Abstract
The complete sequence of the circular 101,016-bp megaplasmid pKB1 from the cis-1,4-polyisoprene-degrading bacterium Gordonia westfalica Kb1, which represents the first described extrachromosomal DNA of a member of this genus, was determined. Plasmid pKB1 harbors 105 open reading frames. The predicted products of 46 of these are significantly related to proteins of known function. Plasmid pKB1 is organized into three functional regions that are flanked by insertion sequence (IS) elements: (i) a replication and putative partitioning region, (ii) a putative metabolic region, and (iii) a large putative conjugative transfer region, which is interrupted by an additional IS element. Southern hybridization experiments revealed the presence of another copy of this conjugational transfer region on the bacterial chromosome. The origin of replication (oriV) of pKB1 was identified and used for construction of Escherichia coli-Gordonia shuttle vectors, which was also suitable for several other Gordonia species and related genera. The metabolic region included the heavy-metal resistance gene cadA, encoding a P-type ATPase. Expression of cadA in E. coli mediated resistance to cadmium, but not to zinc, and decreased the cellular content of cadmium in this host. When G. westfalica strain Kb1 was cured of plasmid pKB1, the resulting derivative strains exhibited slightly decreased cadmium resistance. Furthermore, they had lost the ability to use isoprene rubber as a sole source of carbon and energy, suggesting that genes essential for rubber degradation are encoded by pKB1.
The genus Gordonia was proposed by Tsukamura for coryneform bacteria isolated from sputa of patients with pulmonary disease or from soil (65-67). Gordonia belongs to the so-called CMN group (Corynebacterium, Mycobacterium, and Nocardia) of actinomycetes, which synthesize mycolic acids (13, 59). Gordonia strains also play an important role in bioremediation and biodegradation of pollutants and have attracted much interest in recent years due to their unusual and diverse metabolic capabilities (23, 29, 30, 70). Three strains of Gordonia polyisoprenivorans (2, 37) and the novel species G. westfalica Kb1 (39) were described as bacteria able to degrade natural rubber and synthetic cis-1,4-polyisoprene, which allows species of this genus to serve as model organisms for the investigation of the hitherto unknown biochemical and molecular mechanisms of rubber biodegradation (36). So far, no native plasmids of the genus Gordonia have been detected. Since factors encoded by linear or circular plasmids are often involved in degradation of complex xenobiotics (62), rubber-degrading bacterial strains were screened for the occurrence of extrachromosomal DNA. This publication gives the first example and the complete DNA sequence of a native Gordonia plasmid, which also provides the basis for the development of shuttle vectors that may serve as a novel genetic tool for the study of Gordonia and related genera.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation conditions.
Bacteria and plasmids used in this study are listed in Table 1. All strains of the genera Gordonia and Rhodococcus were grown at 30°C in standard I complex nutrient broth (St-I; E. Merck AG, Darmstadt, Germany), mycobacterial strains were grown at 30°C in Luria-Bertani broth (LB) (52) containing Tween 80 (50 ml/liter of broth), and strains of Streptomyces were cultivated at 30°C in yeast extract-malt extract medium (24). For growth experiments with cis-1,4-polyisoprene as the sole carbon source, G. westfalica Kb1, G. westfalica Kb1-K38, and G. westfalica Kb1-K43 were cultivated at 30°C on mineral salts medium (54). Cells of Escherichia coli were cultivated at 37°C in LB broth. Antibiotics were applied according to the method of Sambrook et al. (52) and as indicated in the text. Carbon sources were added as indicated in the text. Liquid cultures were made in Erlenmeyer flasks and incubated on a horizontal rotary shaker. Solid media were prepared by the addition of agar (15 g/liter).
