Abstract
Pseudomonas syringae pv. phaseolicola synthesizes a non-host-specific toxin, phaseolotoxin, and also synthesizes a phaseolotoxin-resistant ornithine carbamoyltransferase (ROCT) to protect itself from its own toxin. ROCT is encoded by argK, which is expressed coordinately with phaseolotoxin synthesis at 18°C. To investigate the regulatory mechanisms of this system, null mutants were constructed for argK, argF (encoding the phaseolotoxin-sensitive OCTase [SOCT]), and amtA (encoding an amidinotransferase involved in phaseolotoxin synthesis). The argF mutant did not exhibit arginine auxotrophy when grown in M9 medium at 28°C, because under this condition SOCT was replaced by ROCT. This loss of thermoregulation of argK was apparently caused by accumulation of carbamoylphosphate, one of the substrates of SOCT. Carbamoylphosphate, which has a structure similar to that of the inorganic moiety of phaseolotoxin, was used in induction assays with wild-type P. syringae pv. phaseolicola and was shown to be able to induce argK expression in M9 medium at 28°C. These results indicate that argK expression is independent of temperature and is regulated directly by a compound resembling the inorganic moiety of phaseolotoxin.
Pseudomonas syringae pv. phaseolicola is the causal agent of halo blight disease in beans (Phaseolus vulgaris L.). Disease symptoms are typically water-soaked lesions that are surrounded by a chlorotic zone or halo (19). This halo is believed to result from the action of a non-host-specific toxin known as phaseolotoxin [Nδ-(N′-sulfodiaminophosphinyl)-ornithyl-alanyl-homoarginine] (18, 20). Phaseolotoxin is produced when bacteria are grown in minimal medium at temperatures between 18 and 20°C, and it is not detected at 30°C (7, 16, 22, 33).
Phaseolotoxin is a reversible inhibitor of the enzyme ornithine carbamoyltransferase (OCTase; EC 2.1.3.3) (5), which catalyzes the formation of citrulline from ornithine and carbamoylphosphate in the sixth step of the arginine biosynthetic pathway. In planta, phaseolotoxin is readily cleaved by peptidases to release Nδ-(N′-sulfodiaminophosphinyl)-l-ornithine (PSOrn), the major toxic chemical species present in diseased leaf tissue (19). Phaseolotoxin is an effective inhibitor of OCTase activity from plant, mammalian, and bacterial sources and causes a phenotypic requirement for arginine. To protect itself from its own toxin, P. syringae pv. phaseolicola synthesizes a phaseolotoxin-resistant OCTase (ROCT) (6, 10, 11, 25, 34).
The ROCT, which is the product of the argK gene, is expressed under conditions leading to the synthesis of phaseolotoxin, such as growth in minimal medium at 16 to 18°C (6, 8, 21, 34). It has been postulated that in P. syringae pv. phaseolicola the argK gene may be regulated under negative control by a repressor protein synthesized at 28°C (21). This repressor protein may bind to specific motifs (thermoregulatory region [TRR] motifs) that have been postulated to be involved in thermoregulation of phaseolotoxin synthesis (27, 28). It has been observed that Tox− mutants do not seem to express phaseolotoxin immunity, which indicates a coordinated mode of regulation (4, 25). Coordinated regulation of phaseolotoxin synthesis and argK expression could be explained by assuming that a precursor molecule of phaseolotoxin or phaseolotoxin itself could act as an inducer of argK (21).
When P. syringae pv. phaseolicola is grown under conditions that do not promote phaseolotoxin production, the synthesis of arginine is mediated by a phaseolotoxin-sensitive OCTase (SOCT) encoded by the argF gene. SOCT appears to be irrelevant to pathogenicity and to exert a housekeeping function; it is not clear whether SOCT is expressed at 18°C and what relationship (if any) it has with ROCT. Of the two forms of the OCTase present in P. syringae pv. phaseolicola, only SOCT has been shown to be regulated by arginine and complex media (35).
Arginine is a key amino acid in phaseolotoxin synthesis, since it is substrate of an amidinotransferase that catalyzes the synthesis of homoarginine, a constituent of the organic moiety of phaseolotoxin (13, 26). This amidinotransferase is encoded by amtA, whose expression is coordinated with phaseolotoxin synthesis and argK expression. A null mutant of this gene results in a Tox− phenotype (9). The argK and amtA genes are located on a 270-kbp PmeI fragment of the P. syringae pv. phaseolicola chromosome and have a low G+C content compared with the bacterial genome (2, 9). These characteristics and other evidence suggest that the genes involved in phaseolotoxin biosynthesis, together with the argK gene, may have been acquired by P. syringae pv. phaseolicola by horizontal transfer at some point during evolution (31, 32).
Previously reported Tox− mutants obtained by chemical mutagenesis have been shown to be unable to synthesize phaseolotoxin and also to lack ROCT (4, 25). We have obtained a well-characterized amtA mutant with a stringent Tox− phenotype, and we sought to analyze the expression of argK in this mutant, in order to investigate whether expression of argK had been compromised. Contrary to our expectations, argK was overexpressed in the amtA mutant. Therefore, to understand this result and to further investigate the regulation of the system, we decided to construct argK and argF null mutants and to analyze their behavior in terms of growth, phaseolotoxin production, and expression of argK at 18 and 28°C.
