Jehovah's Witnesses and the Historical Evolution of Blood Conservation
Everything that lives and moves will be food for you. … But you must not eat meat that has its lifeblood still in it. For your lifeblood I will surely demand an accounting.
—Genesis 9:3–5, Old Testament, New International Version1
Any Israelite or any alien living among them who eats any blood—I will set my face against that person who eats blood and will cut him off from his people. For the life of a creature is in the blood, and I have given it to you to make atonement for yourselves on the altar; it is the blood that makes atonement for one's life. Therefore I say to the Israelites, “None of you may eat blood, nor may an alien living among you eat blood.”
—Leviticus 17:10, 12, Old Testament, New International1
You must abstain from eating food offered to idols, from consuming blood or the meat of strangled animals, and from sexual immorality.”
—Acts 15:29, New Testament, New LivingTranslation2
Jehovah's Witnesses is a religious organization whose adherents now number more than six million worldwide. The sect was established in 1870 in an effort to return to a pure, unadulterated, scripture-based form of Christianity; consequently, many of the group's doctrines are derived from literal interpretations of the Bible. Though best known for its evangelical efforts, the organization has gained notoriety for its controversial stance on blood transfusions, which its adherents believe to be a violation of God's law. To them, the “life force” resides in the blood, and oral or intravenous ingestion can result in the forfeiture of eternal life and excommunication from the congregation. Because of their beliefs, Jehovah's Witnesses faced resistance and criticism from the medical community. Fearful of undergoing transfusion against their will or without their knowledge and unable to find hospitals willing to treat them in accordance to their religious precepts, members would, at times, risk their health and well-being to avoid medical intervention. The first bloodless surgery program was developed for this population. By coordinating presurgery counseling, specialized equipment, and physicians trained in perioperative and postoperative nonblood therapies for the prevention and treatment of anemia, blood-management programs ensure that patients can access treatment without having to forfeit their beliefs.
The first bloodless surgery program was established to offer Jehovah's Witnesses access to medical treatment in an environment that coincided with their religious belief system. It and other similar programs were designed to be transfusion-free, using a method of blood management that strictly disallows the administration of allogenic cellular transfusions (red blood cells [RBCs], white blood cells, platelets), as well as many serum protein products, despite the risk of patient mortality. The importance of maintaining a transfusion-free environment requires the hospital to implement comprehensive preoperative, intraoperative, and postoperative safety measures as well as patient tracking systems to prevent inadvertent blood product administration to this specific patient population. Ultimately, the program demands a degree of institutional commitment with administrative and operational precision that many hospitals are unable to provide.
Blood-management programs have since advanced to lie on a spectrum from transfusion-free practice to less restrictive alternatives based in a common effort to minimize blood product administration. Their development, which began as an alternative for those with religious beliefs that prohibit the acceptance of blood products, is also driven by the concept that minimizing blood product administration enhances patient safety and reduces the cost and length of hospital stays. Using many of the same clinical strategies to avoid blood product administration, a blood-conservation program can avoid problems with the availability and escalating costs of blood products and take advantage of the fewer complications and increased patient safety associated with decreased allogenic transfusion. In addition, hospitals that are unable to provide transfusion-free programs still have the opportunity to take advantage of the financial and clinical benefits of a blood-management program without the bureaucratic complexities of unconditional prohibition of cellular transfusion that a transfusion-free program would require.
Transfusion—At What Cost?
There is a significant financial cost associated with blood products, generated by the process of collecting blood donations, processing and testing specimens, storage, and transfusion.
Once blood products arrive at a hospital blood bank, other charges are levied: typing ABO; typing Rh; antibody screen; cross-match, immediate spin; cross-match, antiglobulin (Coombs phase); red blood cell antigen screening (per antigen); fresh frozen plasma thawing; cryoprecipitate pooling; and antigen phenotyping.
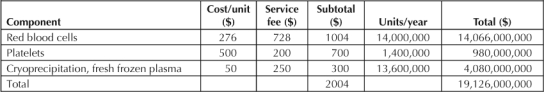
In addition, transportation, handling, and waste management require hospitals to affix additional charges that in the end, can multiply by fivefold the cost of the blood product itself (Table 1).
Table 1.
Additional charges in US dollars to patient: US cost of blood transfusion
The cost of a single unit of blood has quadrupled since the late 1980s, and when the potential cost of adverse events and increased hospital stay is added, the financial burden is immense. Shander3 uses this “cost-of-blood equation” to estimate the cost of transfusion (Figure 1).
Figure 1.
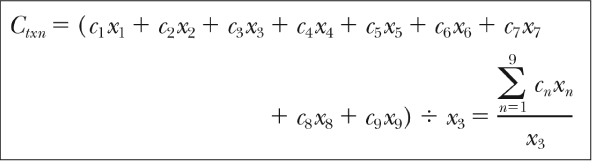
Shander's cost-of-blood equation.3
Ctxn= cost of transfusion; C = cost (eg, donor, production, etc); x = times step repeated.
Reprinted from Surgery 2000 Oct 7;142(4 Suppl), Shander A. Financial and clinical outcomes associated with surgical bleeding complications. p S20-5, Copyright © 2000, with permission from Elsevier.
What this rather complex mathematical equation depicts is how the cost of transfusing (Ctxn) is actually the sum of the individual costs incurred at each step, multiplied by the number of times that each step was repeated. Shander identifies nine important steps: donor cost, production, hospital transfusion preparation, administration of blood products, treatment of adverse outcomes, treatment of transfusion-related disease, litigation, lost productivity, and organization of nationwide hemovigilance systems. What this demonstrates, most importantly, is a global picture of how a single transfusion can incur seemingly unrelated costs.
