Abstract
Introduction
Liver mass is regulated in precise proportion to body mass in health and is restored by regeneration following acute injury. Despite extensive experimental analyses, the mechanisms involved in this regulation have not been fully elucidated. Previous investigations suggest that signals from the bowel may play an important role. The purpose of the studies reported here was to determine the effect of proximal partial small bowel resection on liver mass in a murine model.
Methods
Mice were subjected to a 50% proximal small bowel resection or sham surgery followed by primary anastomosis, then sacrificed at serial times for determination of liver:body mass ratio and analyses of liver tissue.
Results
Liver:body weight ratio was significantly decreased 72 h after small bowel resection, and this decrease correlated with reduced functional liver mass as assessed by determination of total hepatic tissue protein and alanine transaminase (ALT) activity. Liver from bowel-resected animals demonstrated increased expression of LC3-II, a marker of autophagy, and also of pro-apoptotic Bax compared to anti-apoptotic Bcl-2.
Conclusion
These data support a role for signals from the intestine in liver mass regulation, and they have potential implications regarding the pathogenesis of liver injury following small bowel resection.
Keywords: Liver regeneration, Small bowel resection, Autophagy, Apoptosis
Introduction
Liver mass is regulated in precise proportion to body mass in health, and this ratio (liver:body weight) is specifically restored by regeneration following acute injury.1,2 Although extensive analyses have been conducted investigating the mechanisms that regulate liver mass and regeneration, the precise nature of the signals involved has not yet been fully elucidated. We hypothesize that signals derived from the intestine and delivered via the portal circulation contribute to the regulation of liver:body mass. This possibility has previously been suggested. For example, in a prior study from our group, we demonstrated that resection of the proximal 50% of the small intestine was associated with significantly decreased total liver weight.3 Furthermore, the role of the small intestine in regulating recovery of liver mass after injury, i.e. regeneration, has also been investigated with some studies suggesting that the intestinal tract is the source of a humoral factor essential for normal hepatic regeneration after partial hepatectomy,4,5 and others indicating that the small intestine may not be required for this regenerative response.6,7 Finally, recent analyses demonstrating the farnesoid X receptor (FXR)-dependent effects of enterally absorbed bile acids on regulation of liver mass and recovery from injury 8 provide additional support for the role of the small intestine in liver mass regulation. Based on our hypothesis and these data, in the studies reported here we used a mouse experimental model of partial small bowel resection (SBR) to more carefully elucidate the temporal pattern of change in the liver:body mass ratio and investigate the molecular signaling events associated with such change.
Materials and Methods
Animal Husbandry and Surgery
Male, 7–9-week old, 20–30 g C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) were maintained on 12 h dark–light cycles with ad libitum access to standard rodent chow and water, allowed to acclimate to their new environment for at least 7 days prior to surgery, and placed on a preoperative complete liquid diet (Micro-stabilized Rodent Liquid Diet LD101; Purina Mills, St. Louis, MO, USA) 1 day prior to surgery. Proximal partial small bowel resection or sham surgeries were performed as previously described.3,9 Briefly, SBR was performed by transecting the small bowel in two places, 12 cm proximal to the cecum and immediately distal to the duodenum, and removing the ∼12 cm of intervening small bowel (jejunum). Intestinal continuity was subsequently restored with an end-to-end, single-layered, interrupted jejuno-ileal anastomosis using 9–0 monofilament suture. A sham operation was performed by a simple transection of the bowel 12 cm proximal to the cecum and immediately restoring intestinal continuity with a similar anastomosis. After the operation, animals received only water for the first 24 h, followed by the complete liquid diet until sacrifice. Some mice were individually housed to permit quantification of post-operative intake of the liquid diet. Animals were sacrificed at serial times including 1, 2, 3, and 7 days after surgery. At the time of sacrifice, which was by subcutaneous injection of ketamine:xylazine:acepromazine (4:1:1) followed by cervical dislocation, the liver was rapidly removed and weighed, and the left lobe was sectioned with one portion placed in formalin for histology and other portions snap frozen in liquid nitrogen and saved at −80 C for subsequent analysis. In a separate series of experiments, mice were subjected to distal SBR by transecting the small bowel 12 cm proximal to the cecum and at the ileo-cecal junction, removing ∼12 cm of intervening distal small bowel (ileum), and performing a primary jejuno-colonic anastomosis. Three to ten animals were examined at each time point in each surgical group. Animals that appeared ill (pilorection, lethargy) or obstructed (dilated bowel proximal to the anastomosis) at the time of sacrifice and tissue harvest (three out of 54 animals) were excluded from further analysis. No animals died prior to sacrifice. All experiments were approved by the Animal Studies Committee of Washington University and conducted in accordance with institutional guidelines and the criteria outlined in the “Guide for Care and Use of Laboratory Animals” (NIH publication 86–23).