We confirmed that ROCT, which is coded by argK, is essential for P. syringae pv. phaseolicola under conditions of phaseolotoxin synthesis, as it ensures an optimal supply of the arginine required for growth and for phaseolotoxin synthesis. Additionally, we demonstrated that carbamoylphosphate is able to induce argK expression, bypassing the temperature control. Carbamoylphosphate is a compound resembling the inorganic moiety of phaseolotoxin, N′-sulfodiaminophosphinyl, which strongly suggests that argK is being directly regulated by a molecule that may be a precursor of phaseolotoxin and only indirectly by temperature, which directly regulates genes involved in the synthesis of phaseolotoxin.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. P. syringae pv. phaseolicola was grown on King's B medium (KB) (29) or M9 medium (30) at 18 or 28°C. Escherichia coli was grown in Luria-Bertani medium at 37°C. For phaseolotoxin production, P. syringae pv. phaseolicola was grown in M9 medium at 18°C for 48 h. When required, the following supplements were added (with quantities given in micrograms per milliliter): carbenicillin, 100; tetracycline, 10; kanamycin, 50; chloramphenicol, 50; and rifampin, 50.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM103 | Commercial strain used for cloning | 36 |
| DH5αF′ | Commercial strain used for cloning | BRL |
| TOP10F′ | Commercial strain used for cloning | Invitrogen |
| P. syringae pv. phaseolicola | ||
| NPS3121 | Wild type | 29 |
| UIKcat | argK::cat mutant of NPS3121; Tox+ | This study |
| UIFtet | argF::tet mutant of NPS3121; Tox+ | This study |
| AT3 | amtA::aph mutant of NPS3121; Tox− | 9 |
| Plasmids | ||
| pUIKL1 | argK::cat in pU1511 for gene replacement | This study |
| pUIMC1 | argF::tet in pUIGM1 for gene replacement | This study |
| pMW4 | Chloramphenicol cassette | 19 |
| pUIRM504 | Tn5cat delivery plasmid; Tcr Kmr | 14 |
| pUI511 | argK cloned into pUC19 | 21 |
| pUIGM1 | argF cloned into pUC19 | G. Mosqueda, personal communication |
| pUC19 | bla lacZ′ Apr | 36 |
Construction of the P. syringae pv. phaseolicola mutants.
Mutants were obtained by double recombination events, replacing the wild-type gene of interest with the interrupted allele in the P. syringae pv. phaseolicola chromosome.
(i) UIKcat.
The argK gene in plasmid pUI511 (21) was interrupted at the MluI site with a 1.9-kbp SalI-SmaI fragment containing the chloramphenicol resistance gene (cat) from pWM4 (15). The resulting plasmid, pUIKL1, was introduced by electroporation into P. syringae pv. phaseolicola, and chloramphenicol was used to select for double recombination events. The fidelity of the double recombination was confirmed by Southern blot hybridization.
(ii) UIFtet.
The argF gene in plasmid pUIGM1 was interrupted at the NruI site with a 1.9-kbp DraI-HpaI fragment containing the tetracycline resistance gene (tet) from pUIRM504 (14). The resulting plasmid, pUIMC, was introduced by electroporation into P. syringae pv. phaseolicola, and tetracycline was used to select for double recombination events. The fidelity of the double recombination was confirmed by Southern blot hybridization.
Phaseolotoxin bioassays.
Phaseolotoxin production by P. syringae pv. phaseolicola and mutants was assayed by the E. coli growth inhibition assay (33) as previously described (9, 32).
Molecular biology techniques.
Routine techniques were performed as described previously (30). Plasmids and DNA from agarose gels were purified with Qiagen (Valencia, Calif.) columns and kits. Restriction enzymes were used according to the instructions provided. Chromosomal DNA from P. syringae pv. phaseolicola was obtained as described previously (1). DNA fragments used as probes for Southern blotting were labeled with fluorescein by use of the ECF Random Primer Labeling kit, and signal detection was performed with the ECF Signal Amplification system, both from Amersham Pharmacia Biotech (Piscataway, N.J.). For image processing and quantification, a Molecular Dynamics (Sunnyvale, Calif.) Storm 860 apparatus with ImageQuant version 1.1 software was used.
Northern blot analysis.
Total RNA was extracted from P. syringae pv. phaseolicola cells by using the Trizol reagent according to the supplier's instructions (GIBCO). Genomic DNA was removed by digestion with RNase-free DNase (Roche). Samples of total RNA (20 μg) were denatured by treatment with formamide and separated by electrophoresis using 1.5% agarose gels that contained formaldehyde. The RNA was transferred to nylon membranes (Hybond N+; Amersham) and cross-linked by exposure to UV radiation. For hybridization of the nylon membranes, Church buffer was used (30). Hybridization was performed by using the following DNA probes: a 1.2-kbp HincII-EcoRV fragment of argF from plasmid pUIGM and a 1.0-kbp PCR-amplified fragment of argK from plasmid pUI511. The probes were labeled with [α-32P]dCTP by using the RediPrime Random Primer labeling kit (Amersham). Hybridization was carried out overnight at 65°C. The membranes were washed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate for 3 min at room temperature, followed by a wash with 1× SSC-0.1% sodium dodecyl sulfate for 3 min at 65°C. Densitometry of the Northern blotting results was carried out by considering the bands plus the smear as the positive signal. The relative transcript level is related to the value of the positive control, which was defined as 100%.
Induction assays with carbamoylphosphate.
P. syringae pv. phaseolicola grown overnight at 28°C was inoculated into 50 ml of M9 medium to an initial optical density at 600 nm of 0.1. The culture was grown at 28°C to the end of the log phase. At this point, carbamoylphosphate (carbamyl phosphate disodium salt; Sigma) was added to the culture at different concentrations. Samples were collected at the appropriate times and were centrifuged, and the bacterial pellet was used to isolate RNA. The supernatant was used to perform phaseolotoxin assays.
RESULTS
The argK gene is overexpressed in the amtA mutant.