Many of these extra costs can be controlled through the implementation of a blood-management program. Englewood Hospital in Englewood, New Jersey instituted a blood-saving program through which it has decreased RBC transfusions by 20% since 1994. Staff were able to decrease operating room (OR) transfusions to constitute only 5% of total transfusions in 1998, down from 50% in 1994. Instituting such an effort translated into direct financial return. Interestingly, those patients in the blood-conservation program saved even more resources than those who ultimately did not even need a transfusion, illustrating the cost of transfusion even when transfusion is not performed.
Financial burden, although significant, is not the most important cost of allogenic blood-product transfusion. There is an inherent risk of morbidity and even mortality associated with the transfusion of blood products, the safety and efficacy of which rely on the integrity of the blood product as well as the transfusion process itself.
Although transfusion reactions are rare, a small percentage of blood-product recipients experience adverse effects that can be severe. The risk of infection, even if small, is still an important consideration, with transmission of viruses, bacteria, and parasites all possible. Transfusion reactions range from benign to deadly, with various outcomes and frequencies (Tables 2 and 3).4–8
Table 2.

Table 3.
Frequency of hematologic reactions6–8

The cost of allogenic blood-product transfusion can be significant for patients with cancer, who have been shown to experience a higher rate of recurrence of their cancer than those who do not receive allogenic blood. Although the relationship between blood transfusion and cancer recurrence is still open to debate,9 several studies have shown that blood transfusion is a significant independent prognostic factor for cancer recurrence. Tarttar10 found, in one such study, that 40% of 110 patients who received transfusions developed cancer recurrence, compared with 22% of patients who did not receive blood, and that those who did develop recurrence received an average of twice as much blood as patients without recurrence. Makino et al11 found that perioperative blood transfusion enhances the risk of intrahepatic recurrence of hepatocellular carcinoma in patients with portal vein invasion. Although the relationship is still controversial and confounding factors are numerous, it has been documented that blood transfusion causes a complex immunomodulatory reaction that can include immunosuppression and cancer recurrence predisposition.12,13
Furthermore, it has been documented that transfusion can increase risk of infection. One study14 documented that transfusion of >4 U of blood increased the risk of perioperative infection by a factor of 9.28. A meta-analysis15 of 20 articles published between 1986 and 2000, reporting on studies of a total of 13,152 patients (5215 in a transfusion group and 7937 in a nontransfusion group), concluded that blood transfusion is associated with a significantly increased risk of postoperative bacterial infection, while taking into account confounding variables such as age, shock, and wound contamination.
All this potential morbidity adds to the financial burden of blood product administration, as the tests and procedures required for the proper diagnosis and treatment of these adverse effects add a hidden cost to both hospital and patient.
Background: Anemia, Transfusion Safety, and Surgery Mortality
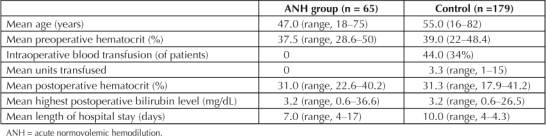
Carson et al16 described a multicenter, retrospective analysis from 1981–1994 that reviewed treatment of 1958 Jehovah's Witnesses patients (older than age 18 years) who declined transfusion, analyzing the effect of anemia and cardiovascular disease (defined as history of angina, myocardial infarction [MI], congestive heart failure [CHF], or peripheral vascular disease) and surgery mortality. The primary outcome was 30-day mortality, with a secondary outcome of 30-day morbidity in the hospital. Mortality rate was 11% in patients with a preoperative hemoglobin level of <10 g/dL, compared with 1.3% in patients with a hemoglobin level of >12 g/dL. Mortality increased linearly to 33.3% with a hemoglobin <6 g/dL. Arrhythmias, respiratory failure, and CHF were the most common complications. These findings establish that overall mortality increases as hemoglobin level decreases and that even mild anemia is associated with some risk of death. This is supported by the findings of another study that showed an inverse correlation between preoperative hemoglobin levels and surgery mortality in surgery patients. A preoperative hemoglobin concentration of <8 mg/dL was associated with a 16.2% increased mortality rate, compared with patients who did not have anemia preoperatively.17
These studies emphasize the danger in not transfusing Jehovah's Witnesses patients in the setting of surgery. Although an attempt can be made to optimize their medical condition and improve their hematocrit levels with treatments such as iron or erythropoietin, these and similar interventions often take time that surgery patients do not have. Going into surgery with anemia that has not been properly addressed is often a reality for patients like Jehovah's Witnesses who refuse transfusions, which places them at a severely increased risk of mortality. Furthermore, because of the dramatic increase in mortality, surgeons may choose not to operate altogether, putting these patients at an even greater disadvantage.
Hébert et al18 determined that a restrictive strategy of blood transfusion is at least as effective as, and possibly superior, to a liberal transfusion strategy in critically ill patients, with possible exception of patients with acute MI and unstable angina. A total of 838 critically ill patients with euvolemia after initial treatment with hemoglobin levels of <9 g/dL were randomized into two groups, one in which a restrictive transfusion strategy was implemented and one in which a liberal strategy was used. The restrictive strategy implemented RBC transfusion at hemoglobin levels of <7 g/dL, and patients' hemoglobin levels were maintained at 7.0 to 9.0 g/dL. The liberal strategy implemented transfusion at <10 g/dL, and patients' hemoglobin was maintained at 10.0 to 12.0 g/dL. The researchers found that 30-day mortality was similar in the two groups, and with the exception of patients with significant cardiac disease, mortality rates were 6% lower (22.3% vs 28.1%) in patients where the restrictive strategy to blood transfusion was implemented, suggesting that not transfusing may be superior to transfusion in many cases. In addition, Carson et al16 concluded that postoperative infections after allogeneic transfusions were associated with a 35% increase in risk of serious bacterial infection and a 52% increased risk of pneumonia.