Total Hepatic Protein and Transaminase Activity Determination
Whole tissue lysates were made from snap frozen liver as previously described.10–13 Protein determination was performed on lysates using the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) and the results used to quantify total protein content in harvested liver. Alanine (ALT) and aspartate (AST) transaminase activity in the liver lysate were determined by the St. Louis Children's Hospital Clinical Laboratory and the results used to quantify total activity of each transaminase in harvested liver.
Histology, Immunohistochemistry, and Cell Size Measurements
Formalin-fixed, paraffin-embedded liver tissue was stained with hematoxylin and eosin. MicroSuite Five Biological Suite (Olympus, Center Valley, PA, USA) was used to quantify hepatocyte cross-sectional area by examination of six different images from each liver, which were obtained with a Nikon Eclipse 80i microscope using a video-assisted computer program (Metamorph, UIC, Dowington, PA, USA). TUNEL staining was performed by the Digestive Disease Research Center Histology Core using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon International/Millipore, Billerica, MA, USA).
Protein Expression Analysis
Forty micrograms aliquots of protein lysate, made as previously described,10–13 were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by electrophoretic transfer to nitrocellulose. Filters were probed with primary antibody (LC3B, Bax, Bcl-2, Caspases 3, 9, 12, PARP, Cell Signaling Technology, Danvers, MA, USA) followed by either a horseradish peroxidase- or fluorophore-conjugated secondary antibody, and then developed using ECL (Amersham, Piscataway, NJ, USA) or the Odyssey Infra-Red Imaging System (LI-COR BioSciences, Lincoln, NE, USA). Densitometric analysis was performed with Scion Image data analysis software (Scion Corporation, Frederick, MD, USA) or with Odyssey System software.
Gene Expression Analysis
Total RNA was analyzed for expression of specific genes of interest using real-time reverse-transcriptase polymerase chain reaction with standardization to the expression of β2-microglobulin as described previously.14 Target-specific forward and reverse primers, from Primer Bank (http://pga.mgh.harvard.edu/primerbank) or published literature,15 include: Bax, forward: 5′-GCT AGC AAA CTG GTG CTC AA-3′, reverse: 5′-TCT TGG ATC CAG ACA AGC AG-3′; Bcl-2 forward: 5′-GTC ACA GAG GGG CTA CGA GT-3′, reverse: 5′-TCA GGC TGG AAG GAG AAG AT-3′; XIAP, forward: 5′-CGA GCT GGG TTT CTT TAT ACC G-3′, reverse: 5′-GCA ATT TGG GGA TAT TCT CCT GT-3′; cIAP1, forward: 5′-TGT GGC CTG ATG TTG GAT AAC-3′, reverse: 5′-GGT GAC GAA TGT GCA AAT CTA CT-3′; cIAP2, forward: 5′-ACG CAG CAA TCG TGC ATT TTG-3′, reverse: 5′-CCT ATA ACG AGG TCA CTG ACG G-3′; survivin, forward: 5′-GAG GCT GGC TTC ATC CAC TG-3′, reverse: 5′-CTT TTT GCT TGT TGT TGG TCT CC-3′; BRUCE, forward: 5′-CGC GGG ACC ATC AAA GTC AT-3′, reverse: 5′-GCA GTG TCT AGC AAC AAG ATC C-3′.
