but had significant fatigue on exertion since age 3 years. At age 11 years he had difficulty running and rising from the floor and serum creatine kinase level was 827 U/L (normal <60 U/L). Prednisone therapy improved his strength but was discontinued because of abdominal pain. Nystatin therapy for thrush worsened the weakness. At age 12 years, a vastus medialis muscle specimen revealed a myopathy associated with necrotic and regenerating fibers, a sural nerve specimen was normal, and a skin biopsy showed EBS and secondary infection. In 1981, at age 15 years, the patient was evaluated at the Mayo Clinic. He now had reduced muscle bulk, bilateral eyelid ptosis, restricted eye movements, and mild facial and moderately severe diffuse cervical and limb muscle weakness, and was areflexic except at the ankles. Nerve conduction studies were normal. Repetitive stimulation at 2 Hz showed a decremental response (67% in hypothenar muscles) that was partially corrected by IV edrophonium chloride. Serratus anterior and intercostal muscles were biopsied. In vitro electrophysiology study of the intercostal specimen by Dr. Edward Lambert revealed reduction of the mean miniature endplate potential amplitude to 50% of normal; the quantal content of the endplate potential was in the low-normal range. Tests for anti-AChR antibodies were negative. A MyS was diagnosed but therapy with pyridostigmine bromide for a year was of no benefit. The weakness progressed more rapidly throughout adolescence and accelerated after routine illnesses. At age 17 years, he could barely walk (figure 1, A and B). He was wheelchair-bound by age 18 years, and respirator-dependent by age 26 years. After age 35 years, he had dysarthria and dysphagia and needed a percutaneous gastrostomy. His cognitive functions and cardiac status remained normal. Subsequently, he became motionless (figure 1C), continued to have skin blisters (figure 1D), communicated with clicks and whispers, failed to respond to 3,4-diaminopyridine combined with pyridostigmine bromide, and died of pneumonia at age 42 years.
Figure 1. Patient photographs.
(A, B) Patient at age 17 years. Note severe asymmetric bilateral ptosis, hyperactive frontalis muscle, facial paresis, open mouth, cubitus valgus, Achilles tendon contractures, and diffuse muscle atrophy. (C, D) Patent at age 41 years. He has a tracheostomy, has facial diplegia, is unable to close his mouth or open his eyes, and shows the chronic skin changes of epidermolysis bullosa simplex. He also has blisters on his lip and tongue and oral moniliasis.
Histochemistry, P2.
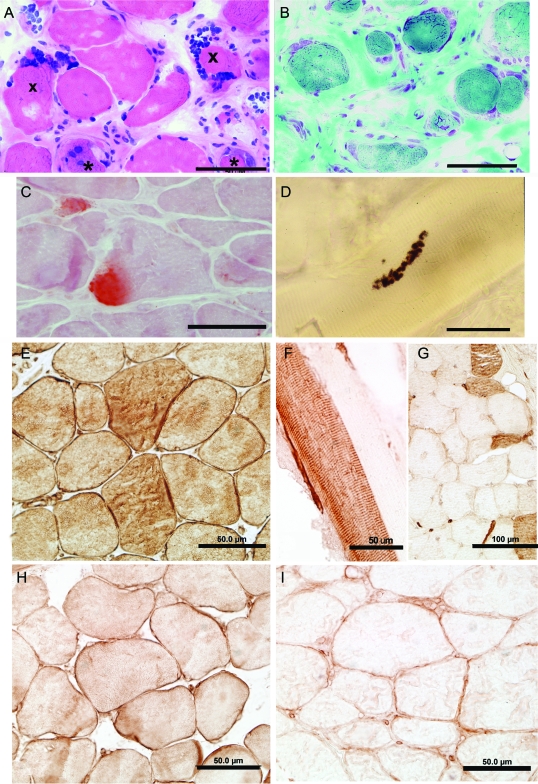
Serratus anterior and intercostal muscle specimens showed similar findings (figure 2, A and B). The muscle fiber diameters varied from 6 μm to ∼120 μm. There was a mild to moderate increase of internal nuclei. Many nuclei were larger than normal and appeared in subsarcolemmal rows or clusters. Some fibers were necrotic or regenerating or subdividing by splitting, or displayed aberrant myofibrils. There was mild to marked (figure 2B) increase of perimysial and endomysial connective tissue. No immunoglobulin G, C3, or C9 deposits were present at patient endplates. In sections reacted for oxidative enzymes, some fibers showed attenuation or an irregular distribution of enzyme activity. Both muscle specimens showed type 1 fiber preponderance. Because plectin deficiency disconnects or weakens the link between the sarcolemma and the underlying cytoskeleton, it likely increases sarcolemmal vulnerability to mechanical stress. We therefore searched for signs of sarcolemmal injury evidenced by subsarcolemmal calcium deposits27 and detected these in scattered fibers in both patients (figure 2C and figure e-1 [on the Neurology® Web site at www.neurology.org]).
