Abstract
Background:
In epilepsy as in other disorders, family history information is often obtained by asking patients about the medical histories of their relatives rather than interviewing or examining the relatives directly. The accuracy of this type of information for epilepsy and other seizure disorders is unclear.
Methods:
This study used data from the Genetic Epidemiology of Seizure Disorders in Rochester study, a population-based investigation including all Rochester, MN, residents born ≥1920 with incidence of unprovoked seizures from 1935 to 1994 (case probands) and control probands matched by age, gender, and prior Rochester residency period. Seizure disorders in the first-degree relatives of case and control probands were ascertained by reviewing the relatives' medical records. Case and control probands were interviewed about seizures in their first-degree relatives using a validated 9-question screening interview. Interviewers were blinded to case-control status.
Results:
Sensitivity of the family history (i.e., proportion of relatives with medical record–documented seizures who screened positive in the proband interview) was 62% (32/52) for epilepsy, 50% (7/14) for isolated unprovoked seizures, and 56% (9/16) for febrile seizures. Sensitivity did not differ by case/control status of the proband. Sensitivity was much higher for probands reporting on their offspring or siblings than their parents. Among relatives with epilepsy, 90% of offspring and 80% of siblings but only 32% of parents screened positive.
Conclusions:
Family histories of epilepsy are reasonably accurate for siblings and offspring, but are underreported in parents. Family histories of other seizure disorders are underreported.
In epilepsy, as in many disorders, family history information is extremely important clinically; it can guide diagnosis, help to evaluate the need for additional diagnostic tests (including genetic tests), and inform risk assessment for genetic counseling.1–4 It is also obviously extremely important for genetic research. However, this information is usually obtained by asking patients about seizure occurrence in their relatives rather than interviewing or examining the relatives directly. For many disorders, this approach is insensitive—many truly affected family members are missed.5–15 Moreover, affected individuals may be more likely than unaffected individuals to report their affected relatives, inflating the apparent genetic effect on the disorder.6,10,15,16
Information about the accuracy of family history data for seizure disorders is very limited. We previously reported that people with epilepsy accurately identified epilepsy in their parents and siblings (sensitivity approximately 90%), but underreported other seizure disorders (isolated unprovoked, febrile, and other acute symptomatic seizures).17 However, in our previous study, the gold standard for diagnosis in the relatives was either the relatives' self-reports about their own seizure histories or the probands' mothers' reports about seizures in other family members. We addressed this limitation in the current study. We ascertained seizure disorders in the relatives of case and control probands by reviewing the relatives' medical records, and interviewed the probands about their relatives' seizure histories using a validated, 9-question screening interview with high sensitivity for identifying epilepsy when administered to people about their own seizure histories.18
METHODS
Data collection.
This study used data from the Genetic Epidemiology of Seizure Disorders in Rochester study (GESDR), a population-based investigation using Rochester Epidemiology Project (REP) resources.19 The GESDR case probands were identified in previous studies20,21 and comprised all residents of Rochester, MN, born ≥1920 who had incidence of either epilepsy (≥2 unprovoked seizures) or an isolated unprovoked seizure from 1935 through 1994. For each case proband, we selected a control proband of the same sex and similar birth year (±5 years) who had no unprovoked seizures before the case's diagnosis date, had visited a REP provider within 1 year of that date, and whose first REP visit was within 1 year of that of the case (i.e., with similar length of prior medical record available for review). We investigated the occurrence of seizure disorders in first-degree relatives (parents, siblings, and offspring) of these case and control probands.
Between 2003 and 2008, we comprehensively reviewed the records of the case probands, to confirm study eligibility and update all information pertaining to clinical diagnosis and classification. We also attempted to interview each surviving case and control proband, using a computer-assisted telephone interview (WinCati, Sawtooth Technologies). Interviews were divided into two parts. Part 1 included a 9-question screening interview regarding lifetime history of seizures in the proband,18 a diagnostic interview to obtain further clinical details in subjects who screened positive (modified from our previously described form),22,23 and a family composition section to obtain identifying and demographic information about each first-degree relative. In part 2, case and control probands were interviewed regarding seizure disorders in each first-degree relative identified in part 1, using the same screen (and diagnostic interview where applicable), repeating the questions for each relative (table 1). Parts 1 and 2 were administered by different interviewers, to ensure that the part 2 interviewer was unaware of part 1 results. Both interviewers were blinded to record review findings.