Statistical Analyses
Data were analyzed using SigmaStat software (SPSS, Chicago, IL, USA). Unpaired Student's t test for pair-wise comparisons was used to compare liver:body mass, dietary intake, cell size, and mRNA and protein expression levels between experimental groups, with significance (alpha) set at 0.05. Data are reported as mean+ standard error.
Results
Functional Liver Mass is Reduced after Proximal and Distal Partial Small Bowel Resection
We have previously reported that liver size is reduced in a murine model of partial small bowel resection (SBR) in which the proximal 50% of mouse small intestine is resected followed by primary anastomosis.3 Based on this study, we wanted to further characterize the temporal regulation of changes in liver mass after SBR. The results of this analysis showed that liver:body mass is unchanged at 24 h after SBR, then declines significantly reaching a nadir 72 h after surgery, and subsequently recovers to near normal by 7 days (Fig. 1a). In order to determine whether this decreased liver mass represented loss of functional hepatic parenchyma, we quantified total protein and alanine transaminase activity (ALT) content in the hepatic tissue recovered at the serial times after surgery. In each case the results showed a significant decrease following SBR compared to sham-operated animals (Fig. 1b and c), indicating that metabolically active liver mass was reduced specifically in response to intestinal resection. Comparable changes were also seen in total hepatic aspartate transaminase activity (AST) after proximal SBR (data not shown). As in the case of liver:body weight, total tissue protein, and transaminase activity demonstrated recovery by 7 days after intestinal resection. Finally, we investigated the effect of a distal 50% SBR on liver weight. The results showed comparable decline in liver: body mass 72 h after surgery (data not shown), indicating that the influence of SBR on liver mass was not dependent upon the site of bowel resected.
Figure 1.
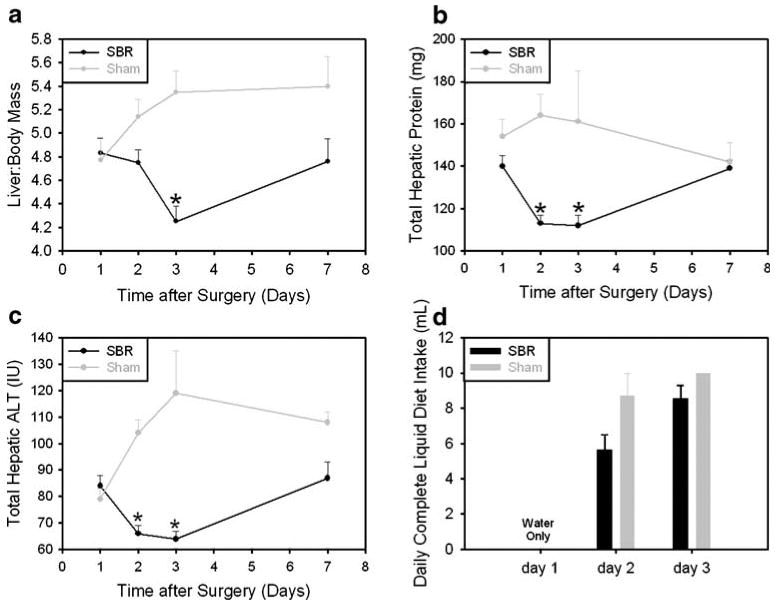
Change in liver:body mass after small bowel resection. a Liver:body mass ratio after proximal and distal small bowel resection (SBR) and sham surgery (*p<0.03). b Total hepatic protein (*p<0.02) and c total hepatic ALT (*p<0.003) after proximal SBR and sham surgery. d Daily complete liquid diet intake after proximal SBR or sham surgery (day 2, p=0.1; day 3, p=0.3).
Dietary Intake is not Significantly Reduced in Mice Subjected to SBR Compared to Control Animals
In order to address the possible contribution of differences in enteral intake between SBR and sham-operated mice to changes in liver:body mass, a subset of operated animals were individually housed for determination of daily liquid diet intake. The results showed a trend towards modestly reduced complete liquid diet intake in SBR versus sham-operated animals on post-operative day 2, which did not achieve statistical significance (p=0.1), and comparable intake between SBR and sham-operated animals on day 3 (p=0.3, Fig. 1d).