Figure 2. Histochemistry and plectin localization studies in patient 2.
(A, B) Note marked variation in fiber size, regenerating fiber elements (asterisks), endomysial fibrosis (B), and clusters of large nuclei at periphery of several fibers. (C) Alizarin red stain reveals focal calcium deposits in 2 fibers. (D) Multiple small cholinesterase-reactive endplate regions arrayed over an extended length of the fiber. Plectin was localized in normal control muscle (E, H) and patient intercostal muscle (F, G, I) with antibody recognizing the plectin rod domain (anti-Rod Ab) (E–G) and antibody recognizing the C-terminal plectin domain (anti-C Ab) (H, I). (E, H) In normal muscle, plectin is localized to the sarcolemma and sarcoplasm with both Abs. The anti-Rod Ab shows plectin-depleted and plectin-positive muscle fibers (F, G), whereas the anti-C Ab shows sarcoplasmic loss and slight sarcolemmal expression of plectin in all muscle fibers (I). Bars indicate 50 μm in all panels except in (G), where they indicate 100 μm.
Plectin immunostains.
These were performed on 6- to 10-μm-thick acetone fixed frozen sections. In 1999, an antibody recognizing the rod domain of plectin (gift from Dr. Owaribe) showed no immunoreactivity in P1 muscle fibers. As this antibody was no longer available, we used the 10F6 antibody directed against the plectin rod domain (anti-Rod Ab), and a C-20 antibody raised against the last 50 C-terminal residues of plectin (anti-C Ab), and immunolocalized plectin in P1, P2, and normal muscle (see Methods). In normal muscle, both antibodies immunostained the sarcolemma, the intermyofibrillar network, capillaries, and vascular smooth muscle (figure 2, E and H); the C-20 Ab also immunostained perineurium and myelinated nerve fibers (not shown). In normal muscle, the anti-Rod Ab (figure 2E) was more reactive than the anti-C antibody (figure 2H) and stained type 1 fibers more intensely than type 2 fibers. In the patients, the anti-Rod Ab demonstrated loss of sarcoplasmic and trace sarcolemmal reactivity in type 1 fibers but, as noted by others,19 type 2 fibers retained plectin positivity (figure 2, F and G, and figure e-2). In contrast, the C-20 Ab revealed no sarcoplasmic and only slight sarcolemmal reactivity in all fibers (figure 2I).
Muscle fiber ultrastructure in P2.
Nuclear abnormalities.