Alternatives to Allogenic Blood-Product Transfusion: Acute Normovolemic Hemodilution
Currently, blood-product avoidance during surgery is centered on the understanding of blood and coagulation systems in surgery patients. Acute normovolemic hemodilution (ANH) is a broad therapeutic initiative that uses these principles of surgical blood systems to accomplish simultaneous removal of the patient's blood and its replacement with a nonblood product.
The protocol for ANH involves the removal of blood from a central line via gravity flow to a citrate phosphate dextrose adenine (CPDA) bag, which remains in continuity with the patient and can be stored for up to six hours at room temperature (to best preserve platelet function) before being placed in a blood cooler for storage at 4° to 6° C. Because the CPDA tube and bag remain in continuity with the patient, the procedure conforms to the religious beliefs of Jehovah's Witnesses. In addition, ANH blood remains in the OR and thus is not required to be registered in accordance with blood banking and storage regulations.
Acute normovolemic hemodilution … accomplishes simultaneous removal of the patient's blood and its replacement with a nonblood product.
ANH blood can be reinfused at the same rate as any other transfused blood product and, if needed urgently, it can be reinfused via rapid transfuser. The volume of blood withdrawn for ANH ranges between 400 and 1500 mL, depending on anticipated blood losses, original hemoglobin level, and patient tolerance; intravascular euvolemia is maintained with 5% albumin and crystalloid, whereas patient heart rate, blood pressure, pulmonary artery pressure, central venous pressure, and blood gases are continuously monitored. Intraoperative transesophageal echocardiography can also be used to monitor cardiac contractility and ventricular filling. In our practice, the lowest hematocrit after ANH was 21%; coagulation profiles were tracked by thromboelastogram, and approved components (factor VIIa, antifibrinolytics, or protamine) were added as indicated for therapy.
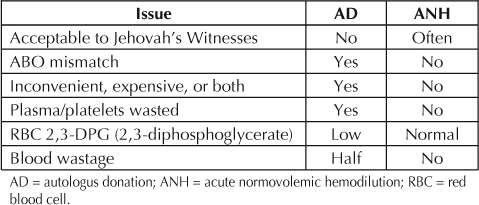
ANH has few contraindications, which include coronary heart disease, significant anemia, and pulmonary hypertension. It successfully creates a state of physiologic anemia that is well tolerated by most patients and generates a cushion against blood loss. Reinfused whole-blood product produced by ANH provides a barrier to hemorrhagic shock and ensures the safety of the patient during a state of predictable blood loss. It effectively lowers the risk of complications of autologous transfusions, including transmission of unknown pathogens, metabolic derangements, and citrate toxicity. It preserves blood bank resources and consequently reduces overall procedure cost (see Table 4 for comparison between autologus blood donation and acute normovolemic hemodilution).
Table 4.
Acute normovolemic hemodilution versus autologous blood donation
Physiology and Safety of Acute Isovolemic Anemia
Weiskopf et al19 described the human response to acute isovolemic anemia in a study of more than 21 volunteers, including 11 patients who were healthy before going under anesthesia. Hemoglobin decreased from 13.1 g/dL to 5 g/dL, with critical oxygen delivery assessed with oxygen consumption, plasma lactate levels, and ST changes on electrocardiographs. The hemodynamic responses to acute isovolemic anemia are shown in Figures 2 through 5.14
Figure 2.
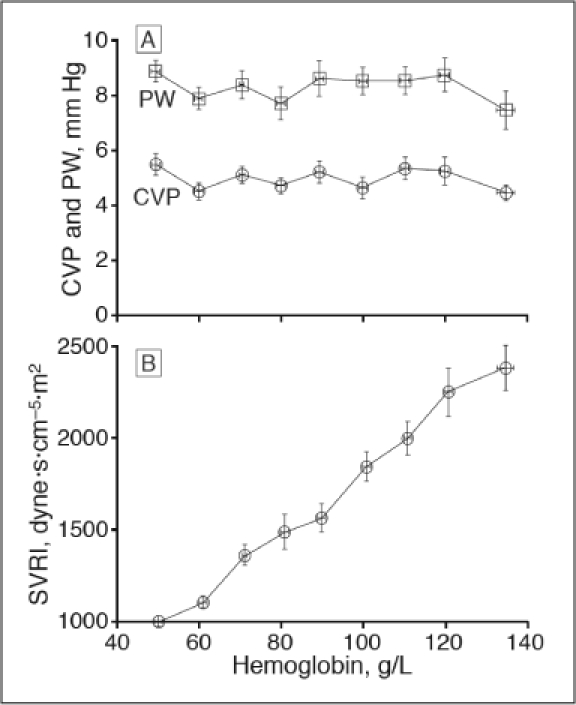
Right heart (central venous pressure [CVP]) and left heart (pulmonary capillary wedge pressure [PW]) did not change (p < 0.05) with acute isovolemic anemia. Acute isovolemic reduction of hemoglobin concentration to 50 g/L decreased systemic vascular resistance index (SVRI; p < .001) by 58%.19
Figure 5.