Table 1.
Questions in family history screening interviewa
Questions were asked for each relative separately, and could be answered no, yes, possible, or don't know. Syntax shown is for living relatives. For deceased relatives, syntax was modified accordingly, e.g., “Did your [he/she] ever have …” instead of “Has [he/she] ever had …”
The phrase “Other than the seizure[s] you had because of a high fever” was added only if the subject responded “yes” or “possible” to question 1.
The gold standard for assessing the validity of the probands' responses to the family history screen was each relative's diagnosis of seizure disorders, based on review of the REP medical records. We obtained this information through a process involving 4 steps. First, we used information from the interview's family composition section to identify the relatives of each case or control. For cases, we also used REP resources to identify relatives independently of the interviews, to facilitate inclusion of the relatives of both interviewed and noninterviewed cases in later studies of familial risks of epilepsy. This involved reviewing the case's medical record for relevant information (e.g., guarantors' names, social work notes, obstetric histories); the proband's mother's medical record for sibling names; male probands' spouses' medical records for offspring names; and other sources (e.g., local newspapers for obituaries and wedding announcements). The case probands' relatives identified through this process matched closely with those reported in the family composition section of the interview.
Second, we used demographic information on each relative to search for the relative's medical record identifiers at all REP providers. Based on previous studies,19 relatives with no identifier at any REP provider were assumed to have never resided locally and were excluded. Relatives who denied permission for use of their medical records in research were also excluded.24
Third, we identified relatives ever assigned any diagnostic code in the REP medical index that was possibly indicative of seizure occurrence, using a comprehensive list including 95 codes from 3 different coding systems in place during the study period.
Fourth, we reviewed the complete medical records of relatives with any such code and a random sample of relatives with none of the codes. This involved initial review by trained nurse abstractors, followed by expert review by study epileptologists (J.R.B., W.A.H.), and included all outpatient and inpatient medical visits and test results (including EEG, neuroimaging, and seizure descriptions) from first to last residency within the local area. Among 156 relatives with none of the codes whose records were reviewed, only 1 (0.6%) had incidence of unprovoked seizures while residing locally, suggesting that the false-negative rate was very low.
Data analysis.
To assess the validity of family history information from proband interviews, we defined a positive screen as a “yes” or “possible” response to any of the 9 questions in the screening interview. Sensitivity was defined as the proportion of relatives with medical record–documented seizures who screened positive in the proband interview, and the false-positive rate as the proportion of relatives who screened positive among those presumed to be seizure-free on record review.
Since the GESDR study was population-based and covered a long time interval (1935–1994), some of the cases and controls were related to each other. Consequently, for 23 relatives, family history reports were obtained from more than one proband. We included only one randomly selected family history report for these relatives. In addition, to account for the nonindependence of different relatives within each family, we used generalized estimating equations (GEE) for analysis.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Boards of the Mayo Clinic Rochester, Olmsted Medical Center, and Columbia University Medical Center. All interviewed subjects gave written informed consent.
RESULTS
The study included 910 case and 939 control probands (including some controls for cases later found to be ineligible). Among these 1,849 subjects, 531 could not be interviewed for family history because they were deceased (n = 294), lost to follow-up (n = 133), too ill or disabled (n = 101), or did not know their biological relatives (n = 3). Thirty-four percent (446/1,318) of remaining subjects were interviewed. Participation rates were higher in cases vs controls (37% [220/593] vs 31% [226/725], p = 0.024), women vs men (38% vs 29%, p = 0.001), and subjects aged ≥50 years vs <50 years (41% vs 28%, p < 0.001). Among the cases, participation rates did not differ significantly between subjects with epilepsy vs isolated unprovoked seizure, but were higher in subjects with onset at ≥20 vs <20 years (47% vs 34%, p = 0.004), or focal vs generalized epilepsy (45% vs 32%, p = 0.031).