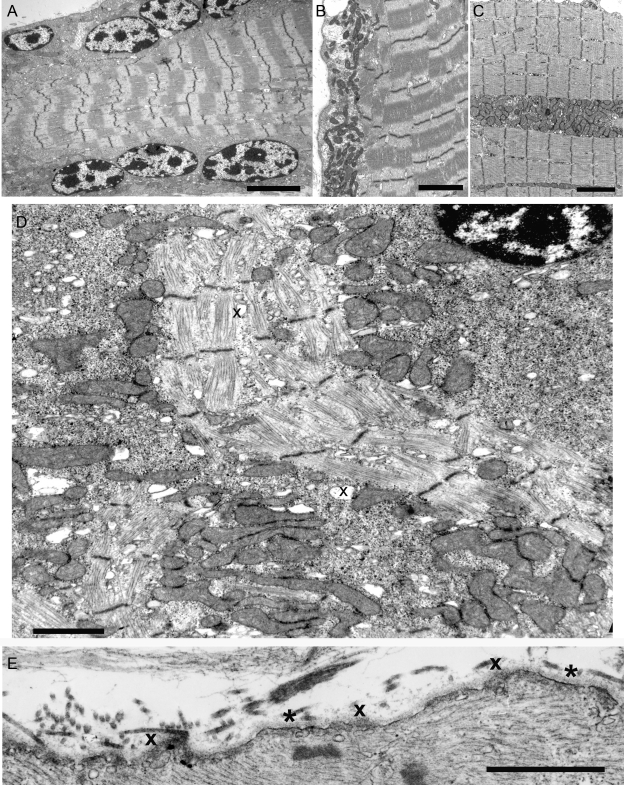
Consistent with light microscopy, numerous muscle fibers harbored subsarcolemmal rows or clusters of large ovoid nuclei containing 3 to 10 large, highly electron dense chromatin deposits (figure 3A). Nuclei in other fiber regions were of normal size and heterochromatic or euchromatic. Some nuclei harbored large clumps of heterochromatin with small islands of euchromatin indicating preapoptotic changes (figure 3D).
Figure 3. Ultrastructural findings in abnormal muscle fibers of patient 2.
(A) Note subsarcolemmal rows of large nuclei harboring multiple prominent chromatin bodies. (B, C) Subsarcolemmal and intrafiber clusters of mitochondria surrounded by fiber regions devoid of mitochondria. (D) Aberrant and disrupted myofibrils surrounded by clusters of mitochondria intermingled with glycogen, ribosomes, and dilated vesicles (x). Note preapoptotic nucleus at upper right. (E) Focal sarcolemma defects due to gaps in the plasma membrane. Where the plasma membrane is absent, the overlying basal lamina is thickened (x). Small vesicles underlie the thickened basal lamina. Asterisks indicate segments of the preserved plasma membrane. Bars = 4 μm in (A), 3 μm (B, C), 1.4 μm in (D), 1 μm in (E).
Mitochondrial abnormalities.
In some fibers, the mitochondria were normally aligned with the Z disk and evenly distributed in the muscle fiber. In other fibers, the mitochondria aggregated into clusters under the sarcolemma (figure 3B), near nuclei, or in other fiber regions (figure 3C), leaving adjacent fiber regions depleted of mitochondria. Some mitochondrial aggregates were interspersed with glycogen granules, ribosomes, and dilated vesicles (figure 3D).
Myofibrillar abnormalities.
Aberrant myofibrils appeared among dislocated organelles in both atrophic and nonatrophic fibers (figure 3D). Disintegration and streaming of Z disks were detected in some fiber regions with or without myofibrillar disarray. A few abnormal fiber regions harbored small nemaline rods. End-stage muscle fibers contained few mitochondria, remnants of Z disks with attached short filaments (Z brushes), and debris. Small membrane-bound vacuoles of different sizes that likely represent dilated components of the sarcoplasmic reticulum appeared in fiber regions with or without other abnormalities (figure 3D).
Sarcolemmal defects.
The focal subsarcolemmal calcium deposits in scattered muscle fibers prompted us to search for sarcolemmal defects at the ultrastructural level and we detected these in some fibers of both patients. Where the plasma membrane was discontinuous, it was often covered by focally thickened basal lamina (figure 3E).
Endplate studies in P2.