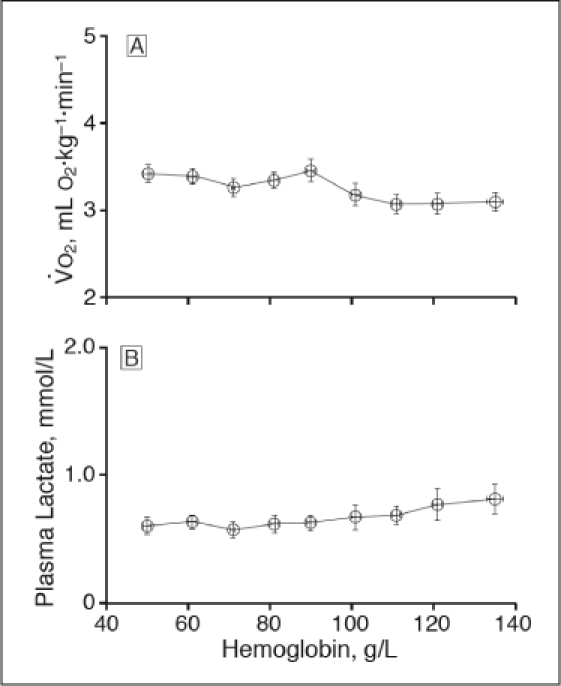
Acute isovolemic reduction of hemoglobin concentration to 50 g/L increased oxygen consumption (VO2, p < .001) but did not change plasma lactate concentration (p = .09).19
Figure 3.
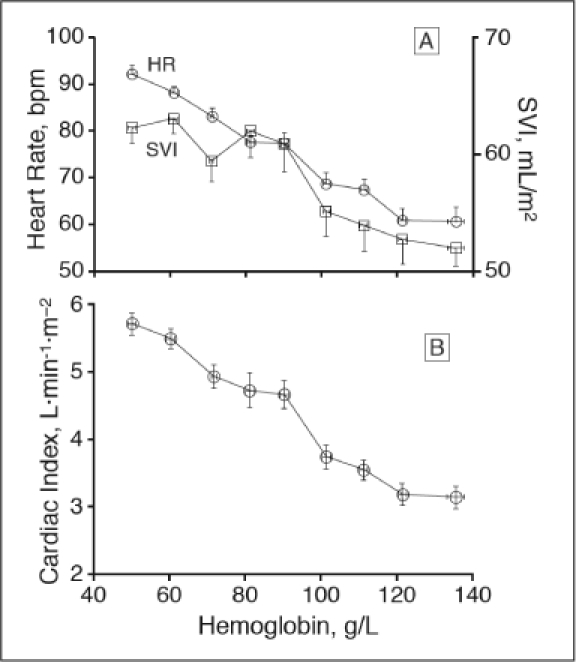
Acute isovolemic reduction of hemoglobin concentration to 50 g/L increased heart rate (p < .001), stroke volume index (p < .001) and cardiac index (p < .001).19
Figure 4.
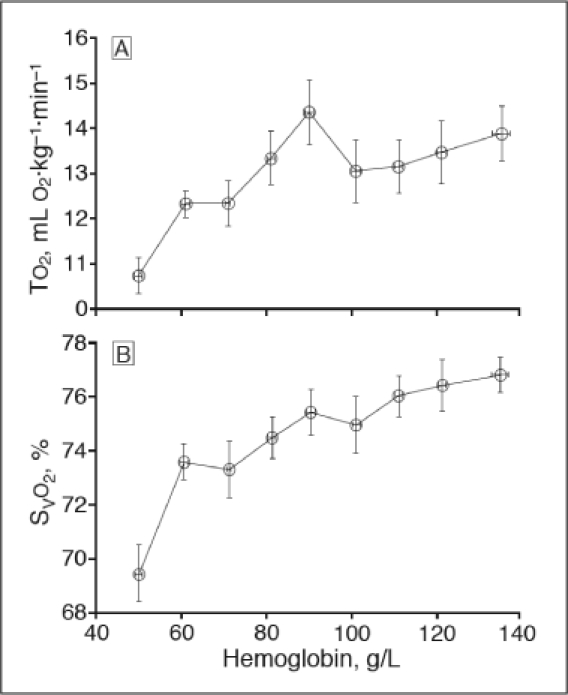
Acute isovolemic reduction of hemoglobin concentration to 50 g/L decreased oxygen transport rate (TO2, p < .001) and mixed venous oxyhemoglobin saturation (SvO2, p < .001).19
ANH decreased systemic vascular resistance and oxygen delivery while increasing heart rate, stroke volume, and cardiac index. Oxygen consumption increased slightly (from 3.07 ± 0.44 mL O2 per kg/min to 3.42 ± 0.54 mL O2 per kg/min) and plasma lactate levels did not change. ANH to hemoglobin levels of 5 mg/dL in conscious healthy resting humans did not produce any inadequate systemic oxygenation. Table 5 summarizes our own experience with acute normovolemic hemodilution in liver resection patients, and emphasizes its potential safety and efficacy.
Table 5.
Acute normovolemic hemodilution in liver resection
The safe lower limit of hematocrit continues to be debated; however, the degree to which ANH is used can be titrated on an individual basis based on patient tolerance. Lower hematocrit levels during surgery may require additional cardiovascular monitoring to assess its impact on systemic functioning; persistent tachycardia and electrocardiographic changes suggestive of MI are usually the first signs of inadequate oxygen delivery as a result of anemia.20 Although ANH is contraindicated in certain patient populations, it can be expected that factors such as age, conditioning, and various comorbidities can limit the degree to which ANH can be tolerated; this has not yet been well established, and further studies are needed to address this matter.