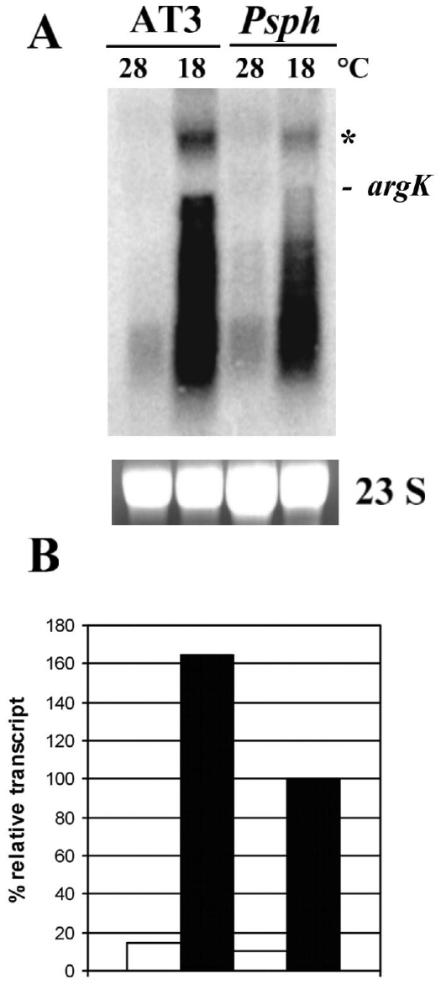
AT3, a mutant in which the amtA gene (coding for the amidinotransferase involved in the synthesis of homoarginine) is affected, and which does not synthesize phaseolotoxin, was obtained previously (9). To determine whether argK was expressed in this mutant background, AT3 was grown under conditions allowing phaseolotoxin production. Total RNA was extracted from this strain and used to identify argK-specific transcripts by Northern blot analysis. Hybridization revealed two different transcripts of around 1.2 and 2.3 kbp as well as a smear, all argK specific; the shorter transcript had the size expected of a monocistronic argK message (Fig. 1). This pattern of hybridization has been observed previously, and alternative transcription start sites have been postulated as a possible explanation (8). The results obtained indicated that despite the Tox− phenotype of strain AT3, not only was argK expressed, but the level of expression was higher than those observed for the wild-type strain.
FIG. 1.
Expression of the argK gene in the amtA mutant background. (A) Northern blot of total RNA from P. syringae pv. phaseolicola (Psph) and the amtA mutant (AT3) grown in rich medium (KB) at 28 and 18°C. P. syringae pv. phaseolicola grown in rich medium (KB) at 18°C was used as a positive control. The blot was hybridized with a radioactively labeled 1-kbp PCR product of argK. 23S rRNA stained with ethidium bromide is shown as a loading control. The signal corresponding to the monocistronic argK message is marked. Asterisk indicates the position of a band of approximately 2.3 kbp corresponding to a possible alternative argK transcript (see the text for details). (B) Densitometric analysis of the Northern blot. The relative transcript level is expressed as a percentage of the transcript level for P. syringae pv. phaseolicola grown in KB at 18°C, which was set at 100%.
Effect of an argK mutation in P. syringae pv. phaseolicola grown under conditions of phaseolotoxin synthesis.
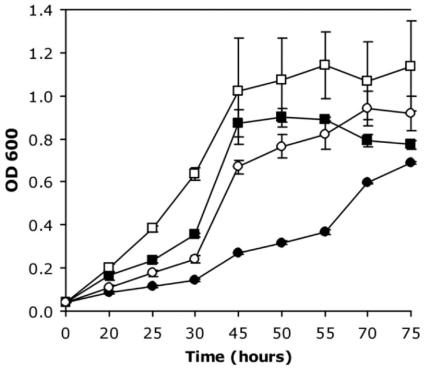
In order to further investigate the regulation of the system, an argK-null mutant, UIKcat, was constructed. To assess its phenotype, this strain was grown under conditions allowing phaseolotoxin production. It might be expected that this mutant strain would not be viable under these conditions because of the loss of immunity due to the absence of a functional ROCT. However, it was observed that the mutant could grow, although at a lower rate than that for the wild-type strain (Fig. 2). Addition of 20 mM arginine to the medium restored growth to wild-type levels, suggesting that the major cause for growth retardation was limitation of this amino acid.
FIG. 2.
Phenotype of the argK mutant grown under conditions of phaseolotoxin synthesis. P. syringae pv. phaseolicola and the argK mutant (UIKcat) were grown in M9 liquid medium at 18°C. Growth was monitored by measuring absorbance at 600 nm. Squares, P. syringae pv. phaseolicola; circles, UIKcat; solid symbols, growth in unsupplemented M9 medium; open symbols, growth in M9 medium supplemented with 20 mM arginine. Each data point is the average from triplicate experiments. OD 600, optical density at 600 nm.
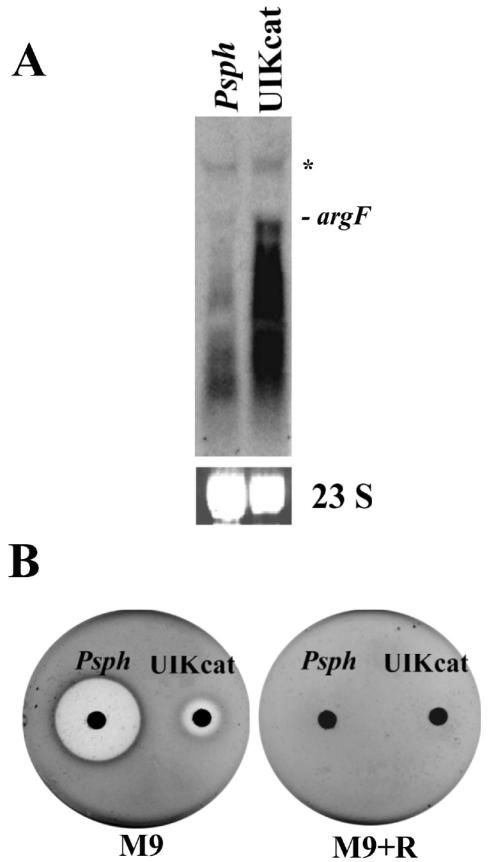
A possible explanation for the observed phenotype was that UIKcat was producing little or no phaseolotoxin, allowing some SOCT activity to be retained. To test this idea, we conducted a bioassay to detect phaseolotoxin in the supernatant of UIKcat grown at 18°C in M9 medium. A small and diffuse inhibition halo was detected (Fig. 3B); this halo was the product of phaseolotoxin, since inhibition could be reversed by the addition of 10 mM arginine to the assay medium.
