Abstract
Background: Data on the rate of positive penicillin skin test (PenST) results over time in large populations are rare. The factors that influence positive PenST results are incompletely understood.
Objectives: We sought to correlate demographic variables to the rate of positive PenST results over time in a large group of patients with a history of penicillin allergy.
Methods: Results from the first test for all patients tested for penicillin allergy in the Kaiser Permanente Health Care Program in San Diego County, CA, between 1995 and 2007 are reported. All patients were tested with penicillin, penicilloyl-poly-lysine, penilloate, penicilloate, and amoxicillin.
Results: There were 255 positive PenST results in 3469 individuals. The rate of positive PenST results declined from >10% to <5% during the 13 years studied. The positive PenST result rate could be accounted for by the year of testing (R2 = 0.56; p = 0.003) without any significant contribution from the patient's age or the time since reaction (TSR). If the TSR was ≤13 years, the relative risk of a positive PenST result was 2.1 (95% confidence interval = 1.6–2.8). If the study subject's age was ≤38 years, the relative risk of a positive PenST result was 2.1 (95% confidence interval = 1.6–2.7). Females reported higher rates of penicillin allergy history than males did (11% compared with 6.6%; p < 0.0001), but there were no significant sex differences in the rate of positive PenST results.
Conclusions: There has been a steady decline in the proportion of positive PenST results between 1995 and 2007, independent of study subject age and TSR. Increasing age and increasing TSR were associated with a lower rate of positive PenST results.
Introduction
Penicillin skin testing has been done in advance of need in large groups of people since penicilloyl-poly-lysine (PPL) became commercially available in the 1970s.1,2 There have been higher rates for positive penicillin skin test (PenST) results reported in recent studies from Europe and the Middle East compared to most recent studies from the US.3–7 The literature on IgE–mediated penicillin allergy has become increasingly difficult to analyze8 for the following reasons: 1) reports of PenST results are marked by differences in populations studied, testing criteria, reagents, and testing methods; 2) some studies have very small sample sizes and include individuals with histories of reactions to nonpenicillin β-lactams;9 3) there are significant disagreements on the concentration of native amoxicillin that should be used for testing;10–12 and 4) there is no international consensus on what constitutes an appropriate panel of PenST reagents.
Another factor contributing to this confusing state is that there may be population variation in the rate of positive PenST results over time. The positive PenST result rate in children in the US has markedly declined since the early 1990s.7 We present data here that was derived from a large population, including both children and adults, studied by a single group of investigators for more than 13 years using the same method of skin testing with a extensive panel of chemically well-defined PenST reagents. In addition to determining variation over time of positive PenST results, we identified clinical predictors of positive results in this population.
Methods
This study was reviewed and approved by the Kaiser Permanente (KP) Southern California institutional review board. All PenSTs were performed by registered nurses from the KP San Diego Allergy Department. Patients were tested either in the outpatient setting or in the hospital. The KP Health Care Program maintains a single comprehensive medical record for each member. The medical records since 2007 are completely electronic. This report complies with the position paper of the European Academy of Allergology and Clinical Immunology on nomenclature for allergy.13
Penicillin Skin Test Reagents
All patients were tested with penicillin (0.01 molar), penicilloyl-poly-lysine (PPL) in the form of Pre-Pen or self-produced PPL (6 × 10−5 molar), penicilloate (0.01 molar), penilloate (0.01 molar), and amoxicillin (0.01 molar).12 Commercially produced PPL (Pre-Pen) became unavailable in the US after September 2004. Penicillin skin testing was done between October 2004 and October 2006 using outdated Pre-Pen, as previously reported.14 All penicillin skin testing done after October 2006 was done with PPL produced and assayed in the KP Southern California Regional Immunology Laboratory, as noted in the Sidebar: The production of penicilloyl-poly-lysine.