A Comprehensive Approach
It would be imprudent to rely on a single drug or sole procedure to preclude transfusion. Rather, a comprehensive approach to blood conservation should be employed. Before surgery, erythropoietin, iron, and/or folate, can be used to increase preoperative RBC mass, a process labeled blood augmentation. ANH can be used in conjunction with intraoperative cell salvage (ICS), a technique that involves collection, washing, and reinfusion of the blood lost during surgery. Although ICS is an effective method for collecting blood from the surgical field, unfortunately, it salvages only the RBCs and not the associated clotting factors or platelets. Used in combination with ANH, which preserves the integrity of both the RBCs and clotting factors, the availability of a significant amount of safe, fresh, autologous blood can be ensured. Surgical skill, appropriate use of cautery, and meticulous technique can be used to combat excessive blood loss in patients. Preoperative and postoperative restriction of blood testing can also effectively minimize blood loss. Elimination of unnecessary laboratory tests, avoiding draws from the central line, and the use of pediatric tubes when blood draws are necessary can limit intensive care unit (ICU) blood draws to 40 mL to 75 mL of blood daily. Blood conservation is a science in which preparation starts preoperatively and continues through the postoperative phase.
Bloodless Surgery at University of Southern California University Hospital, 1997–2005
While it was at the University of Southern California (USC) University Hospital, our transfusion-free practice successfully performed >200 major surgical procedures on Jehovah's Witnesses patients with >99% success rate, and results were detailed in several major publications in journals,21–27 including the Annals of Surgery and the Journal of the American College of Surgeons:
Total operations performed: 850
Number of major procedures: 350
Mean preoperative hematocrit (major): 40%
Mean postoperative hematocrit (major): 34%
Mean hematocrit at discharge (major): 33%
Mean number of days in ICU (major): 3
Mean length (days) of hospital stay: 12
Figure 6 shows the results of the USC experience with transfusion-free liver transplantation between 1995 and 2005. Four patients were alive and well and two patients were reexplored for peritonitis. Mean length of hospital stay was 40.2 days (range, 8–86 days). Two patients died perioperatively; both had associated kidney failure and were receiving dialysis.
Figure 6.
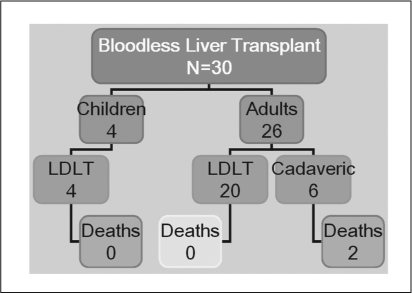
Transfusion-free liver transplantation. University of Southern California University Hospital experience, 1999 to 2005. LDLT = living donor liver transplant.
The efforts made at minimizing transfusion while maintaining hemodynamic stability since the initiation of the transfusion-free surgery program have been extremely successful (Figure 7). The use of ANH, in our experience, has decreased mean length of hospital stay and positively affected other postoperative parameters. In addition to our positive outcomes, we have successfully and dramatically decreased our use of blood and blood products. Since the implementation of transfusion-free services for liver transplantation in patients who are Jehovah's Witnesses, we have successfully implemented similar techniques and programs for a variety of other surgical procedures and subspecialties with similar success.
Figure 7.
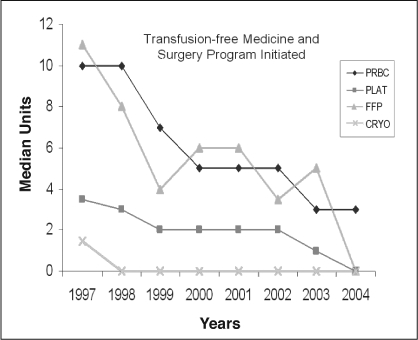
Median perioperative transfusion.
PRBC = packed red blood cells; PLAT = platelets; FFP = fresh frozen plasma; CRYO = cryoprecipitation.
Implementation of Blood-Management Programs: Administrative Schematic
We have entered a new era of patient management in which physicians and hospitals are increasingly accountable for the way that medicine is practiced (transparency; reimbursement tied to outcomes). Hospitals are no longer given a blank check to directly bill insurers for consumables. Instead of relying on direct pass-through costs to insurers, hospital reimbursement formulas are more often being structured as case rates or per diem reimbursement. This translates into fewer pass-through costs, making hospitals directly accountable for resource use. In turn, hospital administrators are now more interested in regulating physicians' use of expensive pharmaceuticals, blood products, and devices and have encouraged them to develop oversight strategies to standardize practice, eliminate outlier use patterns, and enforce the practice of evidence-based medicine. Hospital-based committees—pharmacy and therapeutics, tissue and transfusion, surgical device, and utilization review committees—have been established for data tracking, quality control, and peer review. In this fashion, the casual administration of parenteral nutrition, intravenous antibiotics, mono-clonal antibodies, and blood products, use of surgical devices, and allowance of extended lengths of stay have been curtailed.
Reinfused whole-blood product … ensures the safety of the patient during a state of predictable blood loss.
The success of a blood-management program is predicated on the same logic: Develop a critical pathway for blood conservation (or strict transfusion avoidance), set parameters and safety methods, elaborate on data tracking, use peer review, and set up an education system, and observe the results. The committee and its component parts act to promote and enforce its initiatives with the hospital.
We have established an effective algorithm for the development and execution of a blood-management program that includes both blood-conservation and transfusion-free services. In this way, the algorithm can be used by a hospital for, at the very least, low-complexity blood-conservation services for the mainstream public. On the other hand, the very same algorithm can also be used for those hospitals that choose to pursue the more complex transfusion-free services to cater to the population of Jehovah's Witnesses.
The overall process can be broken down into three separate phases: the initial phase, which is the purview of the process committee; the second phase, overseen by the implementation committee; and the third, or maintenance, phase.
Step 1. Initial Organization
The first step is the formation of a process committee composed of representatives from various hospital services that meet and formulate policies and procedures for:
Administrative identificationa of patient participants depending on their portal of entry (nursing, admitting, outpatient services, blood bank, medical records, OR personnel, Jehovah's Witnesses services director).
Language and legalitya of consent forms that include both refusal and acceptance of blood products (legal, medical records, administration, risk management, Jehovah's Witnesses).