FIG. 3.
(A) Expression of argF in the argK mutant background. Shown is a Northern blot of total RNA from P. syringae pv. phaseolicola (Psph) and the argK mutant (UIKcat) grown in M9 medium at 18°C. The blot was hybridized with a radioactively labeled 1.2-kbp fragment of argF. 23S rRNA stained with ethidium bromide is shown as a loading control. The signal of the size expected for a monocistronic argF message is marked. Asterisk indicates the position of a secondary band of ≈2.2 kbp that also hybridizes with argF. (B) Phaseolotoxin production by the argK mutant. Phaseolotoxin produced by P. syringae pv. phaseolicola and the argK mutant grown in M9 medium at 18°C was evaluated by using the E. coli growth inhibition assay. M9, E. coli JM103 plus M9 medium; M9 + R, E. coli JM103 plus M9 medium supplemented with 10 mM arginine.
Northern blot analysis revealed that argF was strongly expressed in the UIKcat mutant under conditions of phaseolotoxin production (Fig. 3A), supporting the view that residual SOCT activity at suboptimal phaseolotoxin concentrations allowed substantial, although limiting, arginine synthesis.
Effect of an argF mutation in P. syringae pv. phaseolicola grown at 28°C.
To investigate in more detail any possible involvement of argF in the regulatory mechanisms involved in the synthesis of phaseolotoxin, a null mutant was obtained by interrupting the argF gene. After selection of double recombination events for homogenotization, preliminary screening of putative mutants was performed by replicating and growing them in M9 medium at 28°C on the premise that, since argK should not be expressed at this temperature, ROCT would not be present and the mutants should behave as arginine auxotrophs. However, all presumptive double recombinants grew under these conditions. Five independent double recombinants were chosen and retested for their ability to grow in solid and liquid M9 media at 28°C. They were all capable of substantial growth, with a growth rate comparable to that normally observed for the wild-type strain. Their genotypes were also confirmed by Southern blot hybridization, showing that all mutants were bona fide double recombinants (data not shown). One strain, UIFtet, was chosen for further analysis.
If UIFtet was growing at 28°C in M9 medium, then for some reason the ROCT would have to be replacing the function of SOCT, suggesting that a second mechanism, in addition to thermoregulation, was available to allow expression of argK under otherwise nonpermissive conditions.
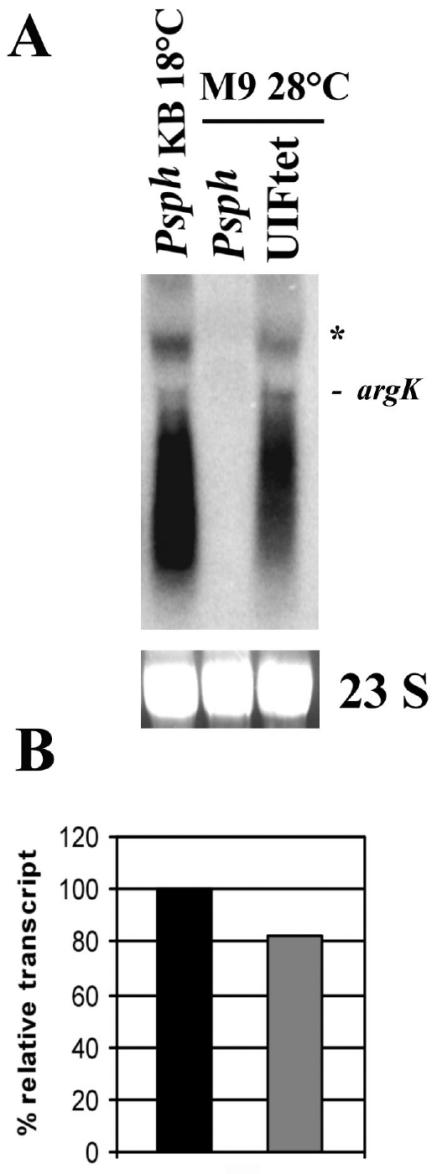
To test this hypothesis, expression from argK in UIFtet grown at 28°C in M9 medium was analyzed by Northern blot analysis. The results obtained confirmed that argK was indeed being transcribed under these conditions (Fig. 4); however, no phaseolotoxin was detected by use of the growth inhibition assay (data not shown).
FIG. 4.
Expression from argK in the argF mutant background. (A) Northern blot of total RNA from P. syringae pv. phaseolicola (Psph) and the argF mutant (UIFtet) grown in M9 medium at 28°C. P. syringae pv. phaseolicola grown in rich medium (KB) at 18°C was used as a positive control. The blot was hybridized with a 1-kbp PCR product of argK labeled with [α-32P]dCTP. 23S rRNA stained with ethidium bromide is shown as a loading control. The signal that has the size expected for a monocistronic argK message is marked. Asterisk indicates the position of a secondary transcript of ≈2.3 kbp. (B) Densitometric analysis of the Northern blot. The relative transcript level is expressed as a percentage of the level of the positive control, which was defined as 100%.
