Increased activity of the sympathoadrenal system plays a major role in the pathogenesis of essential hypertension and end organ damage.1,2 Recent effective antihypertensive strategies have manipulated autonomic nervous control mechanisms including renal nerve ablation.1 Here, we present the first patient in whom refractory hypertension was controlled chronically with deep brain stimulation (DBS) of the ventrolateral periaqueductal gray (PAG)/periventricular gray (PVG) as a primary response and not secondary to associated pathologic changes.
Level of evidence.
This study provides Class IV evidence that chronic electrical stimulation of the PAG/PVG may provide effective treatment for controlling blood pressure in patients with drug-resistant hypertension.
Case report.
A 55-year-old man developed left-sided weakness, and an ischemic stroke affecting the internal capsule was diagnosed (figure e-1A on the Neurology® Web site at www.neurology.org). At hospital admission, hypertension and hypercholesterolemia were diagnosed. In the peristroke period, blood pressure readings ranged from 265/96 to 153/89 mm Hg, and antihypertensive medication was prescribed: atenolol (50 mg), diltiazem (240 mg), perindopril (4 mg), and indapamide (1.25 mg). Aspirin (75 mg) and simvastatin (40 mg) were also prescribed. This medication regimen maintained his blood pressure at 145/69 mm Hg. Four months later, subsequent to multiple dose increases in the quadruple therapy, his blood pressure ranged from 153/87 to 134/72 mm Hg. Unfortunately, although his hemiplegia resolved, he developed a severe left-sided hemibody central pain syndrome that proved refractory to treatment over the following 3 years, leading to referral for DBS to treat his pain.
With use of established protocols3 and a MRI-guided stereotactic technique, the PAG/PVG region was targeted with a DBS quadripolar electrode (model 3387, Medtronic Inc., Minneapolis, MN) (figure e-1B) because it produces analgesia.4 Informed patient consent was acquired, and ethics approval was obtained (Frenchay Hospital, Research/Development Ethics Committee). The patient underwent optimization of stimulation parameters to alleviate his pain, not to treat his blood pressure (e.g., 10 Hz, 210 μs, and 5.4 V). Pain levels were assessed (table e-1). Before surgery and when taking 4 antihypertensive medications, the patient underwent 24-hour ambulatory blood pressure monitoring. Further monitoring at 2, 3, 7, 27, and 33 months occurred after surgery; on/off stimulation testing was performed at 27 months (table e-2A/B, figure e-2).
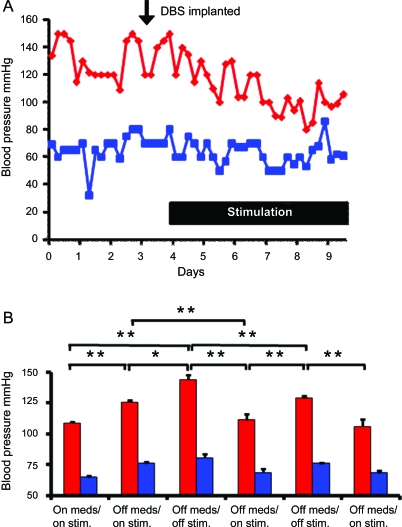
Pain levels decreased initially but returned to presurgical levels at 4 months (table e-1). Serendipitously, immediately after DBS, there was a gradual decrease in blood pressure (trough of 80/53 mm Hg) (figure 1A) that prompted withdrawal of antihypertensive medications. Home monitoring (twice daily readings) for the 8 weeks after surgery showed average blood pressure of 110/65 mm Hg. Twelve weeks postoperatively, blood pressure remained controlled with PAG/PVG stimulation (124/76 mm Hg); this slight increase prompted prescription of Coversyl (perindopril 4 mg and indapamide 1.5 mg). After 33 months, blood pressure was 118/70 mm Hg with Coversyl withdrawn 27 months postoperatively (table e-2A/B, figure e-2).
Figure 1. Activation of the periaqueductal gray (PAG)/periventricular gray (PVG) produced an immediate and pronounced fall in blood pressure (A) and PAG/PVG on/off switching produced repeatable responses in arterial pressure (B).
Blood pressure readings were taken for 3 days before surgery and during deep brain stimulation (DBS) of the PAG/PVG region for 1 week postoperatively (A). Note the immediate fall in blood pressure when stimulation was initiated. The effects of DBS in the PAG/PVG on systolic (red) and diastolic blood pressure (blue) with and without antihypertensive medication (Coversyl) and during on/off stimulation 27 months after the stimulator had been fitted and when pain scores had returned to preoperative levels are shown (B). During the daytime (6:00 am–11:00 pm), readings were taken every 30 minutes and every 60 minutes at nighttime; however, the time spent on each setting was variable and therefore the number of readings ranged from 8 to 75. The average values are presented in the graph. Note the reproducible reductions in arterial blood pressure during stimulation at low frequency (10 Hz). Data are means ± SEM; *p < 0.05; **p < 0.001, paired t test. Meds = Coversyl (perindopril 4 mg and indapamide 1.5 mg); Stim = electrical stimulation of the PAG/PVG.
At 27 months, DBS off-switching increased blood pressure by 18/5 mm Hg (p < 0.01, paired t test); during subsequent on-switching the blood pressure decreased by 33/13 mm Hg (p < 0.01, paired t test). These effects were repeatable (figure 1B, table e-2A/B).