Penicillin Skin Test Method
A buffered saline negative control and a histamine (1 mg/mL for prick tests and 0.1 mg/mL for intradermal tests [ID]) positive control were placed at the start of each round of tests. Drops of each reagent were placed on the outer surface of the upper arm and pricked using a different Duotip-Test device (Lincoln Diagnostics, Inc, Decatur, IL, USA) for each drop. After a 15-minute waiting period, skin prick reactions were read and recorded. The mean diameter of the wheal over the mean diameter of the flare or surrounding erythema was measured in millimeters. Positive responses consisted of a wheal of ≥5 mm in diameter with surrounding erythema greater than the wheal, a negative response to the control solution, and a positive response to histamine. If all test responses were negative by skin prick, then ID testing was performed using the outer surface of the other upper arm. Using the same reagents, we administered 0.02 mL of each reagent intradermally through individual 27-gauge tuberculin syringes. ID test results were also read and recorded after 15 minutes. Positive responses consisted of a wheal of ≥5 mm in diameter with surrounding erythema greater than the wheal, a negative response to the control solution, and a positive response to histamine. If any puncture test result was positive, no ID tests were done with any of the remaining negative reagents.
Oral Challenges
An oral amoxicillin challenge was given to 215 individuals tested between November 16, 1994, and May 28, 1996, who had negative results on PenSTs, as previously reported.12,15 Almost all patients with a negative result after July 16, 2006, were given an oral amoxicillin (250 mg) or penicillin (500 mg) challenge and observed for one hour.
Inclusion and Exclusion Criteria
Patients were offered a PenST if they had a history of a penicillin-associated adverse drug reaction and if it was thought that knowing whether they had positive or negative test results on PenSTs would help in their future clinical treatment. Most patients were tested in advance of acute need for a penicillin-class antibiotic. Patients were not offered a PenST if they had any of the following exclusion criteria: Stevens-Johnson syndrome, toxic epidermal necrolysis, hemolytic anemia, nephritis, hepatitis, or oral and/or skin blisters associated with or attributed to previous penicillin-class antibiotic use. Patients who had a history of anaphylaxis, respiratory problems, hives, local swelling at the site of injection, other rashes, gastrointestinal symptoms, unknown index symptoms, and other mild symptoms not specifically excluded by already-mentioned criteria were tested.
Medical history data were obtained from each study subject at the time of the PenST by the nurse performing the test or the physician treating the patient. Study subjects were asked the following questions, and their medical records were reviewed to confirm or add additional information that the patient could not provide:
How long has it been since your last adverse reaction to a penicillin-class antibiotic? The result was recorded as the time since reaction (TSR) in years.
How long after the first dose of penicillin associated with the last adverse reaction did it take for the first adverse reaction symptom(s) to be noticed? The five choices given were as follows: less than 1 hour, 1 to 24 hours, 25 to 72 hours, 73 or more hours, unknown.
- What type of adverse reaction occurred? Because this was an open-ended question, the answers were sorted into the following eight categories:
- Anaphylaxis—if the word anaphylaxis was offered, if shock occurred, or if more than two organ systems were involved
- Hives—if a pruritic rash occurred, where individual lesions making up the rash lasted <24 hours; angioedema could also occur with the hives
- Local swelling—if only an area around an injection swelled
- Other rashes—if some other nonhive rash occurred
- Gastrointestinal—if only abdominal pain, nausea, vomiting, and/or diarrhea occurred
- Pulmonary—if shortness of breath occurred in isolation
- Other—if any other mild symptoms occurred that were not in categories a through f or in the exclusion criteria noted above
- Unknown—if the patient did not know what happened and if the reaction type could not be determined by medical-record review.
We also attempted to collect data on patients' recollections of the index reaction-associated infection, the specific type of penicillin-class antibiotic used, and the route of penicillin administration. Health Plan demographics were obtained for 2007. Data from all Health Plan patients who had at least one outpatient visit in 2007 were reviewed. Patient-reported drug allergy and intolerance was tabulated.