Induction of argK with carbamoylphosphate in cultures grown at 28°C.
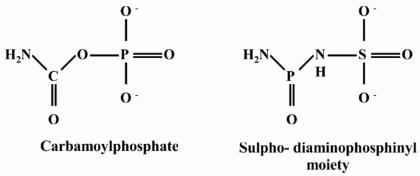
From the results obtained, it appeared that induction of argK was being mediated by a temperature-independent regulatory mechanism. A model of regulation had been proposed previously in which, at the permissive temperature for phaseolotoxin production, an inducer molecule could bind the postulated repressor protein of argK to release it from the argK operator and allow expression of this gene. It had also been proposed that such an inducer could be a precursor of phaseolotoxin or phaseolotoxin itself (21). Therefore, it was possible to imagine that in the mutant growing at 28°C, citrulline synthesis would be blocked and both precursor molecules, ornithine and carbamoylphosphate, could accumulate. On the other hand, the chemical structure of carbamoylphosphate resembled the proposed structure of the inorganic moiety of phaseolotoxin, N′-sulfodiaminophosphinyl (35) (Fig. 5). This idea prompted us to test whether carbamoylphosphate could be used as an inducer of argK expression.
FIG. 5.
Carbamoylphosphate and the inorganic moiety of phaseolotoxin (N′-sulfodiaminophosphinyl) present very similar molecular structures, and both compete for the carbamoylphosphate binding site of OCTase.
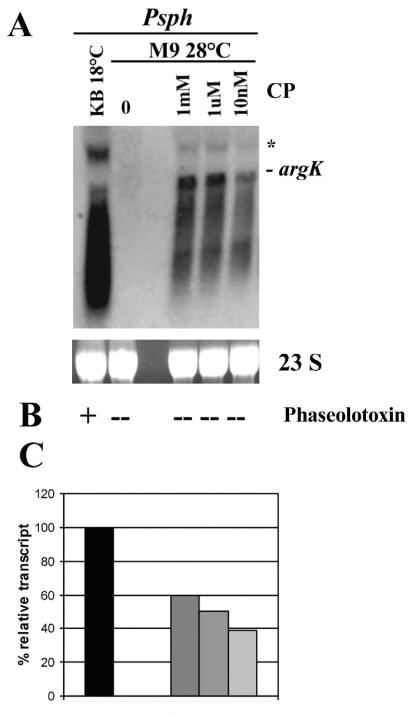
To verify this, P. syringae pv. phaseolicola was grown in M9 medium at 28°C until the end of the logarithmic phase. At this point, carbamoylphosphate was added to the culture at final concentrations of 1, 5, and 10 mM. The cultures were further incubated for 30, 60, and 120 min under agitation at 28°C. Total RNA was extracted from each culture and used to perform Northern blot analysis by using a fragment of the argK gene as the probe. The argK transcript was detected under all the conditions tested (data not shown). Additionally, growth inhibition bioassays were conducted for each sample, but no phaseolotoxin was detected in any case. To investigate any possible dose-respose effect, we conducted a second induction experiment exposing wild-type P. syringae pv. phaseolicola to carbamoylphosphate at concentrations ranging from 1 mM to 10 nM for 30 min. These results show a dose-response pattern, indicating that expression from argK is indeed induced by carbamoylphosphate. The highest level attained was only approximately 60% of the level observed for the wild type growing at 18°C, a finding consistent with carbamoylphosphate functioning as a replacement instead of the original inducer (Fig. 6).
FIG. 6.
Induction of argK expression by carbamoylphosphate under nonpermissive conditions for the synthesis of phaseolotoxin. (A) Northern blot of total RNA from P. syringae pv. phaseolicola (Psph) grown at 28°C in M9 medium supplemented with different concentrations of carbamoylphosphate (CP) (none, 1 mM, 1 μM, and 10 nM) and induced for 30 min. P. syringae pv. phaseolicola grown in rich medium (KB) at 18°C was used as a positive control. The blot was hybridized with a 1-kbp PCR product of argK labeled with [α-32P]dCTP. 23S rRNA stained with ethidium bromide is shown as a loading control. The signal that has the size expected for a monocistronic argK message is marked. Asterisk indicates the position of a secondary transcript of ≈2.3 kbp. (B) Phaseolotoxin synthesis in the supernatant was evaluated by using the E. coli growth inhibition assay. +, presence of phaseolotoxin; −, absence of phaseolotoxin. (C) Densitometric analysis of the Northern blot. The relative transcript level is expressed as a percentage of the level of the positive control, which was defined as 100%.
DISCUSSION
It was previously reported that Tox− mutants of P. syringae pv. phaseolicola also showed impaired ROCT synthesis, suggesting a coordinated response of genes coding for enzymes involved in the synthesis of phaseolotoxin and the argK gene, coding for the ROCT (3, 25). Hernández-Guzmán and Alvarez-Morales had previously obtained a well-characterized Tox− mutant that was unable to synthesize the amidinotransferase required for the biosynthesis of homoarginine, and possibly unable to synthesize enough ornithine, both molecules being required for the synthesis of the tripeptide moiety of phaseolotoxin (9). When we grew this mutant under conditions of phaseolotoxin synthesis to assess the expression of argK, it was clear that not only was the gene expressed, but expression occurred at higher levels than those in the wild-type strain. This result led us to revise other assumptions we had made about this system.
Since regulation of the argK gene, coding for the ROCT, had been proposed to be directly mediated by temperature (28), it became interesting to obtain an argK-null mutant and to assess its phenotype when it was grown under conditions promoting phaseolotoxin synthesis. Under such conditions, this mutant might have been expected to be lethal. Also, it was possible that a null mutation in argF, encoding SOCT, would cause the mutant to behave as an arginine auxotroph when grown at 28°C.