Hepatocyte Size is Reduced After SBR
Morphometric analysis showed a significant reduction in average hepatocellular cross-sectional area in SBR versus sham-operated animals without any apparent differences in liver histology (Fig. 2), suggesting that at least some of the decrease in liver mass results from decreased hepatocyte size.
Figure 2.
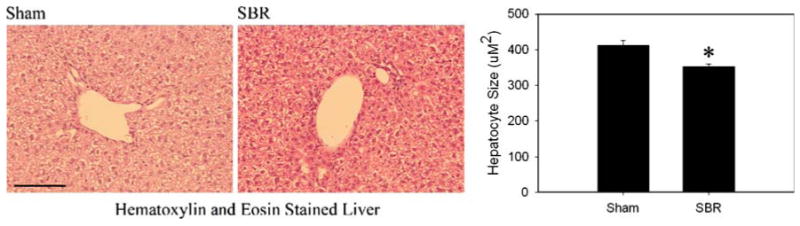
Hepatocyte size after small bowel resection. Hematoxylin and eosin stained sections of mouse liver after sham (left) or SBR surgery (middle) and summary of hepatocellular cross sectional area measurement (right) after proximal SBR and sham surgery (*p<0.02). A 100 μm bar is shown in lower left corner of left panel.
LC3-II is Activated in Liver After SBR
Based on the SBR-induced changes in hepatocyte size, we next examined whether autophagic signaling is activated in livers from mice subjected to partial bowel resection. Autophagy is a process by which eukaryotic cells degrade and remove redundant or defective proteins and organelles.16,17 This pathway can be stimulated by nutrient deprivation 17 and is a central regulator of cell growth and size.18 Autophagic activation can be detected by protein immunoblot analysis of LC3, a component of the autophagic machinery that undergoes lipidation during such activation.19 This modification results in a change in electrophoretic mobility of LC3 on denaturing SDS-PAGE analysis from the slower migrating parent molecule (LC3-I) to the faster migrating modified form (LC3-II). Immunoblot analysis of whole protein lysates of liver recovered at serial times after SBR showed significantly increased levels of LC3-II at 48 h after surgery compared to sham-operated animals (Fig. 3a and b). These data suggest that hepatic autophagy may be activated after resection of the proximal small bowel.
Figure 3.
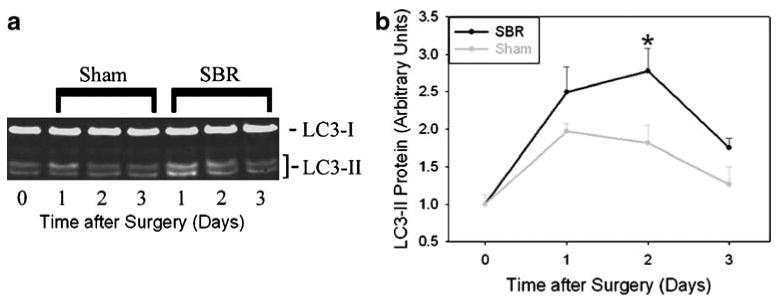
Increased hepatic expression of LC3-II, an autophagic marker, after small bowel resection. a Representative protein immunoblot and b summary of densitometric analysis showing relative LC3-II expression in liver after proximal SBR and sham surgery (*p<0.03).
The Ratio of Bax:Bcl-2 Expression is Increased After SBR
Apoptosis represents another mechanism by which organ size can be regulated;20 therefore, we investigated whether hepatic apoptotic signaling is activated in liver after SBR. First we examined expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 mRNA and protein expression. The results showed a significant increase in the ratio of Bax:Bcl-2 protein expression 72 h after proximal SBR (Fig. 4a–c). Despite this significant change in expression favoring increased apoptosis, we did not observe any corresponding increase in hepatic TUNEL staining, Caspase 3, 9, or 12 activation, or PARP cleavage (data not shown). Together, these observations raise the possibility that apoptotic progression may be inhibited in liver by specific mechanisms after SBR, for example by induction of expression of inhibitor of apoptosis (IAP) family members.21,22 To address this possibility, we quantified hepatic mRNA expression of several IAPs. The results showed that hepatic expression of the IAPs XIAP, cIAP1, cIAP2, survivin, or BRUCE was not significantly increased after SBR versus sham surgery, though several showed a trend towards increased expression after SBR (Fig. 5).