Statistical Analysis
Hypothesis testing for continuous variables was by means of Student's t-test (two groups) and analysis of variance ([ANOVA] more than two groups) and for categoric variables by χ2. Relationships between year of study and rate of positive results on skin tests, and between year of study and mean patient age were determined by simple linear regression. Results were expressed as the average change per year on the basis of the regression coefficient, with the adjusted R2, ANOVA, F, and p value of the models also presented. Independent predictors of positive findings for skin test reactivity were determined by means of stepwise multiple linear regression. In this model, the dependent variable was the percentage of positive penicillin skin test results, and the independent variables were mean age of skin-tested patients, mean TSR, and year tested. Because of potential colinearity, a forward stepwise algorithm was used. Nominal statistical significance was set at p = 0.05. All statistical analyses were performed using SAS version 9.1 statistical software (SAS Institute, Inc, Cary, NC, USA).
Results
Penicillin skin testing was performed on 3469 unique individuals between November 16, 1994, and January 21, 2008, including 3158 previously reported on.12,14–19 Study cohort demographics are reported in Table 1. Of 3469 study subjects tested, 255 had positive results on PenSTs. Of 411,543 Health Plan patients seen during 2007, 37,059 (9.0%) reported a history of penicillin allergy. More females, 11.0%, than males, 6.6%, reported a history of penicillin allergy (p < 0.0001). Males accounted for 33.2% of the Health Plan patients with a history of penicillin allergy in 2007 and 30.8% of the patients undergoing PenST over the course of the study (Table 1). The proportion of positive PenSTs, including the subgroup with positive results on penicillin puncture, was not significantly different between females and males (Table 1). TSR data was not available for 355 (10.2%) study subjects.
Table 1.
Study subject demographics
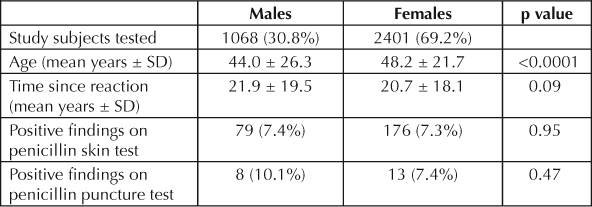
There were 36 adverse reactions, both subjective and objective, during testing, 13 (5.1%) in the group with positive PenST results and 23 (0.72%) in the group with negative PenST results (p < 0.001). In the former group, there were 21 (8.2%) who had positive results on penicillin puncture. There were 4 (19.0%) adverse reactions in the PenST group with positive findings on puncture and 9 (3.9%) in the PenST ID group with positive findings (p = 0.0024). None of the testing-associated reactions were severe. Fewer than half received any treatment. Seven were treated with epinephrine and antihistamines, five were treated with antihistamines only, two received ammonia inhalant, and the rest received no treatment.
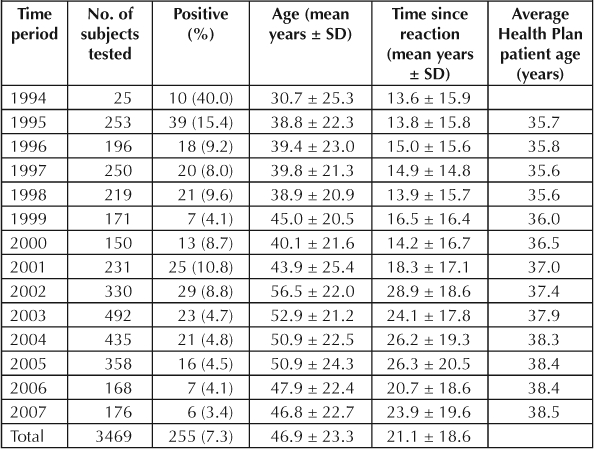
The percentage of positive findings on PenSTs by year of test, along with mean age of tested study subject, TSR, and mean age of Health Plan patients, are shown in Table 2. There was a significant decrease in the rate of positive findings on PenSTs with time (R2 = 0.561; F = 14.069; p = 0.0032). Study subjects were progressively older at an average rate of 1.18 years per year over the 13 years studied (R2 = 0.556; F = 13.783; p = 0.0034). The average age of Health Plan patients increased by only 0.22 years per year. After 2001, a greater emphasis was placed on testing hospitalized individuals who were older than average Health Plan patients (data not shown).