Testing these ideas, we found that an argK mutant unable to produce ROCT was capable of growing and producing phaseolotoxin when cultured in minimal medium at 18°C, although at reduced levels compared to those of the wild-type strain. Northern blot data for this strain showed that expression of the argF gene was taking place under these conditions; this could account for the phenotype observed. It is known that in Pseudomonas aeruginosa the argF gene is negatively regulated by arginine and the repressor molecule ArgR (23, 24); the same situation has been shown to occur in P. syringae pv. phaseolicola (J. L. Hernández-Flores, K. Lopez-Lopez, R. Garcidueñas-Piña, A. Jofre-Garfias, and A. Alvarez-Morales, unpublished data). Therefore, when the argK mutant UIKcat is grown at 18°C, there is no ROCT to provide the arginine required to sustain growth; thus, the levels of this amino acid must be low and the argF operon should be derepressed. However, even though the enzyme is being synthesized, its enzymatic activity is severely reduced due to the presence of phaseolotoxin, and the consequence of this reduction is a steady low level of arginine and a constant state of derepression for argF, which we observe in Fig. 3.
When we tested the phenotype of the argF mutant strain UIFtet, cultured in minimal medium at 28°C, it did not behave as we expected, that is, like an arginine auxotroph. Suppression selected by the culture conditions, such as a second mutation in the repressor molecule negatively regulating argK, could also result in such a phenotype. However, suppressors are expected to appear at very low frequencies, whereas the phenotype observed represented the behavior of the whole mutant population.
The fact that strain UIFtet was capable of growing at 28°C indicated that argK was escaping thermoregulation and being expressed. Northern blot experiments using an argK-derived probe showed that indeed the argK gene was being expressed to levels that could account for the phenotype observed.
Why was argK derepressed at 28°C? We had previously postulated that a precursor of phaseolotoxin, or the toxin itself, could act as an inducer that would bind to the postulated repressor molecule when cells were grown at 18°C, relieving repression. The fact that argK was being expressed in the amtA mutant indicated that homoarginine could be ruled out as an inducer, since this molecule is not produced by this strain. Ornithine and alanine, the other two amino acids present in the organic moiety, were not likely candidates, because of their ubiquity. A molecule that could play this role might be the inorganic moiety of phaseolotoxin, the N′-sulfodiaminophosphinyl group. The chemical structure of this molecule strongly resembles the structure of carbamoylphosphate, and this molecule, when bound to ornithine to form PSOrn, results in a transition state analogue of a unstable intermediate catalyzed by OCTase (12, 17).
We assumed that in the argF mutant grown at 28°C, lacking SOCT, the reaction synthesizing citrulline from carbamoylphosphate and ornithine would not proceed and these precursors would then accumulate. Could derepression of argK be induced by the accumulated carbamoylphosphate? As expected, P. syringae pv. phaseolicola growing in minimal medium at 28°C did not show any expression from argK; however, upon addition of carbamoylphosphate to the medium, argK was derepressed even though the temperature was kept at 28°C and phaseolotoxin could not be detected. This finding indicates that carbamoylphosphate has an effect only on the argK gene, not on those genes involved in biosynthesis of the toxin.
This result strongly suggests the following: (i) argK is subject to induction with negative control, where the inducer molecule appears to be the N′-sulfodiaminophosphinyl group resembling carbamoylphosphate; (ii) argK is not directly regulated by temperature, but coordination with phaseolotoxin synthesis is mediated through the synthesis of the inducer, which occurs at the lower permissive temperatures.
Based on this idea, we can then attempt to explain the phenotype of argK overexpression in the amtA mutant when it is cultured at 18°C. Amidinotransferase is an enzyme that transfers the amidino group of l-arginine to l-lysine to produce homoarginine and ornithine (13); both of these amino acids are present in the tripeptide moiety of phaseolotoxin. Therefore, in the absence of amidinotransferase, the N′-sulfodiaminophosphinyl group may accumulate because of the lack of homoarginine and possibly limiting amounts of ornithine. The accumulated N′-sulfodiaminophosphinyl molecule could then be responsible for the continuous expression of argK, resulting in overexpression of amtA in this mutant.
It has previously been suggested that the postulated repressor molecule that acts on argK may also exert a regulatory action on other genes involved in the synthesis of phaseolotoxin, because mobility shift experiments show that a protein present only in cells growing at 28°C binds specifically both to the TRR motifs and to fragments derived from argK (28). DNA fragments carrying the TRR motif have been shown to override thermoregulation, presumably due to the titration of the repressor molecule (27, 28). However this is not the case when only fragments derived from argK are used; in this case, lower affinity of the repressor for these DNA regions in argK has been postulated as responsible for this lack of titration (28). Our results indicate that argK is not directly thermoregulated, whereas some other genes involved in the synthesis of phaseolotoxin seem to respond to temperature. Therefore, it is possible that more than one repressor protein is found in cells growing at 28°C: one species recognizing the TRR motif and responding to temperature changes, and a second species, a repressor molecule, acting on the argK gene, presumably with an allosteric site for recognition of the inorganic moiety of phaseolotoxin and with only a limited affinity for sequences similar to the TRR motif.