Figure 4.
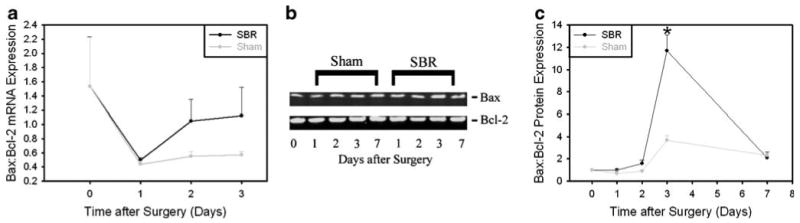
Increased Bax:Bcl-2 expression after small bowel resection. a mRNA expression analysis, b representative protein immunoblot, and c summary of densitometric analysis of protein expression showing relative Bax:Bcl-2 expression in liver after proximal SBR and sham surgery (*p<0.001).
Figure 5.
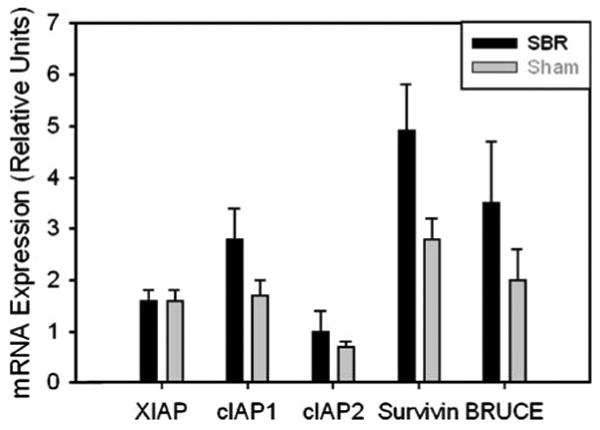
Hepatic mRNA expression of XIAP, cIAP1, and cIAP2, survivin, and BRUCE after small bowel resection. Expression is shown in proportion to expression in liver from unoperated animals.
Discussion
The precision with which the liver:body mass ratio is regulated in health and restored by regeneration after injury has been recognized for thousands of years, as indicated by the legend of Prometheus from ancient Greek mythology,23 and extensive experimental analyses have been conducted to identify the responsible mechanisms. Nevertheless, the specific signals that regulate liver:body mass with such remarkable fidelity have not been fully elucidated, and further studies are needed before the potential benefits that such understanding might offer towards clinical management of patients with liver diseases are fully realized. Several lines of evidence suggest that important signals involved in regulating liver mass may come from the small intestine. For example, some studies have shown that hepatic regeneration is impaired after extensive SBR,4,5 and we have previously reported that liver mass is decreased after proximal SBR.3 Based on these observations, the studies reported here were conducted in order to further characterize the changes that occur in liver:body mass after partial small bowel resection, including determination as to whether such changes involve both physical and functional liver mass and identification of candidate mechanistic mediators of this effect. The results showed that following proximal SBR, liver:body mass is significantly decreased with comparably decreased total hepatic tissue protein and transaminase activity, indicating that SBR results in loss of functional, metabolically active liver tissue. A similar decrease in liver:body mass was noted after distal SBR, indicating that the mechanisms responsible for decreased liver mass in this model do not depend on region-specific functions of the small intestine. For example, these mechanisms are not likely to depend on bile-acid-dependent regulation of FXR activity, which has been shown to regulate liver mass 8 but depends primarily on active reabsorption of bile acids in the distal small intestine.24 Our data also showed that by 7 days after SBR liver:body mass, total hepatic transaminase activity, and total hepatic protein had recovered. This timing of recovery correlates with, and may result from, signals derived from the intestinal adaptive response, whose onset is detectable as early as 24 h after SBR and which plateaus 7 days after such surgery.25,26