In the cases (where relatives were identified for both interviewed and noninterviewed individuals), participation rates did not differ between those with and without a family history of epilepsy, based on review of the relatives' medical records (37.6% vs 38.2%, p = 0.92). Also, in the relatives of interviewed controls, the incidence rate of epilepsy, as determined from review of the relatives' medical records, was very similar to the incidence rate in the general population (standardized incidence ratio, adjusted for age, sex, and secular period = 0.99, 95% confidence interval 0.45–1.88). These analyses argue against selection bias related to family history in either cases or controls.
The 446 interviewed probands provided information for 2,936 first-degree relatives. For some of these relatives, medical record review was impossible: 774 had no contact with a REP provider; 1,294 were last seen by a REP provider ≥1 year before the proband interview and thus could subsequently have had seizures that were not documented in the REP records; and 44 either denied permission for research review or had uninformative records. Among the remaining relatives, 726 were presumed to be seizure-free, because they either had no REP diagnosis codes suggestive of seizures or were determined to be seizure-free on record review. Ninety-eight relatives had medical record–documented seizures. Two were excluded because seizure onset was after the family history interview was completed, leaving 96 affected relatives for analysis.
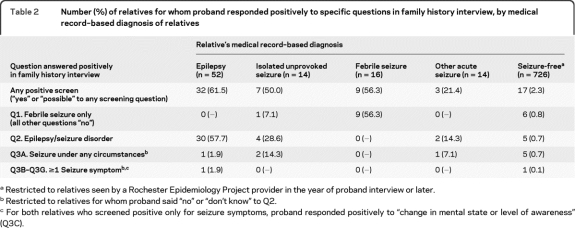
Table 2 shows the distribution of positive responses by probands to specific questions in the screening interview, according to the relatives' medical record–based diagnoses. Sensitivity of the family history data was 62% (32/52) for relatives with epilepsy, but lower for those with isolated unprovoked seizures (50%), febrile seizures (56%), or other acute symptomatic seizures (21%). Among the relatives presumed to be seizure-free, only 2% screened positive.
Table 2.
Number (%) of relatives for whom proband responded positively to specific questions in family history interview, by medical record–based diagnosis of relatives
Restricted to relatives seen by a Rochester Epidemiology Project provider in the year of proband interview or later.
Restricted to relatives for whom proband said “no” or “don't know” to Q2.
For both relatives who screened positive only for seizure symptoms, proband responded positively to “change in mental state or level of awareness” (Q3C).
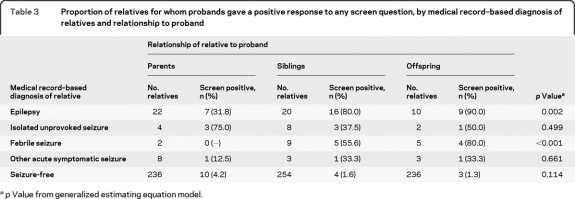
Among relatives with medical record–documented epilepsy, the proportion who screened positive was 90% in offspring, 80% in siblings, and only 32% in parents (p = 0.002, table 3). Similarly, among relatives with febrile seizures, the proportion who screened positive was 80% in offspring but lower in siblings (56%) and parents (0). Among relatives presumed to be seizure-free, the proportion who screened positive (false-positives) was greater for parents (4.6%) than siblings (1.6%) or offspring (1.3%).
Table 3.
Proportion of relatives for whom probands gave a positive response to any screen question, by medical record–based diagnosis of relatives and relationship to proband
p Value from generalized estimating equation model.