Tracking of product administration, including both allogeneic and ANH blood and blood products (physician leaders, data tracking, blood bank, quality assurance or performance improvement).
Education and awareness programs for nurses, physicians, and ancillary personnel to foster better understanding of the blood-conservation strategies as well as of the religious beliefs and preferencesa of their patients (physician leaders, continuing medical education, nursing, marketing).
Step 2. Implementation
Step 2 occurs once the process committee has formalized the policies and procedures that govern patient traffic, blood-product allocation, and legal issues. Of course, the actions of the process committee are crucial if a transfusion-free program is implemented, and the need for proper patient tracking and identification, as well as the appropriate legal documents and procedures, cannot be stressed enough. However, these more intricate tasks are less important in the setting of establishing a more simple blood-management program. The implementation committee is responsible for:
Educational certification programs, including a formal physician testing process that covers clinical and administrative information related to the program. This certification will allow physicians' entry into the program (physician leaders, continuing medical education group).
Awareness programs that provide promotional and broad public education to Jehovah's Witnesses or the general public. This includes a yearly symposium about the nature of the program and other services offered (marketing, business development, physician leaders, Jehovah's Witnesses liaison services).
Performance improvement projects to identify and track decreases in blood product use, increases in ANH and preoperative blood-augmentation practices, and corresponding changes in costs (quality assurance or performance improvement, data tracking, pharmacy services).
Marketing and business development composed of Web site development, managed care relations, marketing, and liaison services for the Jehovah's Witnesses community (marketing, business development).
Peer review and standards that establish an evidence-based standard for general blood-product administration and the review of unusual cases (blood bank and laboratory, physician leaders, quality assurance or performance improvement).
Step 3. Maintenance
The maintenance phase is a continuation of the committees, procedures, and standards of care created by the implementation team. It is carried out by the various committees and should be supervised by a core group of physicians and professionals particularly interested in promoting the standards of transfusion-avoidance practice (Figure 8).
Figure 8.
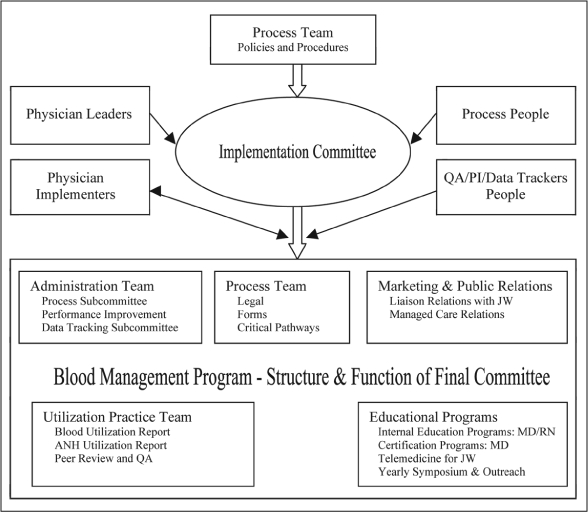
Structure of an implementation team for transfusion-avoidance practices.
ANH = acute normovolemic hemodilution; JW = Jehovah's Witness; PI = performance improvement; QA = quality assessment.
Practical Development of a Hospital Level Blood-Management Program
Implementation of a blood-management program should be done in steps, allowing both medical and hospital staff to adapt to the new policies and procedures in a methodical manner. This is accomplished through work done by the initial team meeting, setting implementation guidelines, and addressing other issues.
Initial Team Meeting
The initial team meeting is crucial to the successful establishment of the blood-management program. The goal of the meeting is to establish an action plan and an adequate, realistic time frame for policy implementation. With this goal in mind, the following tasks should be completed in this meeting:
Outline the action plan with a specific timeframe to ensure adequate completion; present the action plan and timeframe to the action-team, department directors, and relevant medical staff
Present findings from surgery and critical care to the initiative team
Initiate clinical and medical staff education programs; consider inviting a physician speaker to conduct medical staff education
Obtain and establish proper approval for new blood-conservation strategies; consider scheduling ad hoc medical staff committee meeting(s) to expedite approval process
Establish and implement new policies, including transfusion triggers
Review the results-tracking tools
Assess baseline spending and management of potential savings targets; keep goal in mind to share savings target and quality matrices with physicians and hospital staff monthly
Outline an action plan to shift autologous blood donation to freestanding blood banks
Identify necessary physician involvement to ensure proper patient notification
Develop and share implementation guidelines (see section on this topic)
Celebrate success as milestones are met.
After the initial team meeting and successful creation and implementation of a plan and realistic timeline, the following steps should be considered crucial key principles:
- Establishment of a hospital-based committee that deals with transfusion and blood banking to monitor costs and quality indicators that include:
- - Cross-match-to-transfusion (C:T) ratios by specialty
- - Transfusion reactions
- - Annual blood usage by category and cost per unit
- - Cell salvage and subsequent autotransfusion
- - Top five surgical procedures that routinely use blood products
- - Current use and costs associated with ICS
- - Baseline use of cell salvage by surgical procedure
- - High-volume physicians in Orthopedic, Spine, Cardiovascular, and Oncology Departments that routinely type and cross for blood product
- - Top five diagnoses requiring blood transfusion, including number of units routinely typed and crossed per procedure vs infused.
Provision of the hospital staff with educational sessions on blood conservation and management programs
Ensuring that the chief executive office (CEO), chief nursing officer, and physician initiative champions are involved and supportive of efforts. This critical step is essential to the success of the program
Implementation of perioperative blood-conservation guidelines and considerations such as ICS, autologous transfusion, and ANH
Adoption of a general hospital policy on universal avoidance of blood transfusiona [Jehovah's Witnesses]
Sharing of working definitions and “frequently asked questions” sheets with key stakeholders.