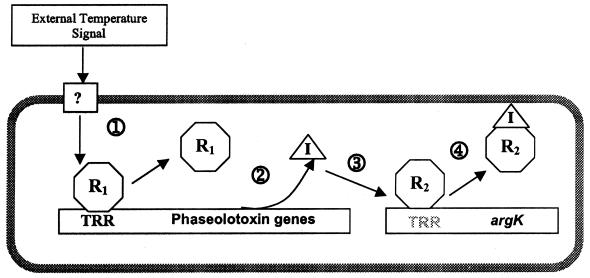
From the data obtained as part of this work, we propose a working model for the induction of genes involved in phaseolotoxin synthesis in P. syringae pv. phaseolicola growing at 18°C. This model includes the following steps (Fig. 7). (i) Temperature downshifts are sensed through an as yet unidentified mechanism. The signal is processed and transduced to some effector molecule that will act upon the repressor (R1 in Fig. 7) of phaseolotoxin genes. (ii) At 28°C, genes involved in the synthesis of phaseolotoxin, such as amtA, are negatively regulated by a repressor molecule (R1) that may bind to TRR motifs; when the temperature is downshifted to 16 to 18°C, the signal is processed and relieves repression mediated by R1. These genes are then actively expressed. (iii) The gene products from the phaseolotoxin genes synthesize both the inorganic (I) and peptide moieties of phaseolotoxin. (iv) The inorganic moiety resembling carbamoylphosphate acts as an inducer of argK by binding to an argK-specific repressor molecule (R2) and removing it from its DNA target sequence. (v) Expression of argK takes place, and ROCT is synthesized to provide arginine and, via the amidinotransferase reaction, homoarginine and ornithine for cell growth and phaseolotoxin synthesis.
FIG. 7.
Model for the induction of genes involved in phaseolotoxin synthesis in P. syringae pv. phaseolicola growing at 18°C. The question mark represents an unknown signal-transducing element; TRR, specific motifs that have been postulated to be involved in the thermoregulation of phaseolotoxin synthesis; R1, a repressor molecule that may bind to TRR motifs; I, inorganic moiety of phaseolotoxin (N′-sulfodiaminophosphinyl); R2, an argK-specific repressor molecule. For an explanation of the model, see the text.
This model can account for the results we have obtained and discussed in the present work, as well as for other observations, such as the phenotypes observed for some Tox− mutants that do not produce ROCT. In this case the mutations may be affecting the synthesis of the inorganic moiety, and therefore no inducer is available to relieve repression of argK.
Further work to investigate the thermoregulation of genes involved in the synthesis of phaseolotoxin, as well as to characterize genes that may be involved in the synthesis of the inorganic moiety, is currently under way in our laboratory.
Acknowledgments
This work was funded through grants from the National Council for Science and Technology—Mexico (CONACYT) to A. Alvarez and a fellowship to K. López.
We thank Juan Carlos Vaca for helpful discussions and suggestions and Gustavo Hernández for critical reading of the manuscript.
REFERENCES
- 1.Chen, W.-P., and T.-T. Kuo. 1993. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 21:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Ita, M. E., R. Marsch-Moreno, P. Guzmán, and A. Alvarez-Morales. 1998. Physical map of the chromosome of the phytopathogenic bacterium Pseudomonas syringae pv. phaseolicola. Microbiology 144:493-501. [DOI] [PubMed] [Google Scholar]
- 3.Durbin, R. D. 1991. Bacterial phytotoxins: mechanism of action. Experientia 47:776-783. [Google Scholar]
- 4.Durbin, R. D., and P. J. Langston-Unkefer. 1988. The mechanisms for self-protection against bacterial phytotoxins. Annu. Rev. Phytopathol. 26:313-329. [Google Scholar]
- 5.Ferguson, A. R., and J. S. Johnston. 1980. Phaseolotoxin: chlorosis, ornithine accumulation and inhibition of ornithine carbamoyltransferase in different plants. Physiol. Plant Pathol. 16:269-275. [Google Scholar]
- 6.Ferguson, A. R., J. S. Johnston, and R. E. Mitchell. 1980. Resistance of Pseudomonas syringae pv. phaseolicola to its own toxin, phaseolotoxin. FEMS Microbiol. Lett. 7:123-125. [Google Scholar]
- 7.Goss, R. W. 1970. The relation of temperature to common and halo blight of beans. Phytopathology 30:258-264. [Google Scholar]
- 8.Hatziloukas, E., and N. J. Panopoulos. 1992. Origin, structure and regulation of argK, encoding the phaseolotoxin-resistant carbamoyltransferase in Pseudomonas syringae pv. phaseolicola, and functional expression of argK in transgenic tobacco. J. Bacteriol. 174:5895-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández-Guzmán, G., and A. Alvarez-Morales. 2001. Isolation and characterization of the gene coding for the amidinotransferase involved in the biosynthesis of phaseolotoxin in Pseudomonas syringae pv. phaseolicola. Mol. Plant-Microbe Interact. 14:545-554. [DOI] [PubMed] [Google Scholar]
- 10.Jahn, O., J. Sauerstein, and G. Reuter. 1987. Characterization of two ornithine carbamoyltransferases from Pseudomonas syringae pv. phaseolicola, the producer of phaseolotoxin. Arch. Microbiol. 147:174-178. [DOI] [PubMed] [Google Scholar]
- 11.Jahn, O., J. Sauerstein, and G. Reuter. 1985. Detection of two ornithine carbamoyltransferases in a phaseolotoxin-producing strain of Pseudomonas syringae pv. phaseolicola. J. Basic Microbiol. 25:543-546. [DOI] [PubMed] [Google Scholar]
- 12.Langley, D. B., M. D. Templeton, B. A. Fields, R. E. Mitchell, and C. A. Collyer. 2000. Mechanism of inactivation of ornithine transcarbamoylase by Nδ-(N′-sulfodiaminophosphinyl)-l-ornithine, a true transition state analogue? crystal structure and implications for catalytic mechanism. J. Biol. Chem. 275:20012-20019. (Erratum, 275:30746.) [DOI] [PubMed] [Google Scholar]