For relatives with epilepsy, we examined sensitivity according to attributes of the probands and relatives (table 4). Sensitivity did not differ between case and control probands or according to proband education. However, sensitivity was significantly greater for female vs male probands (74% vs 43%), and for probands aged <50 vs ≥50 years at the time of interview (78% vs 48%). Among case probands, sensitivity was unrelated to probands' age at first unprovoked seizure or epilepsy type, but was greater for probands with vs without a history of convulsive seizures (65% vs 14%).
Table 4.
Proportion of relatives with epilepsy for whom proband gave a positive response to any screen question, by proband and relative characteristics
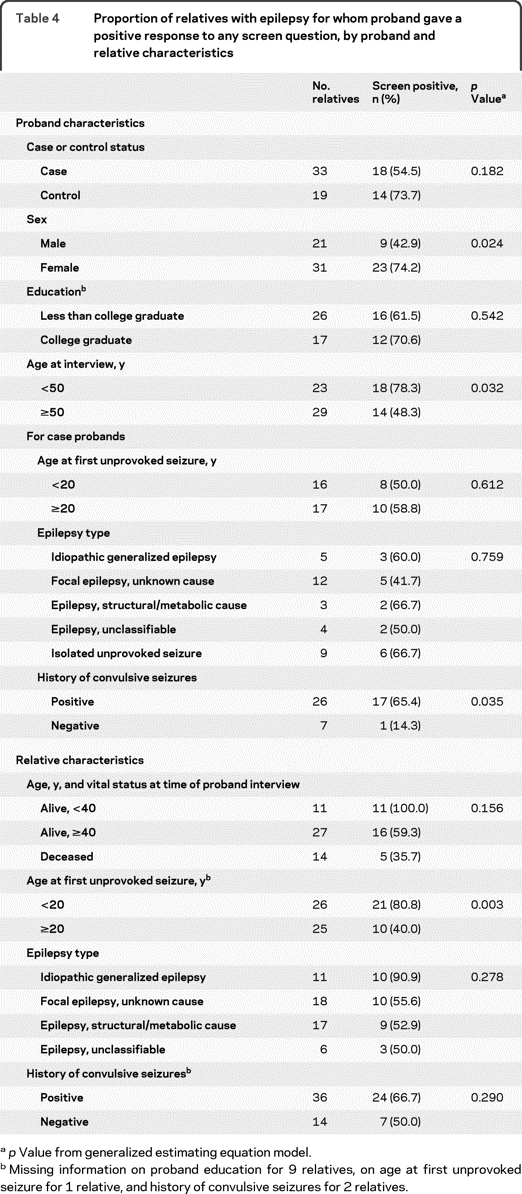
p Value from generalized estimating equation model.
Missing information on proband education for 9 relatives, on age at first unprovoked seizure for 1 relative, and history of convulsive seizures for 2 relatives.
Sensitivity was greater for relatives whose first seizure occurred at age <20 vs ≥20 years (81% vs 40%), for relatives with idiopathic generalized epilepsy (IGE) vs other epilepsy types (91% vs 54%), and, although not significantly, for relatives aged <40 years (100%) vs ≥40 years (59%), or deceased (36%) at the time of proband interview.
Given the markedly higher sensitivity for siblings and offspring than for parents, we examined the potential for confounding by relative type in the relations of sensitivity to the other variables. The distribution of relative types did not differ by proband case/control status, education, age at interview, or diagnostic features (epilepsy type, age at onset, or history of convulsive seizures). However, the proportion of affected relatives who were siblings or offspring was greater for female vs male probands (74% vs 33%), and for relatives aged <40 years vs ≥40 (100% vs 56%) years at interview, with onset <20 vs ≥20 years (73% vs 40%), or with IGE vs other epilepsy types (91% vs 49%). To control for potential confounding resulting from these differences, we examined the strongest predictors of sensitivity within strata defined by relationship to the proband (table 5). Although power was limited by small sample size, the results generally confirmed those from the unstratified analyses. However, the difference in sensitivity between female and male probands was much diminished, suggesting it was largely due to confounding by relative type.