Table 2.
Penicillin skin test results by year of test
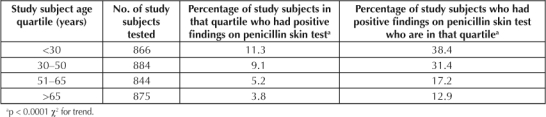
The prevalence of positive findings on PenSTs was highest in younger patients and decreased significantly (p < 0.0001 χ2 for trend) with advancing age, as displayed in Table 3A. Nearly 70% of patients with positive findings on PenSTs were ≤50 years old. Half of the study subjects with positive findings on PenSTs were ≤38 years old. If a patient reported an age of <38 years (median), the relative risk of a positive finding on a PenST was 2.1 (95% confidence interval [CI] = 1.6–2.7).
Table 3A.
Relationship of subject age (by quartile) to the prevalence of positive findings on penicillin skin tests
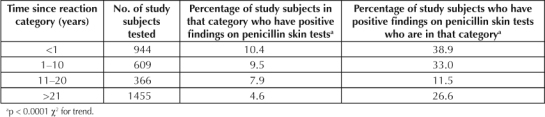
The relationship between the TSR and the prevalence of positive findings on PenSTs is displayed in Table 3B. Patients with positive findings on PenSTs had shorter TSRs (13.3 ± 15.4 years) than patients with negative PenSTs (21.7 ± 18.7 years; p = 0.0001). Half of the study subjects with positive findings on PenSTs had a TSR ≤6 years. One quarter of the study subjects with positive findings on PenSTs had a TSR ≤3 months. Ten percent of the study subjects with positive findings on PenSTs had a TSR ≥38.2 years. If a patient reported a TSR ≤13 years (mean) the relative risk of a positive finding on a PenST was 2.1 (95% CI = 1.6–2.8).
Table 3B.
Relationship of time since reaction to the prevalence of positive findings on penicillin skin tests
Given that both study subject age and TSR correlated to PenST results and the overall population studied was older as the study progressed, a stepwise linear regression was performed to see if the year of testing had an independent effect on the proportion of patients with a positive finding on a PenST in a given year. The rate of positive findings could be accounted for by the year of testing (R2 = 0.56; p = 0.003) without any significant contribution from the patient's age or the TSR.
The prevalence of positive findings on PenSTs was highest in younger patients and decreased significantly … with advancing age …
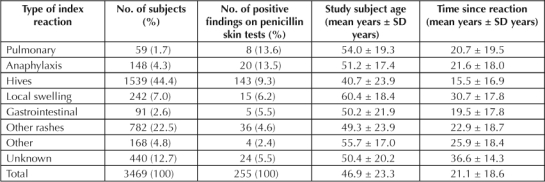
The relationships among the type of index reaction to proportion of positive findings on PenSTs, patient age, and TSR are displayed in Table 4. Time to onset of the index adverse reaction to penicillin was not significantly associated with PenST outcome (n = 2279; χ2 = 4.05; p = 0.26). The index reaction type was related to a positive result for a PenST (Table 3; n = 3029; χ2 = 34.28; p < 0.0001). There was a significant relationship between age and the index reaction type (ANOVA; F = 40.85; p < 0.0001). The most pronounced difference was noted between individuals reporting hives (mean age, 40.7 years) compared with those reporting local swelling (mean age, 60.4 years). There was also a significant relationship noted between the TSR and the index reaction type (ANOVA; F = 35.72; p < 0.0001). Again, the most pronounced differences were seen between study subjects reporting hives (mean TSR, 15.5 years) and those reporting local swelling (mean TSR, 30.7 years). Subjects reporting anaphylaxis were intermediate (mean TSR, 21.6 years).
Table 4.