Synaptic contacts on the muscle fibers, visualized by the cholinesterase reaction, consisted of multiple small EP regions arrayed over an extended length of the muscle fiber (figure 2D). This finding has been observed in other patients with ongoing destruction and remodeling of the postsynaptic region.31
Colocalization of plectin and AChR by fluorescence microscopy showed strong expressions of plectin and AChR at normal EPs. At patient EPs AChR expression was not appreciably attenuated but plectin expression was barely perceptible (figure e-3).
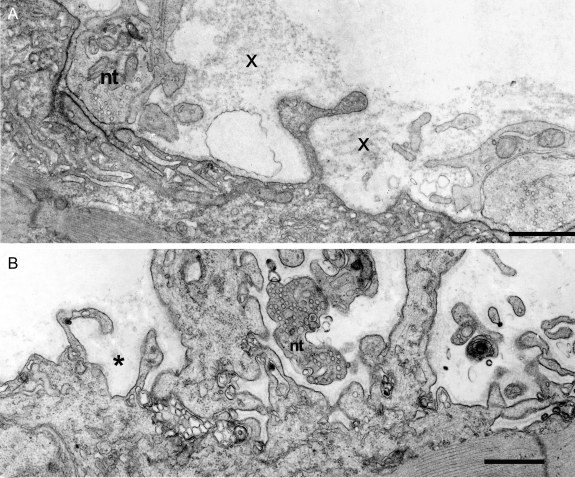
On electron microscopy, some EP regions appeared normal but many had an abnormal conformation, displaying one or more of the following: partial occupancy of the postsynaptic region by nerve terminals, small nerve terminals, atrophic and remnants of degenerated junctional folds resulting in highly simplified postsynaptic regions, and nerve sprouts near degenerating EPs (figure 4 and figure e-4). Table e-1 shows the frequency of the observed conformational changes. Table e-2 shows morphometry revealing reduced size of presynaptic and postsynaptic EP components.
Figure 4. Abnormal endplate (EP) regions in patient 2.
(A) The imaged EP regions show partial occupancy of the postsynaptic region by the nerve terminal and remnants of degenerate folds (x). The nerve terminal (nt) occupies only part of the postsynaptic region. Degenerate remnants of the folds (x) appear over the simplified postsynaptic region from which folds were lost. Dark reaction product on postsynaptic membrane shows acetylcholine receptor localization with peroxidase labeled α-bungarotoxin. (B) Small nerve terminal occupies only part of a highly simplified postsynaptic region. Asterisk indicates remnants of basal lamina that surrounded preexisting folds. Bars = 1 μm.
Genetic analysis.
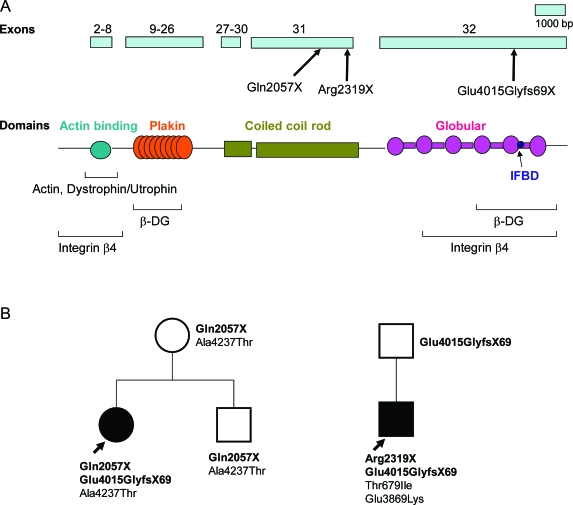
Genetic analysis was challenging owing to the very large size of the PLEC transcript (∼14 Kb vs ∼11 Kb for dystrophin), the very large exons 31 (3381 bp) and 32 (6219 bp), multiple splice variants of exon 1, and polymorphisms that may be race-dependent. Eventually we detected 2 truncating mutations in each patient (figure 5). P1 harbors a stop codon mutation at nucleotide 6169 in exon 31 (c.6169C>T/p.Gln2057X), and a duplication at nucleotide 12043 in exon 32 that predicts 68 missense codons followed by a stop codon (c.12043dupG/p.Glu4015GlyfsX69). P2 harbors a previously reported nonsense mutation at nucleotide 6955 in exon 31 which generates a stop codon (c.6955C>T/p.Arg2319X),16 and the same duplication mutation detected in P1 (figure 5B). Presence of the pathogenic mutation was confirmed at the cDNA level in both patients. Both stop codon mutations abrogate, and the c.12043dupG mutation disrupts, the IF binding site and one of the two β-dystroglycan and integrin β4 binding sites (figure 5A).