Table 5.
Proportion of relatives with medical record–documented epilepsy for whom probands gave a positive response to any screen question, stratified by relationship to proband
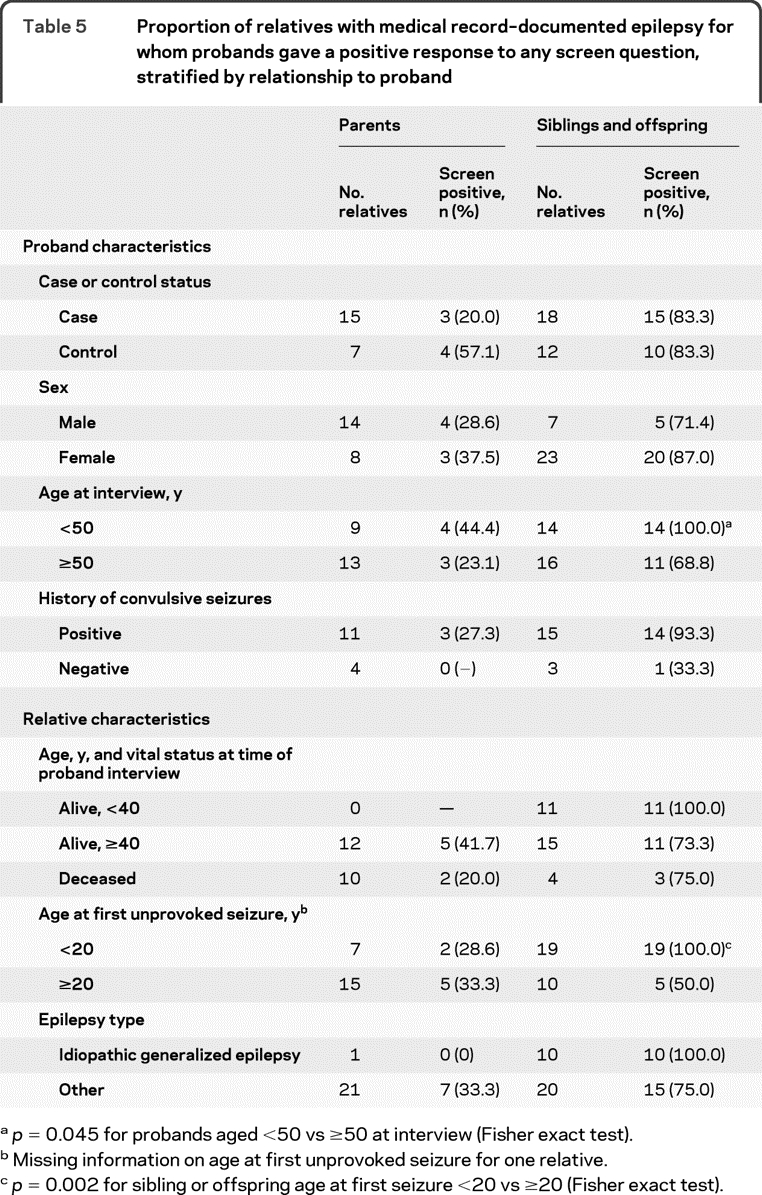
p = 0.045 for probands aged <50 vs ≥50 at interview (Fisher exact test).
Missing information on age at first unprovoked seizure for one relative.
p = 0.002 for sibling or offspring age at first seizure <20 vs ≥20 (Fisher exact test).
DISCUSSION
To our knowledge, this is the most comprehensive analysis to date of the accuracy of family history data on epilepsy and other seizure disorders. The 83% sensitivity of family history of epilepsy in offspring and siblings is encouraging, and higher than in several other neurologic disorders, where sensitivity has been <50% (e.g., migraine,6,7 essential tremor,9,14 and adult-onset dystonia13).