Relationship between the type of index reaction to the proportion of positive findings on penicillin skin tests, study subject age, and time since reaction
Patient recollection of the infection associated with the index reaction and the specific penicillin-class antibiotic used were too poor to produce a meaningful analysis.
Oral challenges with amoxicillin or penicillin were given to 311 individuals with negative results on PenSTs after July 16, 2006. Data on study subjects given oral challenges after testing before July 16, 2006, have been previously reported.12,15 There were six (1.9%) acute subjective reactions reported; five patients noted itching, but no visible rash was present; and one patient reported chest tightness but had completely normal spirometry results while symptomatic. There were also 5 (1.6%) delayed-onset reactions reported, all rashes starting from 5 to 30 hours after the oral challenge. Several were treated with oral antihistamines, and there were no severe reactions.
Discussion
We find in a large, well-characterized group of individuals with a history of penicillin allergy that the rate of positive results on PenSTs has decreased since 1995. The rate of positive findings on PenSTs was lower in older patients and in those with longer TSRs, but the decreasing rate of positive findings on PenSTs was independent of these variables. A partial explanation for these observations may lie in the changes over time in the route and frequency of outpatient antibiotic use. Parenteral antibiotic use has become rare in the outpatient setting, where most antibiotic use occurs. Consistent with this, patients with histories of local reactions to penicillin injections were the oldest group of patients studied. Overall outpatient oral antibiotic use has also decreased significantly in our Health Plan (data not shown). Reduced use of antibiotics over time, especially by the parenteral route, could help explain the overall decrease in positive results on PenSTs over time as well as the relationship between positive results on PenSTs and both older age and longer TSRs.
Even though the rate of positive findings on PenSTs is decreasing and is lowest in older patients, positive test results still occur. Thus, the PenST is a very useful clinical tool in older individuals who are more likely to be hospitalized and, when in the hospital, much more likely to require antibiotics.18
We did not see higher rates of positive findings on PenSTs in women, as reported recently by Park et al.6 We did see overall similar low rates of positive findings on PenSTs in adults. For patients undergoing penicillin skin testing between June 2, 2002, and June 30, 2004, Park et al reported 64 (3.7%) positives from 1722 valid test results. When they reanalyzed their data using 5 mm as the threshold for a positive finding on a PenST as we did in this study, they did not see a significant difference between males, 8/724 (1.1%) and females 19/988 (1.9%) (Miguel Park, MD personal communication, September 2008).a
Penicillin skin testing as we describe is safe.20 However, a few individuals with positive results on PenSTs, 7% to 10%, also have positive results on PenST puncture tests and thus are extremely allergic. These individuals are likely to have a testing-associated reaction and may benefit from an oral antihistamine given as soon as the positive puncture finding is apparent. This is also the reason we do not perform any ID tests on an individual with any positive puncture results.9,21 Our use of amoxicillin at about 2 mg/mL or 0.01 molar, compared with the 20 to 25 mg/mL used by some other investigators, helps explain two findings. First, it may explain the very high rates of false positive results on PenSTs recently noted by Goldberg and Confino-Cohen,5 where only 6.6% of individuals with positive results on PenSTs responded to an oral challenge, only mild rashes were seen, and no severe reactions to the oral challenges occurred. Second, it might explain the relatively high rates of systemic testing reactions reported by some European investigators.21 If 0.02 mL of a 25-mg/mL solution of amoxicillin is used for ID testing, it results in 0.5 mg of systemic antibiotic exposure, which may be enough to cause a reaction. Additionally concentrated amoxicillin solutions have to be very basic because the solubility of amoxicillin in water at physiologic pH is only about 4.0 mg/mL, which may contribute to a nonspecific irritant effect. The use of a mean wheal diameter of 5 mm with erythema greater than wheal as the positive test result cutoff reduced the rate of false positive results on PenSTs in our study.
The Production of Penicilloyl-poly-lysine.