- 13.Markisch, U., and G. Reuter. 1990. Biosynthesis of homoarginine and ornithine as precursors of the phytoeffector phaseolotoxin by the amidinotransfer from arginine to lysine catalyzed by an amidinotransferase in Pseudomonas syringae pv. phaseolicola. J. Basic Microbiol. 30:425-433. [Google Scholar]
- 14.Marsch-Moreno, R., G. Hernández-Guzmán, and A. Alvarez-Morales. 1998. pTn5cat: a Tn5-derived genetic element to facilitate insertion mutagenesis, promoter probing, physical mapping, cloning, and marker exchange in phytopathogenic and other Gram-negative bacteria. Plasmid 39:205-214. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf, W. W., and B. L. Wanner. 1993. Construction of new β-glucuronidase cassettes for making transcriptional fusions and their use with new methods for allele replacement. Gene 129:17-25. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell, R. E. 1978. Halo blight of beans: toxin production by several Pseudomonas phaseolicola isolates. Physiol. Plant Pathol. 13:37-49. [Google Scholar]
- 17.Mitchell, R. E. 1991. Implications of toxins in the ecology and evolution of plant pathogenic microorganisms: bacteria. Experientia 47:791-803. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell, R. E. 1976. Isolation and structure of a chlorosis-inducing toxin of Pseudomonas phaseolicola. Phytochemistry 15:1941-1947. [Google Scholar]
- 19.Mitchell, R. E., and R. L. Bieleski. 1977. Involvement of phaseolotoxin in halo blight of beans. Transport and conversion to functional toxin. Plant Physiol. 60:723-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, R. E., W. P. Niemczura, O. C. H. Kwok, and S. S. Patil. 1984. Inhibitors of ornithine carbamoyltransferase from Pseudomonas syringae pv. phaseolicola. Tetrahedron Lett. 25:3931-3934. [Google Scholar]
- 21.Mosqueda, G., G. Van den Broeck, O. Saucedo, A.-M. Bailey, A. Alvarez-Morales, and L. Herrera-Estrella. 1990. Isolation and characterization of the gene from Pseudomonas syringae pv. phaseolicola encoding the phaseolotoxin-insensitive ornithine carbamoyltransferase. Mol. Gen. Genet. 222:461-466. [DOI] [PubMed] [Google Scholar]
- 22.Nüske, J., and W. Fritsche. 1989. Phaseolotoxin production by Pseudomonas syringae pv. phaseolicola: the influence of temperature. J. Basic Microbiol. 29:441-447. [DOI] [PubMed] [Google Scholar]
- 23.Park, S.-M., C.-D. Lu, and A. T. Abdelal. 1997. Cloning and characterization of argR, a gene that participates in regulation of arginine biosynthesis and catabolism in Pseudomonas aeruginosa PAO1. J. Bacteriol. 179:5300-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, S.-M., C.-D. Lu, and A. T. Abdelal. 1997. Purification and characterization of an arginine regulatory protein, ArgR, from Pseudomonas aeruginosa and its interactions with the control regions for the car, argF, and aru operons. J. Bacteriol. 179:5309-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peet, R. C., and N. J. Panopoulos. 1987. Ornithine carbamoyltransferase genes and phaseolotoxin immunity in Pseudomonas syringae pv. phaseolicola. EMBO J. 6:3585-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuter, G. 1989. Enzymatic regulation of microbial phytoeffector biosynthesis, p. 271-281. In M. E. Bushell and U. Gräfe (ed.), Bioactive metabolites from microorganisms. Elsevier, Amsterdam, The Netherlands.
- 27.Rowley, K. B., D. E. Clements, M. Mandel, T. Humphreys, and S. S. Patil. 1993. Multiple copies of a DNA sequence from Pseudomonas syringae pathovar phaseolicola abolish thermoregulation of phaseolotoxin production. Mol. Microbiol. 8:625-635. [DOI] [PubMed] [Google Scholar]
- 28.Rowley, K. B., R. Xu, and S. S. Patil. 2000. Molecular analysis of thermoregulation of phaseolotoxin-resistant ornithine carbamoyltransferase (argK) from Pseudomonas syringae pv. phaseolicola. Mol. Plant-Microbe Interact. 13:1071-1080. [DOI] [PubMed] [Google Scholar]
- 29.Saettler, A. W., N. W. Schaad, and D. A. Roth (ed.). 1989. Detection of bacteria in seed and other planting material. APS Press, St. Paul, Minn.
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis (ed.). 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Sawada, H., S. Kanaya, M. Tsuda, F. Suzuki, K. Azegami, and N. Saitou. 2002. A phylogenomic study of the OCTase genes in Pseudomonas syringae pathovars: the horizontal transfer of the argK-tox cluster and the evolutionary history of OCTase genes on their genomes. J. Mol. Evol. 54:437-457. [DOI] [PubMed] [Google Scholar]
- 32.Sawada, H., F. Suzuki, I. Matsuda, and N. Saitou. 1999. Phylogenetic analysis of Pseudomonas syringae pathovars suggests the horizontal gene transfer of argK and the evolutionary stability of the hrp gene cluster. J. Mol. Evol. 49:627-644. [DOI] [PubMed] [Google Scholar]
- 33.Staskawicz, B. J., and N. J. Panopoulos. 1979. A rapid and sensitive assay for phaseolotoxin. Phytopathology 69:663-666. [Google Scholar]
- 34.Staskawicz, B. J., N. J. Panopoulos, and N. J. Hoogenraad. 1980. Phaseolotoxin insensitive ornithine carbamoyltransferase of Pseudomonas syringae pv. phaseolicola: basis for immunity to phaseolotoxin. J. Bacteriol. 142:720-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Templeton, M. D., P. A. Sullivan, and M. G. Shepherd. 1984. The inhibition of ornithine transcarbamoylase from Escherichia coli W by phaseolotoxin. Biochem. J. 224:379-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]