Discussion.
PAG/PVG stimulation can produce a large, sustained lowering of blood pressure in a patient with refractory hypertension, which seems to be efficacious because all antihypertensive medication could be withdrawn. The hypotensive response was independent of the pain relief provided by DBS because it persisted after the patient's pain scores returned to preoperative levels. This finding is important because, for the first time, pain relief was differentiated from a persistent hypotensive response.
The quadripolar electrode used (figure e-1B) is likely to create current spread beyond the targeted PAG area; however, we have no way to quantify this. Although we cannot rule out stimulation spread to the contralateral PAG/PVG region, we believe it is unlikely that thalamic nuclei will have been affected because no side effects associated with thalamic stimulation were observed. We found that stimulating the PAG/PVG at 10 Hz provided optimal pain relief, which is consistent with another report,5 whereas frequencies greater than 50 Hz worsened the pain. A 10 Hz frequency would be expected to have an excitatory effect on PAG/PVG neurons, thereby inducing analgesia and hypotension as described in animals.6 In rats, ventrolateral PAG stimulation evoked hypotension associated with peripheral vasodilatation indicative of sympathetic vasomotor withdrawal.6 Indeed, the ventrolateral PAG projects to and terminates in regions containing sympathetic premotor neurons including the raphe magnus, gigantocellular reticular nucleus, rostral ventrolateral medulla (including C1 adrenergic neurons), locus ceruleus, A5 cell group, and paraventricular and lateral hypothalamic nuclei.7 We propose that any of these structures may mediate the hypotension. Whichever pathway is involved, it would need to be inhibitory in function, as proposed earlier.6
With hypertension in so many patients being resistant to multiple drug therapy, alternative strategies are needed. Manipulating endogenous autonomic blood pressure control mechanisms such as ablating renal nerves1 has provided long-term therapeutic responses in patients with severe refractory hypertension. The present data and those of others1,4 indicate that manipulating the autonomic nervous system is a powerful approach for chronic control of hypertension in humans.
Refractory hypertension may relate to high cerebral vascular resistance that triggers hypertension via a Cushing response, thereby ensuring adequate blood flow to the brain.8 Consistent with this prediction, there was complete occlusion of the right internal carotid artery and a 50%–60% stenosis of the left in our patient (figure e-3). Intriguingly, PAG stimulation is known to improve cerebral perfusion, at least in the rat.9 This result leads us to conclude that DBS of the PAG/PVG provides an opportunity to elucidate central mechanisms controlling blood pressure in patients with refractory hypertension, which might include improvement of cerebral perfusion and reductions in sympathetic activity.
Supplementary Material
Footnotes
Disclosure: Dr. Patel has received speaker honoraria from Boston Scientific and Medtronic, Inc. Dr. Javed has served as a consultant for Renishaw plc. Dr. Khan and Dr. Papouchado report no disclosures. Dr. Pickering receives publishing royalties from Oxford University Press; and receives research support from Astellas Pharma Inc. and is a Wellcome Trust Senior Clinical Fellow. Dr. Malizia has received speaker honoraria or funding for travel from Medtronic, Inc, Lundbeck Inc., Eli Lilly and Company, and Servier; serves on the editorial board of Neuropsychopharmacology; serves as a consultant for Bristol-Myers Squibb; receives research support from Schering-Plough Corp. (Organon), Bristol Primary Care Trust, Avon Wiltshire Partnership Trust, North Bristol NHS Trust, and TUDOR Trust; and holds stock in GlaxoSmithKline. Dr. Paton receives research support from the British Heart Foundation.
Supplemental data at www.neurology.org
References
- 1. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009;373:1275–1281 [DOI] [PubMed] [Google Scholar]
- 2. Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension 2009;54:690–697 [DOI] [PubMed] [Google Scholar]
- 3. Pickering AE, Thornton SR, Love-Jones SJ, Steeds C, Patel NK. Analgesia in conjunction with normalisation of thermal sensation following deep brain stimulation for central post-stroke pain. Pain 2009;147:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pereira EA, Wang S, Paterson DJ, Stein JF, Aziz TZ, Green AL. Sustained reduction of hypertension by deep brain stimulation: sustained reduction of hypertension by deep brain stimulation. J Clin Neurosci 2010;17:124–127 [DOI] [PubMed] [Google Scholar]
- 5. Bittar RG, Kar-Purkayastha I, Owen SL, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci 2005;12:515–519 [DOI] [PubMed] [Google Scholar]
- 6. Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal grey: a correlative functional and anatomical study. Brain Res 1991;541:206–215 [DOI] [PubMed] [Google Scholar]
- 7. Farkas E, Jansen AS, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res 1998;792:179–192 [DOI] [PubMed] [Google Scholar]
- 8. Paton JFR, Dickinson CJ, Mitchell G. Harvey Cushing and the regulation of blood pressure in giraffe, rat and man: introducing ‘Cushing's mechanism.’ Exp Physiol 2009;94:11–17 [DOI] [PubMed] [Google Scholar]
- 9. Nakai M, Maeda M. Nitrergic cerebral vasodilatation provoked by the periaqueductal grey. Neuroreport 1996;7:2571–2574 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.