Penicilloyl-poly-lysine (PPL) was produced as follows: All chemicals were obtained from Sigma Chemicals (St Louis, MO; www.sigmaaldrich.com). A solution of 2 mmol of lysine (0.25 g) in the form of poly-l-lysine hydrobromide (Sigma Chemicals P0879; molecular weight, 1–5 kDa) in 50 mL of sterile deionized water was made by stirring until everything was completely dissolved in the water. An equal molar amount of potassium penicillin G (Sigma Chemicals P8721), 2 mmol (0.0746 g), was slowly added into the solution during continuous stirring. The pH was adjusted to 11.5 with 5N NaOH. The mixture was continuously stirred at room temperature for 90 minutes. A three-times molar excess of succinic anhydride (Sigma Chemicals 28-5500), 6 mmol (0.06 g), was slowly added to the solution with continued stirring. The pH was maintained at 11.0 for 1 hour while stirring continued. Another 0.6 g of succinic anhydride was added into the solution. Stirring continued as pH was maintained at 9.5 for 1 hour. The last 0.6 g of succinic anhydride was added into the solution. Stirring continued as pH was maintained at 9.5 for a final hour. The mixture was transferred into a Spectrum/Por #6 MW cutoff 1K dialysis tubing (Spectrum Laboratories, Inc; Rancho Dominguez, CA; www.spectrapor.com). The PPL was dialyzed against 4-L baths at 4°C, with daily buffer changes, for 7 days. On days 1 and 2, 0.002 M Tris, at a pH of 8.5, with 5 g of BioRad 50W-X2 resin was used. On days 3 to 7, 0.15N NaCl with no resin was used. The dialyzed solution was filtered through a 0.22-μm Millipore (Billerica, MA, USA) filter. The PPL solution was placed into sterile tubes and lyophilized to obtain PPL powder. The PPL was assayed using the penamaldate (HgCl2 titration) method to determine the moles of penicillin bound.1 The molar concentration was calculated using an extinction coefficient of 22.325 for the penicilloyl moiety at 282 nm, pH = 7.6. The penicilloyl-bound concentration (M) = 500{[Amax (3 + 0.02N)/3] – Aini}/22.325 b, where Amax is the maximum absorbance observed at 282 nm, Aini is the initial absorbance at 282 nm, N is the number of 20-μL portions of 0.007% HgCl2 solution (3.5 mg of HgCl2 in 50 mL water) added, and b = the width of the cuvette. A dilution factor of 500 was chosen on the basis of the transfer of 10 μL of the PPL test solution into 5 mL of phosphate-buffered saline (PBS) at a pH of 7.6. The assay was performed using 3 mL of the penamaldate-PPL-PBS mixture in a standard 3-mL quartz cuvette, b = 1 cm. Samples were then tested for endotoxin. Sterility was verified using blood agar plates and a BBL-enriched thioglycolate anaerobic broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The PPL was then diluted with sterile PBS to the desired concentration of 6 × 10−5 molar. The PPL was stored at 4°C until ready for skin testing. The PPL solution has been found to be stable for >2 years at 4°C.
1United States Phamacopeia and National Formulary 2004. Rockville, MD: United States Pharmacopeial Convention, 2003 Nov. p 228.
The use of an oral challenge after a negative skin test result is safe. Our oral challenge reaction rates after a negative PenST result are about an order of magnitude lower than recently reported by European investigators.10,22 About 3% of individuals with a history of penicillin allergy and a negative finding on a PenST will report some sort of adverse reaction, generally mild, after a therapeutic course of a penicillin-class antibiotic. Some of these delayed-onset reactions may be T-cell mediated. In our study, only rarely did an individual report a delayed-onset reaction—one to two days later—at the site of ID tests with negative findings. Our rates of delayed-type hypersensitivity reactions after PenSTs are also about an order of magnitude lower than seen by European investigators, who used much higher amoxicillin concentrations.10,22 Their rate of positive findings on PenST puncture was higher than ours, but their overall rate of positive findings on PenSTs was very similar to ours.