Figure 5. Plectin domains and identified PLEC variants.
(A) Schematic representation of PLEC exons 2–32 indicating identified patient mutations and binding domains associated with C- and N-terminal regions of plectin.39 (B) Family analysis of P1 and P2 shows transmission of pathogenic mutations (bold face) and polymorphisms.
Both patients also harbored polymorphisms not listed in the SNP database (see figure 5B). In P1, p.Ala4237Thr was deemed a polymorphism because it appeared together with Gln2057X in the unaffected mother. In P2, p.Thr679Ile was present in 1 of 60 African Americans and p.Glu3869Lys in 1 of 100 African Americans, although both variants were absent in 200 Caucasians.
It has been suggested that expression of the rodless plectin transcript may mitigate the plectinopathy phenotype.32 We therefore confirmed presence of the rodless domain by sequencing cDNA and used real-time PCR to compare the relative abundance of the rodless transcript in P1, P2, and 3 normal controls. The rodless transcript/full transcript ratio was 0.15 in P1, 0.32 in P2, and 0.22 ± 0.03 (mean ± SD) in 3 controls.
DISCUSSION
Although each patient carries a nonsense mutation in PLEC exon 31 and an identical frameshift mutation in exon 32, the tempo of the disease was faster in P2 than in P1. In P2, EBS presented at age 6 weeks, MyS at age 3 years, and he lost ambulation by age 18 years. In P1, MyS presented at age 9 years, EBS at age 18 years, and she can still take a few steps at age 31 years. It has been suggested that expression of the rodless transcript can mitigate the phenotype in patients who carry mutations in the plectin rod domain.32 However, real-time PCR indicates that expression of the rodless transcript was higher in the more severely affected P2. Thus in the patients studied by us the abundance of the rodless transcript was not a reliable indicator of the clinical phenotype.
Dislocation of the fiber organelles apparent at the ultrastructural level has multiple predictable consequences. Abnormal alignment and displacement of the myofibrils weakens or eliminates their contractile strength; separation of mitochondria from myofibrils renders energy delivery to contracting myofibrils inefficient. The eccentrically positioned large nuclei with multiple chromatin deposits may be dysfunctional or inefficient in their translational activities and in nuclear-cytoplasmic trafficking when not adjacent to organelles or fiber domains they subserve. Injury to the inadequately supported plasma membrane is evidenced by subsarcolemmal calcium deposits and sarcolemmal defects in a proportion of the fibers (see figure 2C, figure 3E, and figure e-1). Most of these were smaller than those in Duchenne dystrophy27,33 but they still likely contribute to fiber injury and, if large, they may contribute to fiber necrosis.