The following recommendations should be implemented to realize prompt benefit to patient and hospital:
Decreasing transfusion trigger to 7.0 g/dL
Monitoring usage to establish baseline
Ensuring that departmental leaders assess staff participation and identify those with concerns about blood conservation. Address these issues on an individual basis
Selecting a multidisciplinary committee of medical staff leadership that includes staff and physicians from anesthesia, nursing, the laboratory, the blood bank, surgery, oncology, and pharmacy
Preparing for an initial meeting. Collect for review all policies related to blood and blood-product administration. Data collected should include cell-saver setup, supply cost per procedure, amount of blood collected, and amount of blood transfused, labor cost associated with cell saver (hospital staff or outside contractor such as perfusion or anesthesia)
Establishing a tracking tool. Determine the frequency of blood draws in critical care environment
Considering the use of low-volume draw primary tubes and appropriate instrumentation in the laboratory for accuracy, reproducibility, fast turnaround times, and microvolume analysis to reduce incidence of ICU anemia
Determining use of autologous blood (number of units received vs transfused). Identify steps to move autologous blood donation to local blood banks to reduce hospital's indirect expense for donation and preparation
Establishing a blood-management team weekly meeting schedule. The goals should be to adopt blood-management standards to facility policy and to expedite policy approvals through appropriate hospital and medical staff committees for review and approval.
The safe lower limit of hematocrit continues to be debated …
Implementation Guidelines
The development of specific implementation guidelines will help expedite the establishment of a complete and effective blood-management program. These are some implementation guidelines that we recommend:
Ensuring adequate comprehension of hospital's current blood and blood product transfusion policies, practices, and actual utilization
Monitoring monthly blood spending, expired blood products, and existing cross match to C:T ratios
Implementing regular communication with hospital/medical staff concerning progress of program; CEO to write letter(s) to hospital and medical staff supporting blood-management conservation program
Reviewing blood requirements for all laboratory testing
Developing minimum and average blood volume for each laboratory test
Reviewing blood collection tubes and catalogue for proper tube and volume draw measurements; make recommendations to lab and medical directors
Setting up purchasing protocols for new tubes
Creating education plans using charts and posters; consider enlisting vendor representatives to provide visual aids
Scheduling educational events to introduce new procedures and tubes for low-volume blood collection
Creating plan to continuously monitor educational objectives and evaluate success of program; report to quality management committees on outcome.
Other Issues
Cell Salvage: Cell salvage is a blood-collection process that allows the surgical team to collect blood lost during a procedure, filter it, and return it to the patient intraoperatively. Fresh, autologous blood is recommended as a patient's first option for blood transfusion in surgery; however cell salvage is a technique that can be of particular benefit in a blood-conservation program. If an institution does not already have a cell-salvage protocol in place, we make the following recommendations to help establish an effective cell-salvage program:
Reviewing blood bank and OR records to identify procedures regularly requiring blood transfusion to create a list of procedures that might require cell-salvage equipment
Properly training appropriate staff to manage and monitor equipment. Monitoring may be done by a perfusionist, nurse, or OR tech. The filtering and return of blood is traditionally the responsibility of the circulating nurse, anesthesiologist, or perfusionist
Reviewing the current management and use of cell-salvage technology; cell-salvage equipment vendors may help assist with staff education as well as provide current information on benefits to blood-conservation strategies
Using cell-saver log to begin data collection.
Anesthesia Management: Clear communication with the anesthesiologist is vital to the management and maintenance of a patient's hematologic system. The role of the anesthesiologist in blood management is critical, given his or her involvement in intraoperative cell salvage and ANH. During transfusion-free surgery, anesthesia should maintain the normovolemic status and handle the potential need for hyperoxic ventilation and controlled hypotensive anesthesia. For example, a low central venous pressure may markedly reduce blood loss during parenchymal transection for liver resection. Similarly, blood loss during spinal surgery can be significantly curtailed by relative hypotensive anesthesia. The avoidance of hypothermia can help avoid temperature-related coagulopathy.
Conclusion
As physicians and health care professionals bound by an ethical and professional responsibility to treat patients of all faiths and beliefs, we found ourselves developing a new science, a practice that was able to maintain safety in a transfusion-free setting while performing some of the most complex and intricate surgical procedures. Through our experience, we were able to develop a process that efficiently and effectively establishes a transfusion-free program. What we realized, however, is that there are benefits to practicing in a setting that lies on a spectrum closer to being transfusion-free, where specific and calculated efforts are made to reduce the amount of allogeneic blood that is transfused, even for those who have no moral obligation for avoidance. Through effective blood management that minimizes the use of allogeneic blood and blood products, there is increased patient safety, better resource allocation, and minimization of financial burden. A strategy similar to establishing a transfusion-free program can be simplified, as outlined in this paper, and implemented to create a blood-management program that works in a similar manner, without some of the legal and moral complexities involved in maintaining a transfusion-free program. Blood management is an enlightened approach to medical and surgical treatment and is based on proven and effective methods. It can be used not only to benefit the Jehovah's Witness population but also to benefit the general population, through better use of resources.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
Footnotes
a Indicates steps that apply only to transfusion-free programs.
Figures 2–5 Reprinted from Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998 Jan 21;279(3):217–21. With permission from the American Medical Association © 1998. All rights reserved.