Penicillin skin testing as we describe with oral challenge is an effective way to allow the majority of individuals with a history of penicillin allergy to subsequently take penicillin-class antibiotics. The longer an allergic individual goes without exposure to an allergen, the more likely an allergy is to become clinically insignificant. This was demonstrated well with latex allergy starting in the late 1980s through the early 2000s.23 The inverse relationship demonstrated in our current study between TSR and the rate of positive results on PenSTs is consistent with this paradigm as well.
Penicillin skin testing as we describe with oral challenge is an effective way to allow the majority of individuals with a history of penicillin allergy to subsequently take penicillin-class antibiotics.
In summary, our data suggest that true penicillin allergy, as defined by medical history, positive results on a PenST, and clinically significant reactions with re-exposure may be decreasing over time. We believe that this makes it even more important to identify the increasing majority of patients who have a history of an adverse reaction to penicillin but are not currently allergic. We thus encourage the use of penicillin skin testing and oral challenge as we describe to improve patient safety. We encourage the pharmaceutical industry to provide PPL, penilloate, and penicilloate to use in testing worldwide. Our data suggest that testing would be particularly helpful for older individuals, who are more likely to benefit from the use of penicillin-class antibiotics and less likely to have positive results on a PenST.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.




Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
Footnotes
a Department of Medicine, Division of Allergic Diseases, Mayo Clinic, Rochester, MN.
References
- Green GR, Rosenblum AH, Sweet LC. Evaluation of penicillin hypersensitivity: value of clinical history and skin testing with penicilloyl-polylysine and penicillin G. A cooperative prospective study of the penicillin study group of the American Academy of Allergy. J Allergy Clin Immunol. 1977 Dec;60(6):339–45. doi: 10.1016/0091-6749(77)90064-1. [DOI] [PubMed] [Google Scholar]
- Mendelson LM, Ressler C, Rosen JP, Selcow JE. Routine elective penicillin skin testing in children and adolescents: study of sensitization. J Allergy Clin Immunol. 1984 Jan;73(1 Part 1):76–81. doi: 10.1016/0091-6749(84)90487-1. [DOI] [PubMed] [Google Scholar]
- Palma-Carlos ML, Palma-Carlos AG, Medina M. “In vivo” and “in vitro” tests in the diagnosis of Beta-lactams allergy. Eur Ann Allergy Clin Immunol. 2007 May;39(5):157–61. [PubMed] [Google Scholar]
- Matheu V, Pérez E, González R, et al. Assessment of a new brand of determinants for skin testing in a large group of patients with suspected beta-lactam allergy. J Investig Allergol Clin Immunol. 2007;17(4):257–60. [PubMed] [Google Scholar]
- Goldberg A, Confino-Cohen R. Skin testing and oral penicillin challenge in patients with a history of remote penicillin allergy. Ann Allergy Asthma Immunol. 2008 Jan;100(1):37–43. doi: 10.1016/S1081-1206(10)60402-4. [DOI] [PubMed] [Google Scholar]
- Park MA, Matesic D, Markus PJ, Li JT. Female sex as a risk factor for penicillin allergy. Ann Allergy Asthma Immunol. 2007 Jul;99(1):54–8. doi: 10.1016/S1081-1206(10)60621-7. [DOI] [PubMed] [Google Scholar]
- Jost BC, Wedner HJ, Bloomberg GR. Elective penicillin skin testing in a pediatric outpatient setting. Ann Allergy Asthma Immunol. 2006 Dec;97(6):807–12. doi: 10.1016/S1081-1206(10)60973-8. [DOI] [PubMed] [Google Scholar]