Our studies also identified a molecular marker of autophagic activation (LC3-II) increased in liver tissue recovered from mice subjected to bowel resection, raising the possibility that autophagy may contribute to loss of liver tissue in this model. Autophagy is an ancient, evolutionarily conserved mechanisms by which eukaryotic cells degrade and remove redundant, senescent, or defective proteins and organelles.16,17 This pathway has been shown to regulate cell size,18 which is intriguing in light of our observation that partial bowel resection also results in decreased hepatocellular cross-sectional area. Autophagy is increasingly being studied because of emerging links between its dysregulation and human diseases, including liver diseases. For example α1-antitrypsin deficiency associated liver disease has been associated with increased hepatocellular autophagy.27 In addition, autophagy has recently been implicated as a candidate mediator of acute liver cell damage in patients with anorexia nervosa.28 Given the established link between nutritional deprivation and autophagic activation,17 these data highlight the possibility that, despite comparable post-operative dietary intake in SBR versus sham-operated mice, the bowel resected animals may suffer from some degree of relative nutritional deprivation related to reduced small bowel absorptive capacity. Alternatively, the small bowel may be the source of a non-nutritive signal that regulates autophagy. Based on the data presented here, future studies should investigate the impact of pharmacological or genetic interventions that disrupt autophagy on regulation of liver:body mass after SBR.
Finally, our studies also showed increased hepatic expression of the pro-apoptotic protein Bax in proportion to the anti-apoptotic protein Bcl-2 after partial bowel resection. However, our inability to detect evidence of hepatic activation of downstream markers of apoptotic activation, such as caspase 3 and 9 activation, PARP cleavage, or TUNEL staining raised the possibility that hepatic apoptotic progression may be specifically inhibited in this setting. To address this we investigated hepatic mRNA expression of several IAP family members. None of these was identified as significantly up-regulated after SBR. It is intriguing to note that hepatocellular apoptosis was not identified in anorexia nervosa patients with acute liver injury.28 Nevertheless, the functional role that apoptosis plays in the changes that occur in functional liver mass after small bowel resection remains to be further defined, and future analyses investigating the effects of genetic or pharmacological disruption of apoptotic pathways on changes in liver mass after SBR may provide additional insight.
Conclusion
The data presented here define the temporal pattern of change in functional liver mass after SBR in an experimental mouse model, and identify autophagy and, perhaps, apoptosis as candidate mediators of this effect. These data raise the interesting possibility that similar signaling events may effect hepatic mass and function after partial bowel resection in human patients, which could contribute to liver dysfunction after extensive small bowel resection, for example in neonates with necrotizing enterocolitis.
Acknowledgments
The authors thank Dr. Dennis Dietzen for his assistance with transaminase activity determinations on mouse liver lysates.
These studies were supported by grants from National Institutes of Health (RO1 DK52712 (DAR), DK53234 (BWW); T32-54294C (SWL)) and CDHNF/TAP (DAR), and by the Digestive Disease Research Core Center at Washington University School of Medicine (P30DK052574).
Footnotes
Presented at 2009 SSAT Annual Meeting, June 3, 2009, Chicago, IL
Contributor Information
Zhaohua Qiu, Department of Pediatrics, Washington University School of Medicine, St. Louis, MO, USA.