The lower sensitivity in parents (32%) than in siblings or offspring may be explained by diminished recall of diagnoses that occurred long ago, since many of the parents may have had epilepsy as children and achieved remission.25 The lower sensitivity for seizure disorders other than epilepsy (isolated unprovoked, febrile, and other acute symptomatic seizures) probably results from their lower severity and chronicity, as we reported previously.17 However, 80% (4/5) of offspring with febrile seizures were correctly reported to be affected. Also, in relatives with febrile seizures, all positive screens were in response to the question asking specifically about febrile seizures, suggesting that probands were able to distinguish febrile seizures from other seizure disorders.
Family information bias, a greater awareness of family history in cases than in controls, is a significant concern in case-control studies.6,10,15,16 Hence the lack of difference in sensitivity between cases and controls in our study was surprising. Interestingly, a recent review of the validity of family history data from 37 studies of various disorders also failed to find consistent evidence for a difference between cases and controls.1
Sensitivity was greater for relatives who were younger at the time of proband interview, had epilepsy onset <20 years of age, or had IGE, suggesting that family history interviews may miss older relatives, those with later onset, and those with epilepsy types other than IGE. We found no evidence that family history was recalled differently when it occurred in the context of an identified risk factor (e.g., stroke or head trauma). Female probands initially appeared to have higher sensitivity than males, but this was largely attributed to a greater proportion of siblings or offspring among the affected relatives of female probands, reflecting the higher risk of epilepsy in the offspring, and to some extent the siblings, of female vs male probands.26,27
Our study has several strengths. The cases and controls were ascertained from a population-based incidence study. Diagnoses were based on review of the relatives' medical records, carried out independently of family history interviews, and interviewers were blinded to case-control status and record review findings. We used a previously validated questionnaire with high sensitivity when administered to individuals regarding their own medical histories.18 We used GEE models for analysis to control for correlations among members of the same family.
Several potential limitations should also be considered. Only 34% of eligible subjects were interviewed, raising the possibility of selection bias relating to awareness of family history. Our findings argue against this possibility because participation rates were the same in individuals with and without a family history, as determined from medical record review. A family history could not have influenced participation in individuals who were not aware of it. Hence among individuals with a family history, but unaware of it, participation rates should be the same as in those without a family history. The overall participation rate among individuals with a family history is a weighted average of the rates in those who were and were not aware of it. This implies the rate must also have been the same in individuals with a family history who were aware of it as in those without a family history.
Diagnoses of seizure disorders based on REP medical record review, which served as the gold standard in this study, are subject to error but better than expected in many other settings. Our diagnoses were not based on recorded diagnoses alone, but on expert review of original data (e.g., seizure descriptions, EEG, neuroimaging, other medical history) by study epileptologists (J.R.B. and W.A.H.). Among relatives classified as having epilepsy based on medical record review, 89% were seen by neurologists at the Mayo Clinic, and thus detailed data were available for review in most cases. Nevertheless, the available information was sometimes limited.
If some relatives were incorrectly classified as having epilepsy based on record review, yet probands correctly reported they were unaffected, we could have underestimated sensitivity. However, this problem was probably minor because the diagnoses were likely to be correct in those classified as affected by our study epileptologists.
If some affected relatives were missed through record review, yet probands correctly reported them to have had seizures, we could have overestimated the false-positive rate (i.e., proportion reported to have had seizures, among relatives presumed to be seizure-free). The small numbers of relatives with seizure disorders other than epilepsy may have reflected underascertainment of these outcomes, because the diagnostic codes used to screen the relatives' medical records were assembled to maximize sensitivity for identifying epilepsy, the major focus of our study. However, similar to family history data for many disorders,1 the false-positive rate was very low in our study (2%, table 2), so overestimation is probably not extensive.
The unique features of our study population may limit the generalizability of our findings. The subjects included were overwhelmingly white non-Hispanic, and unusually well-educated compared with the general US population. Because of proximity to the Mayo Clinic and other REP healthcare providers, the population has unusually good access to medical care and may be better informed about seizure disorders than in other settings. Other populations may also differ in the rate of concealment of epilepsy diagnoses by family members, or the rates of misdiagnosis or lack of diagnosis. Given the associations of sensitivity with attributes of the probands and relatives, sensitivity could differ in settings with different distributions of these factors.