References
- Genesis 9:3-5; Leviticus 17:10, 12; The Holy Bible, New International Version. Colorado Springs (CO): IBS-STL Global; 1973, 1978, 1984. [Google Scholar]
- Acts 15:29. The Holy Bible, New Living Translation. Wheaton (IL): Tyndale House Publishers, Inc; 1996, 2004. [Google Scholar]
- Shander A. Financial and clinical outcomes associated with surgical bleeding complications. Surgery. 2000 Oct 7;142(4 Suppl):S20–5. doi: 10.1016/j.surg.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retro-virus Epidemiology Donor Study. N Engl J Med. 1996 Jun 27;334(26):1685–90. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- Zou S, Fujii K, Johnson S, et al. ARCNET Study Group Prevalence of selected viral infections among blood donors deferred for potential risk to blood safety. Transfusion. 2006 Nov;46(11):1997–2003. doi: 10.1111/j.1537-2995.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis. 1983 Jul;128(1):185–9. doi: 10.1164/arrd.1983.128.1.185. [DOI] [PubMed] [Google Scholar]
- Pineda AA, Vamvakas EC, Gorden LD, Winters JL, Moore SB. Trends in the incidence of delayed hemolytic and delayed serologic transfusion reactions. Transfusion. 1999 Oct;39(10):1097–103. doi: 10.1046/j.1537-2995.1999.39101097.x. [DOI] [PubMed] [Google Scholar]
- Capon SM, Goldfinger D. Acute hemolytic transfusion reaction, a paradigm of the systemic inflammatory response: new insights into pathophysiology and treatment. Transfusion. 1995 Jun;35(6):513–20. doi: 10.1046/j.1537-2995.1995.35695288773.x. Erratum in: Transfusion 1995 Sep;35(9):794. [DOI] [PubMed] [Google Scholar]
- Donohue JH, Williams S, Cha S, et al. Perioperative blood transfusions do not affect disease recurrence of patients undergoing curative resection of colorectal carcinoma: a Mayo/North Central Cancer Treatment Group study. J Clin Oncol. 1995 Jul;13(7):1671–8. doi: 10.1200/JCO.1995.13.7.1671. [DOI] [PubMed] [Google Scholar]
- Tartter PI. The association of perioperative blood transfusion with colorectal cancer recurrence. Ann Surg. 1992 Dec;216(6):633–8. doi: 10.1097/00000658-199212000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Yamanoi A, Kimoto T, El-Assal ON, Kohno H, Nagasue N. The influence of perioperative blood transfusion on intrahepatic recurrence after curative resection of hepatocellular carcinoma. Am J Gastroenterol. 2000 May;95(5):1294–300. doi: 10.1111/j.1572-0241.2000.02028.x. [DOI] [PubMed] [Google Scholar]
- Tartter PI, Heimann TM, Aufses AH., Jr. Blood transfusion, skin test reactivity, and lymphocytes in inflammatory bowel disease. Am J Surg. 1986 (Mar);151(3):358–61. doi: 10.1016/0002-9610(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007 Nov;21(6):327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res. 2002 Feb;102(2):237–44. doi: 10.1006/jsre.2001.6330. [DOI] [PubMed] [Google Scholar]
- Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003 May;54(5):908–14. doi: 10.1097/01.TA.0000022460.21283.53. [DOI] [PubMed] [Google Scholar]
- Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996 Oct 19;348(9034):1055–60. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988 Apr 2;1(8588):727–9. doi: 10.1016/s0140-6736(88)91536-x. [DOI] [PubMed] [Google Scholar]
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999 Feb 11;340(6):409–17. doi: 10.1056/NEJM199902113400601. Erratum in: N Engl J Med 1999 Apr 1;340(13):1056. [DOI] [PubMed] [Google Scholar]
- Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998 Jan 21;279(3):217–21. doi: 10.1001/jama.279.3.217. Erratum in: JAMA 1998 Oct 28;280(16):1404. [DOI] [PubMed] [Google Scholar]
- Murray D. Acute normovolemic hemodilution. Eur Spine J. 2004 Oct;13(Suppl 1):S72–5. doi: 10.1007/s00586-004-0755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour N, Gagandeep S, Mateo R, et al. Live donor liver transplantation without blood products: strategies developed for Jehovah's Witnesses offer broad application. Ann Surg. 2004 Aug;240(2):350–7. doi: 10.1097/01.sla.0000133352.25163.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour N, Gagandeep S, Bramstedt KA, et al. To do or not to do: living donor hepatectomy in Jehovah's Witnesses: single institution experience of the first 13 resections. Am J Transplant. 2005 May;5(5):1141–5. doi: 10.1111/j.1600-6143.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- Jabbour N, Gagandeep S, Thomas D, et al. Transfusion-free techniques in pediatric live donor liver transplantation. J Pediatr Gastroenterol Nutr. 2005 Apr;40(4):521–3. doi: 10.1097/01.mpg.0000157590.23126.fd. [DOI] [PubMed] [Google Scholar]
- Jabbour N, Gagandeep S, Mateo R, Sher L, Genyk Y, Selby R. Transfusion free surgery: single institution experience of 27 consecutive liver transplants in Jehovah's Witnesses. J Am Coll Surg. 2005 Sep;201(3):412–7. doi: 10.1016/j.jamcollsurg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Jabbour N, Gagandeep S, Mateo R, Sher L, Selby R, Genyk Y. Live donor liver transplantation in children—guidelines for transfusion free techniques. Hepatobiliary Pancreat Dis Int. 2004;6(Suppl 1):67. [Google Scholar]
- Jabbour N, Gagandeep S, Peilin AC, et al. Recombinant human coagulation factor VIIa in Jehovah's Witness patients undergoing liver transplantation. Am Surg. 2005 Feb;71(2):175–9. [PubMed] [Google Scholar]
- Strum E, Jabbour N, Gagandeep S, Selby R. Transplantation in Jehovah's Witness population: bloodless surgery allows options. Seminars in Anesthesia Perioperative Medicine and Pain. 2004 Mar;23(1):66–70. [Google Scholar]