- Torres MJ, Blanca M. Penicillin allergy. Allergy. 2003;58(5):452. doi: 10.1034/j.1398-9995.2003.00585.x. [DOI] [PubMed] [Google Scholar]
- Nolan RC, Puy R, Deckert K, O'Hehir RE, Douglass JA. Experience with a new commercial skin testing kit to identify IgE-mediated penicillin allergy. Internal Med J. 2008 May;38(5):357–61. doi: 10.1111/j.1445-5994.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- Bousquet PJ, Kvedariene V, Co-Minh HB, et al. Clinical presentation and time course in hypersensitivity reactions to beta-lactams. Allergy. 2007 Aug;62(8):872–6. doi: 10.1111/j.1398-9995.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- Solensky R. Hypersensitivity reactions to beta-lactam antibiotics. Clin Rev Allergy Immunol. 2003 Jun;24(3):201–20. doi: 10.1385/CRIAI:24:3:201. [DOI] [PubMed] [Google Scholar]
- Macy E, Richter PK, Falkoff R, Zeiger R. Skin testing with penicilloate and penilloate prepared by an improved method: amoxicillin oral challenge in patients with negative skin test responses to penicillin reagents. J Allergy Clin Immunol. 1997 Nov;100(5):586–91. doi: 10.1016/s0091-6749(97)70159-3. [DOI] [PubMed] [Google Scholar]
- Johansson SG, Hourihane JO, Bousquet J, et al. EAACI (the European Academy of Allergology and Clinical Immunology) nomenclature task force A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature talk force. Allergy. 2001 Sep;56(9):813–24. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. Erratum in: Allergy 2001 Dec;56(12):1229. [DOI] [PubMed] [Google Scholar]
- Macy E, Lin CK, Goldberg B. Penicilloyl-polylysine stability and clinical use over time. Perm J. 2007 Fall;11(4):10–1. doi: 10.7812/tpp/07-063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy E. Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. J Allergy Clin Immunol. 1998 Aug;102(2):281–5. doi: 10.1016/s0091-6749(98)70097-1. [DOI] [PubMed] [Google Scholar]
- Macy E, Burchette RJ. Oral antibiotic adverse reactions after penicillin testing: multi-year follow-up. Allergy. 2002 Dec;57(12):1151–8. doi: 10.1034/j.1398-9995.2002.23700.x. [DOI] [PubMed] [Google Scholar]
- Macy E, Mangat R, Burchette RJ. Penicillin skin testing in advance of need: multiyear follow-up in 568 test result–negative subjects exposed to oral penicillins. J Allergy Clin Immunol. 2003 May;111(5):1111–5. doi: 10.1067/mai.2003.1385. [DOI] [PubMed] [Google Scholar]
- Macy E, Roppe L, Schatz M. Routine penicillin skin testing in hospitalized patients with a history of penicillin allergy. Perm J. 2004 Summer;8(3):20–4. doi: 10.7812/tpp/04.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy E. Penicillin skin testing in pregnant women with a history of penicillin allergy and group B streptococcus colonization. Ann Allergy Asthma Immunol. 2006 Aug;97(2):164–8. doi: 10.1016/S1081-1206(10)60007-5. [DOI] [PubMed] [Google Scholar]
- Macy E. Risks of penicillin skin testing. Ann Allergy Asthma Immunol. 2000 Nov;85(5):330–1. doi: 10.1016/S1081-1206(10)62539-2. [DOI] [PubMed] [Google Scholar]
- Co Minh HB, Bousquet PJ, Fontaine C, Kvedariene V, Demoly P. Systemic reactions during skin tests with beta-lactams: a risk factor analysis. J Allergy Clin Immunol. 2006 Feb;117(2):466–8. doi: 10.1016/j.jaci.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Bousquet PJ, Pipet A, Bousquet-Rouanet L, Demoly P. Oral challenges are needed in the diagnosis of beta-lactam hypersensitivity. Clin Exp Allergy. 2008 Jan;38(1):185–90. doi: 10.1111/j.1365-2222.2007.02867.x. [DOI] [PubMed] [Google Scholar]
- Reunala T, Alenius H, Turjanmaa K, Palosuo T. Latex allergy and skin. Curr Opin Allergy Clin Immunol. 2004 Oct;4(5):397–401. doi: 10.1097/00130832-200410000-00011. [DOI] [PubMed] [Google Scholar]