The approach we used to collect family history data is too time-consuming to be practical in most clinical settings. Sensitivity with the usual clinical query (i.e., “Have any of your relatives ever had seizures or epilepsy?”), or for more distant relatives, is very likely to be lower than that observed here, and remains to be evaluated. Asking about each relative individually and probing for symptoms possibly reflecting seizures are likely to improve sensitivity. The number of false-positives is likely to be small enough that additional information (e.g., from direct interviews with relatives) can be collected for confirmation. To obtain family history information on parents, it may be useful to request that patients ask their parents directly about seizure occurrence in the past.
ACKNOWLEDGMENT
The authors thank Jane Emerson, RN, Melissa Petersen, RN, Diane Carlson, RN, Ann Van Oosten, and Thomas Bitz for assistance with data collection; Keith Onken for programming of the CATI instrument; Gillian Crockford for assistance with data processing; and the study participants for their generous contribution of time to the study.
Footnotes
- GEE
- generalized estimating equation
- GESDR
- Genetic Epidemiology of Seizure Disorders in Rochester
- IGE
- idiopathic generalized epilepsy
- REP
- Rochester Epidemiology Project
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Ruth Ottman.
DISCLOSURE
Dr. Ottman serves on the scientific advisory board for and holds stock options in Trigeminal Solutions, Inc; has received funding for travel from the International League Against Epilepsy, Fondazione Ettore Majorana E Centro, National Epifellows Forum, the National Institute of Mental Health, and Coriell Institute for Medical Research; serves as a consultant to Ortho-McNeil Janssen Scientific Affairs, LLC.; and has received research support from the NIH. Dr. Barker-Cummings has received research support from the NIH. Dr. Leibson has received research support from NLP International Corporation, the AJ and Sigismunda Palumbo Foundation, the American Diabetes Association, and the NIH (NIDRR). Dr. Vasoli receives research support from the NIH. Dr. Hauser serves on a scientific advisory board for King Pharmaceuticals; has served as a consultant to Pfizer Inc, Valeant Pharmaceuticals International, Schwartz Biomedical, LLC, and Lundbeck, Inc. (Ovation Pharmaceuticals, Inc.); serves on the editorial boards of Acta Neurologica Scandinavia, Neuroepidemiology, and Epilepsy Research; and has received research support from the CDC, the NIH, and the Hotchkiss Neurological Institute. Dr. Buchhalter serves on scientific advisory boards for the NIH; serves on the editorial board of Clinical Neurology News; served on the speakers' bureau for UCB; and receives research support from Lundbeck Inc., Pfizer Inc, and the NIH.