Electron microscopy of the EPs indicates that plectin deficiency targets the junctional folds for destruction. When sarcomeres contract and relax, the extrajunctional sarcolemma bulges and relaxes but the junctional folds maintain a constant architecture.34 This mandates enhanced rigidity of the junctional folds and renders them especially vulnerable to mechanical stress. Thus loss of cytoskeletal support of the junctional folds due to the plectin deficiency, as depicted in figure e-3, readily explains the progressive destruction of the folds. Destruction of junctional folds decreases the density or eliminates the voltage-gated Na+ channels which are concentrated in troughs between the folds35,36 and this increases the threshold for action potential generation.37 Destruction of the folds also decreases the input resistance of the postsynaptic membrane and thereby the amplitude of synaptic potential.38 Widening of the synaptic space reduces the concentration of acetylcholine before it reaches the junctional folds. The combination of these factors decreases quantal efficacy and compromises the safety margin of neuromuscular transmission. The relentless progression of the myasthenic symptoms in both patients implies that formation of new EP regions eventually fails to compensate for the ongoing destructive changes.
Why only a proportion of the muscle fibers is affected in a given muscle, and why some EPs are more severely affected than others, remain unanswered questions. It is uncertain that it can be attributed to preserved plectin expression in type 2 fibers, as shown by the 10F6 anti-Rod antibody. First, the anti-C terminal antibody showed nearly complete plectin deficiency in all muscle fibers in both patients. Second, the anti-rod domain antibody showed either no plectin reactivity in any fiber, or preserved immunoreactivity in type 1 instead of type 2 fibers.19 The fiber type specificities of the different anti-rod domain antibodies in plectinopathy are presently unexplained.
It is also unclear why some patients with EBS-MD have myasthenic symptoms and others do not. Possibly myasthenic weakness of the limb muscles is masked by the MD, or is overlooked in severely weak patients, but fatigable weakness of the oculobulbar muscles would be unlikely to go unrecognized. It also is not known whether some mutations are more prone to result in the MyS phenotype than others.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. Neill Graff-Radford and Nilufer Ertekin-Taner for anonymated DNA samples from African American control subjects.
- Ab
- antibodies
- AChR
- acetylcholine receptor
- anti-C Ab
- antibody recognizing the C-terminal plectin domain
- anti-Rod Ab
- antibody recognizing the plectin rod domain
- EBS
- epidermolysis bullosa simplex
- EP
- endplate
- IF
- intermediate filament
- IgG
- immunoglobulin G
- MD
- muscular dystrophy
- MyS
- myasthenic syndrome
- P1
- patient 1
- P2
- patient 2.
Supplemental data at www.neurology.org
DISCLOSURE
Dr. Selcen reports no disclosures. Dr. Juel has received research support from Alexion Pharmaceuticals, Inc. Dr. Hobson-Webb has served as a consultant for Novella Clinical Inc. and has received research support from Genzyme Corporation and AANEM Foundation. Dr. Smith reports no disclosures. Dr. Stickler has received research support from Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention (CDC) and has served as an expert witness in a legal proceeding. Ms. Bite reports no disclosures. Dr. Ohno has received Grants-in-Aid from the Japan Society for the Promotion of Science, the Ministry of Health, Labour and Welfare, and the Japan Science and Technology Agency. Dr. Engel serves as an Associate Editor of Neurology; receives publishing royalties for Myology 3rd ed. (McGraw-Hill, 2004); and has received research support from the NIH and the Muscular Dystrophy Association.