Shannon W. Longshore, Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA; Department of Surgery, University of California, Davis Medical Center, Sacramento, CA, USA
Brad W. Warner, Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA
David A. Rudnick, Department of Pediatrics, Washington University School of Medicine, St. Louis, MO, USA; Department of Developmental Biology, Washington University School of Medicine, St. Louis, MO, USA
References
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson LA, O'Brien DP, Kemp CJ, Williams JL, Dunke-Jacobs E, Erwin CR, Warner BW. Intestinal and hepatic response to combined partial hepatectomy and small bowel resection in mice. Am J Surg. 2002;183:435–440. doi: 10.1016/s0002-9610(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Szuch P, Levine M, Fisher ER. A portal blood factor as the humoral agent in liver regeneration. Science. 1971;171:575–577. doi: 10.1126/science.171.3971.575. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Szuch P, Levine M, Saffer E, Fisher ER. The intestine as a source of a portal blood factor responsible for liver regeneration. Surg Gynecol Obstet. 1973;137:210–214. [PubMed] [Google Scholar]
- 6.Bucher NL, Swaffield MN. Regeneration of liver in rats in the absence of portal splanchnic organs and a portal blood supply. Cancer Res. 1973;33:3189–3194. [PubMed] [Google Scholar]
- 7.Poirier RA, Cahow CE. Role of the small intestine in liver regeneration. Am Surg. 1974;40:555–557. [PubMed] [Google Scholar]
- 8.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 9.Helmrath MA, Erwin CR, Warner BW. A defective EGF-receptor in waved-2 mice attenuates intestinal adaptation. J Surg Res. 1997;69:76–80. doi: 10.1006/jsre.1997.5033. [DOI] [PubMed] [Google Scholar]
- 10.Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proc Natl Acad Sci U S A. 2001;98:8885–8890. doi: 10.1073/pnas.151217998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Y, Shikapwashya ON, Shteyer E, Dieckgraefe BK, Hruz PW, Rudnick DA. Delayed hepatocellular mitotic progression and impaired liver regeneration in early growth response-1-deficient mice. J Biol Chem. 2004;279:43107–43116. doi: 10.1074/jbc.M407969200. [DOI] [PubMed] [Google Scholar]
- 12.Clark A, Weymann A, Hartman E, Turmelle Y, Carroll M, Thurman JM, Holers VM, Hourcade DE, Rudnick DA. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol Immunol. 2008;45:3125–3132. doi: 10.1016/j.molimm.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turmelle YP, Shikapwashya O, Tu S, Hruz PW, Yan Q, Rudnick DA. Rosiglitazone inhibits mouse liver regeneration. The FASEB Journal. 2006;20:2609–2611. doi: 10.1096/fj.06-6511fje. [DOI] [PubMed] [Google Scholar]
- 14.Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40:1322–1332. doi: 10.1002/hep.20462. [DOI] [PubMed] [Google Scholar]
- 15.Dziarmaga A, Hueber PA, Iglesias D, Hache N, Jeffs A, Gendron N, Mackenzie A, Eccles M, Goodyer P. Neuronal apoptosis inhibitory protein is expressed in developing kidney and is regulated by PAX2. Am J Physiol Renal Physiol. 2006;291:F913–F920. doi: 10.1152/ajprenal.00004.2006. [DOI] [PubMed] [Google Scholar]
- 16.Hussey S, Terebiznik MR, Jones NL. Autophagy: healthy eating and self-digestion for gastroenterologists. J Pediatr Gastroenterol Nutr. 2008;46:496–506. doi: 10.1097/MPG.0b013e3181617895. [DOI] [PubMed] [Google Scholar]
- 17.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 18.Vellai T, Bicsak B, Toth ML, Takacs-Vellai K, Kovacs AL. Regulation of cell growth by autophagy. Autophagy. 2008;4:507–509. doi: 10.4161/auto.5670. [DOI] [PubMed] [Google Scholar]
- 19.Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 20.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal N. Prometheus's vulture and the stem-cell promise. N Engl J Med. 2003;349:267–274. doi: 10.1056/NEJMra020849. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci. 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 25.Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 26.Warner BW, Erwin CR. Critical roles for EGF receptor signaling during resection-induced intestinal adaptation. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S68–S73. doi: 10.1097/01.mpg.0000226393.87106.da. [DOI] [PubMed] [Google Scholar]
- 27.Teckman JH, Perlmutter DH. Retention of mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G961–G974. doi: 10.1152/ajpgi.2000.279.5.G961. [DOI] [PubMed] [Google Scholar]
- 28.Rautou PE, Cazals-Hatem D, Moreau R, Francoz C, Feldmann G, Lebrec D, Ogier-Denis E, Bedossa P, Valla D, Durand F. Acute liver cell damage in patients with anorexia nervosa: a possible role of starvation-induced hepatocyte autophagy. Gastroenterology. 2008;135:840–848. doi: 10.1053/j.gastro.2008.05.055. [DOI] [PubMed] [Google Scholar]