REFERENCES
- 1. Berg AO, Baird MA, Botkin JR, et al. National Institutes of Health State-of-the-Science Conference Statement: family history and improving health. Ann Intern Med 2009;151:872–877 [DOI] [PubMed] [Google Scholar]
- 2. Helbig KL, Bernhardt BA, Conway LJ, Valverde KD, Helbig I, Sperling MR. Genetic risk perception and reproductive decision making among people with epilepsy. Epilepsia 2010;51:1874–1877 [DOI] [PubMed] [Google Scholar]
- 3. Ottman R, Hirose S, Jain S, et al. Genetic testing in the epilepsies: report of the ILAE Genetics Commission. Epilepsia 2010;51:655–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family history in public health practice: a genomic tool for disease prevention and health promotion. Annu Rev Public Health 2010;31:69–87 [DOI] [PubMed] [Google Scholar]
- 5. Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry 1977;34:1229–1235 [DOI] [PubMed] [Google Scholar]
- 6. Ottman R, Hong S, Lipton RB. Validity of family history data on severe headache and migraine. Neurology 1993;43:1954–1960 [DOI] [PubMed] [Google Scholar]
- 7. Russell MB, Fenger K, Olesen J. The family history of migraine: direct versus indirect information. Cephalalgia 1996;16:156–160 [DOI] [PubMed] [Google Scholar]
- 8. Devi G, Marder K, Schofield PW, Tang MX, Stern Y, Mayeux R. Validity of family history for the diagnosis of dementia among siblings of patients with late-onset Alzheimer's disease. Genet Epidemiol 1998;15:215–223 [DOI] [PubMed] [Google Scholar]
- 9. Louis ED, Ford B, Wendt KJ, Ottman R. Validity of family history data on essential tremor. Mov Disord 1999;14:456–461 [DOI] [PubMed] [Google Scholar]
- 10. Elbaz A, McDonnell SK, Maraganore DM, et al. Validity of family history data on PD: evidence for a family information bias. Neurology 2003;61:11–17 [DOI] [PubMed] [Google Scholar]
- 11. Marder K, Levy G, Louis ED, et al. Accuracy of family history data on Parkinson's disease. Neurology 2003;61:18–23 [DOI] [PubMed] [Google Scholar]
- 12. Fogelson DL, Nuechterlein KH, Asarnow RF, Payne DL, Subotnik KL. Validity of the family history method for diagnosing schizophrenia, schizophrenia-related psychoses, and schizophrenia-spectrum personality disorders in first-degree relatives of schizophrenia probands. Schizophr Res 2004;68:309–317 [DOI] [PubMed] [Google Scholar]
- 13. Martino D, Aniello MS, Masi G, et al. Validity of family history data on primary adult-onset dystonia. Arch Neurol 2004;61:1569–1573 [DOI] [PubMed] [Google Scholar]
- 14. Prakash KM, Tan EK. Validity of family history in essential tremor. Parkinsonism Relat Disord 2008;14:151–153 [DOI] [PubMed] [Google Scholar]
- 15. Milne BJ, Caspi A, Crump R, et al. The validity of the family history screen for assessing family history of mental disorders. Am J Med Genet B Neuropsychiatr Genet 2009;150B:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sackett DL. Bias in analytic research. J Chronic Dis 1979;32:51–63 [DOI] [PubMed] [Google Scholar]
- 17. Ottman R, Hauser WA, Susser M. Validity of family history data on seizure disorders. Epilepsia 1993;34:469–475 [DOI] [PubMed] [Google Scholar]
- 18. Ottman R, Barker-Cummings C, Leibson CL, Vasoli VM, Hauser WA, Buchhalter JR. Validation of a brief screening instrument for the ascertainment of epilepsy. Epilepsia 2010;51:191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–274 [DOI] [PubMed] [Google Scholar]
- 20. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993;34:453–468 [DOI] [PubMed] [Google Scholar]
- 21. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota Mayo. Clin Proc 1996;71:576–586 [DOI] [PubMed] [Google Scholar]
- 22. Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians' diagnoses. Epilepsia 1990;31:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ottman R, Lee JH, Hauser WA, et al. Reliability of seizure classification using a semistructured interview. Neurology 1993;43:2526–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melton LJ., 3rd The threat to medical-records research. N Engl J Med 1997;337:1466–1470 [DOI] [PubMed] [Google Scholar]
- 25. Ottman R, Lee JH, Hauser WA, Risch N. Birth cohort and familial risk of epilepsy: the effect of diminished recall in studies of lifetime prevalence. Am J Epidemiol 1995;141:235–241 [DOI] [PubMed] [Google Scholar]
- 26. Ottman R, Annegers JF, Hauser WA, Kurland LT. Higher risk of seizures in offspring of mothers than of fathers with epilepsy. Am J Hum Genet 1988;43:257–264 [PMC free article] [PubMed] [Google Scholar]
- 27. Ottman R, Hauser WA, Susser M. Genetic and maternal influences on susceptibility to seizures: an analytic review. Am J Epidemiol 1985;122:923–939 [DOI] [PubMed] [Google Scholar]