REFERENCES
- 1. Elliott CE, Becker B, Oehler S, Castanon MJ, Hauptmann R, Wiche G. Plectin transcript diversity: identification and tissue distribution of variants with distinct first coding exons and rodless isoforms. Genomics 1997;42:115–125 [DOI] [PubMed] [Google Scholar]
- 2. Fuchs P, Zorer M, Rezniczek GA, et al. Unusual 5′ transcript complexity of plectin isoforms: novel tissue-specific exons modulate actin binding activity. Hum Mol Genet 1999;8:2461–2472 [DOI] [PubMed] [Google Scholar]
- 3. Konieczny P, Fuchs P, Reipert S, et al. Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J Cell Biol 2008;181:667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banwell BL, Russel J, Fukudome T, Shen X-M, Stilling G, Engel AG. Myopathy, myasthenic syndrome, and epidermolysis bullosa simplex due to plectin deficiency. J Neuropathol Exp Neurol 1999;58:832–846 [DOI] [PubMed] [Google Scholar]
- 5. McLean W, Pulkkinen L, Smith F, et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev 1996;10:1724–1735 [DOI] [PubMed] [Google Scholar]
- 6. Smith FJ, Eady R, Leigh I, et al. Plectin deficiency results in muscular dystrophy and epidermolysis bullosa simplex. Nat Genet 1996;13:450–457 [DOI] [PubMed] [Google Scholar]
- 7. Chavanas S, Pulkkinen L, Gache Y, et al. A homozygous nonsense mutation in the PLEC1 gene in patients with epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest 1996;98:2196–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pulkkinen L, Smith F, Shimizu H, et al. Homozygous deletion mutations in the plectin gene (PLEC1) in patients with epidermolysis bullosa simplex associated with late-onset muscular dystrophy. Hum Mol Genet 1996;5:1539–1546 [DOI] [PubMed] [Google Scholar]
- 9. Gache Y, Chavanas S, Lacour J, et al. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest 1996;97:2289–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mellerio J, Smith F, McMillan J, et al. Recessive epidermolysis bullosa simplex associated with plectin mutations: infantile respiratory complications in two unrelated cases. Br J Dermatol 1997;137:898–906 [PubMed] [Google Scholar]
- 11. Dang M, Pulkkinen L, Smith F, McLean W, Uitto J. Novel compound heterozygous mutations in the plectin gene in epidermolysis bullosa with muscular dystrophy and the use of protein truncation test for detection of premature termination codon mutations. Lab Invest 1998;78:195–204 [PubMed] [Google Scholar]
- 12. Takizawa Y, Shimizu H, Rouan F, et al. Four novel plectin gene mutations in Japanese patients with epidermolysis bullosa and muscular dystrophy disclosed by heteroduplex scanning and protein truncation tests. J Invest Dermatol 1999;112:109–112 [DOI] [PubMed] [Google Scholar]
- 13. Shimizu H, Takizawa Y, Pulkkinen L, et al. Epidermolysis bullosa simplex associated with muscular dystrophy: phenotype-genotype correlations and review of the literature. J Am Acad Dermatol 1999;41:950–956 [DOI] [PubMed] [Google Scholar]
- 14. Rouan F, Pulkkinen L, Meneguzzi G, et al. Epidermolysis bullosa: novel and de novo premature termination codon and deletion mutations in the plectin gene predict late-onset muscular dystrophy. J Invest Dermatol 2000;114:381–387 [DOI] [PubMed] [Google Scholar]
- 15. Bauer JW, Rouan F, Kofler B, et al. A compound heterozygous one amino-acid insertion/nonsense mutation in the plectin gene causes epidermolysis bullosa simplex with plectin deficiency. Am J Pathol 2001;158:617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi Y, Rouan F, Uitto J, et al. Plectin deficient epidermolysis bullosa simplex with 27-year-history of muscular dystrophy. J Dermatol Sci 2005;37:87–93 [DOI] [PubMed] [Google Scholar]
- 17. Pfendner E, Rouan F, Uitto J. Progress in epidermolysis bullosa: the phenotypic spectrum of plectin mutations. Exp Dermatol 2005;14:241–249 [DOI] [PubMed] [Google Scholar]
- 18. Bolling MC, Pas HH, De Visser M, et al. PLEC1 mutations underlie adult-onset dilated cardiomyopathy in epidermolysis bullosa simplex with muscular dystrophy. J Invest Dermatol 2010;130:1178–1181 [DOI] [PubMed] [Google Scholar]
- 19. McMillan JR, Akiyama M, Rouan F, et al. Plectin defects in epidermolysis bullosa simplex with muscular dystrophy. Muscle Nerve 2007;35:24–35 [DOI] [PubMed] [Google Scholar]
- 20. Rezniczeck GA, Walko G, Wiche G. Plectin defects lead to various forms of epidermolysis bullosa simplex. Dermatol Clin 2009;28:33–41 [DOI] [PubMed] [Google Scholar]
- 21. Niemi K, Sommer H, Kero M, Kanerva L, Haltia M. Epidermolysis bullosa simplex associated with muscular dystrophy with recessive inheritance. Arch Dermatol 1988;124:551–554 [PubMed] [Google Scholar]
- 22. Fine J-D, Stenn J, Johnson L, Wright T, Bock H, Horiguchi Y. Autosomal recessive epidermolysis bullosa simplex. Arch Dermatol 1989;125:931–938 [DOI] [PubMed] [Google Scholar]
- 23. Doriguzzi C, Palmucci L, Mongini T, et al. Congenital muscular dystrophy associated with familial junctional epidermolysis bullosa letalis. Eur Neurol 1993;33:454–460 [DOI] [PubMed] [Google Scholar]
- 24. Engel AG. The muscle biopsy. In: Engel AG, Franzini-Armstrong C. eds. Myology, 3rd ed. New York: McGraw-Hill; 2004: 681–690 [Google Scholar]
- 25. Engel AG, Lambert EH, Howard FM. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc 1977;52:267–280 [PubMed] [Google Scholar]
- 26. Sahashi K, Engel AG, Lambert EH, Howard FM., Jr Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol 1980;39:160–172 [DOI] [PubMed] [Google Scholar]
- 27. Bodensteiner JB, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology 1978;28:439–446 [DOI] [PubMed] [Google Scholar]
- 28. Gautron J. Cytochimie ultrastructurale des acétylcholinestérases. Microscopie 1974;21:259–264 [Google Scholar]
- 29. Engel AG. Quantitative morphological studies of muscle. In: Engel AG, Franzini-Armstrong C. eds. Myology, 2nd ed. New York: McGraw-Hill; 1994:1018–1045 [Google Scholar]
- 30. Engel AG, Lindstrom JM, Lambert EH, Lennon VA. Ultrastructural localization of the acetylcholine receptor in myasthenia gravis and in its experimental autoimmune model. Neurology 1977;27:307–315 [DOI] [PubMed] [Google Scholar]
- 31. Engel AG, Lambert EH, Mulder DM, et al. A newly recognized congenital myasthenic syndrome attributed to a prolonged open time of the acetylcholine-induced ion channel. Ann Neurol 1982;11:553–569 [DOI] [PubMed] [Google Scholar]
- 32. Nastsuga K, Nishie W, Akiyama M, et al. Plectin expression patterns determine two distinct subtypes of epidermolysis bullosa simplex. Hum Mutat 2010;31:308–316 [DOI] [PubMed] [Google Scholar]
- 33. Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology 1975;25:1111–1120 [DOI] [PubMed] [Google Scholar]
- 34. Ruff RL. Effects of length changes on Na+ current amplitude and excitability near and far from the end-plate. Muscle Nerve 1996;19:1084–1092 [DOI] [PubMed] [Google Scholar]
- 35. Flucher BE, Daniels MP. Distribution of Na+ channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron 1989;3:163–175 [DOI] [PubMed] [Google Scholar]
- 36. Ruff RL, Whittlesey D. Na+ current density and voltage dependence in human intercostal muscle fibers. J Physiol 1992;458:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruff RL, Lennon VA. How myasthenia gravis alters the safety factor of neuromuscular transmission. J Neuroimmunol 2008;15:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin AR. Amplification of neuromuscular transmission by postjunctional folds. Proc R Soc Lond B 1994;258:321–326 [DOI] [PubMed] [Google Scholar]
- 39. Konieczny P, Wiche G. Muscular integrity: a matter of interlinking distinct structures via plectin. In: Lang NG. ed. The Sarcomere and Skeletal Muscle Disease. New York: Springer; 2009:165–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.