TABLE 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source (strain no.) |
|---|---|---|
| Strains | ||
| Gordonia species | ||
| G. alkanivorans HKI 0136 | Hexadekane-degrading wild type | 33 (DSM 44369) |
| G. amicalis IEGMT | Dibenzothiophene-desulfurizing strain | 30 (DSM 44461T) |
| G. bronchialis | (DSM 43247T) | |
| G. desulfuricans 213E | Benzothiophene-desulfurizing wild type | 29 (NCIMB 40816) |
| G. nitida LE31T | 3-Ethylpyridine- and 3-methylpyridine-degrading strain | 70 (DSM 44499) |
| G. polyisoprenivorans Kd2 | cis-1,4-Polyisoprene-degrading wild type | 37 (DSM 44302) |
| G. polyisoprenivorans VH2 | cis-1,4-Polyisoprene-degrading wild type | 2 (DSM 44266) |
| G. polyisoprenivorans Y2K | cis-1,4-Polyisoprene-degrading wild type | 2 |
| G. sputi | (DSM 43896T) | |
| G. terrae | Ethyl t-butyl ether-degrading strain | 23 (DSM 43249T) |
| G. westfalica Kb1/Kb2 | cis-1,4-Polyisoprene-degrading wild type | 38 (DSM 44215) |
| G. westfalica Kb1-K12 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K34 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K35 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K36 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K37 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K38 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K39 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K40 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K41 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K42 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K43 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K44 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K45 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| G. westfalica Kb1-K46 | pKB1-deficient mutant of G. westfalica Kb1 | This work |
| Mycobacterium species | ||
| M. smegmatis mc2155 | Transformation-efficient mutant | 58 |
| Rhodococcus species | ||
| R. opacus PD630 | Wild type; TAG+ | (DSMZ 44193) |
| R. rhodochrous RNMS1 | Wild type | 61 (ATCC 13808T) |
| R. ruber | Wild type; PHA+ TAG+ | 22 |
| Streptomyces species | ||
| S. coelicolor A3(2) | Wild type | 56 (DSMZ 40783) |
| S. lividans TK23 | Wild type | John Innes Institut, Norwick, England |
| E. coli strains | ||
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi1 hsdR17(rK− mK+) supE44 relA1 λ−lac [F′ proAB lacIqlacZΔM15 Tn10 (Tcr)] | 12 |
| E. coli S17-1 | thi1 proA hsdR17(rK− mK+) recA1 tra gene of plasmid RP4 integrated into the chromomsome | 57 |
| E. coli W3110 | Wild type | 20 |
| E. coli RW3110 | zntA | 50 |
| Plasmids | ||
| pASK3 | E. coli vector | IBA-GmbH, Göttingen, Germany |
| pBBR1MCS-1 | CmrlacPOZ′, Mob | 32 |
| pBBR1MCS-2 | KmrlacPOZ′, Mob | 31 |
| pBBR1MCS-3 | TcrlacPOZ′, Mob | 31 |
| pBBR1MCS-4 | Apr, lacPOZ′, Mob | 31 |
| pBBR1MCS-5 | Gmr, lacPOZ′, Mob | 31 |
| pBBRKB1:XhoI7 | Kmr, lacPOZ′, Mob, oriV pKB1 | This work |
| pDBMCS-2 | KmrlacPOZ′, Mob, oriV pKB1 | This work |
| pDBMCS-5 | GmrlacPOZ′, Mob, oriV pKB1 | This work |
Determination of metal tolerance.
For the gram-positive bacteria of the genera Gordonia, Rhodococcus, and Mycobacterium, St-I agar plates containing 100 to 800 μM CdCl2 were prepared in a 0.1 M stock solution of CdCl2 H2O (E. Merck AG) which was sterilized by autoclaving. The plates were inoculated with the bacterial strains and tolerance for cadmium was evaluated after incubation for 3 days at 30°C. Resistance of E. coli strains was determined by use of dose-response curves generated by growth in Lennox medium (Becton-Dickinson, Sparks, Md.). The medium contained 50 μg of anhydrotetracycline per liter to induce expression of the plasmid-encoded genes in these strains. Overnight cultures of E. coli strains were used to inoculate parallel cultures with increasing metal concentrations. Cells were cultivated for 16 h with shaking at 37°C, and the optical density was determined at 600 nm.
Cadmium uptake experiments.
Cadmium uptake experiments were performed with E. coli cells in Tris buffer (10 mM; pH 7.0) by filtration, as published previously (44). The cells were cultivated in Tris-buffered mineral salts medium in the presence of 2 g of glucose per liter and 1 g of yeast extract per liter, up to 100 Klett units, when 200 μg of anhydrotetracycline per liter was added, and incubation was continued with shaking for 3 h at 30°C. Cells were harvested by centrifugation, washed, and suspended in 10 mM Tris-HCl buffer (pH 7.0). Radioactive 109Cd2+ (87.4 GBq/g) was added at a concentration of 10 μM, incubation was continued with shaking at 30°C, and the metal content in washed cells (dry weight) was determined at various time points by use of an equilibration curve. Background binding was not subtracted.
Isolation, analysis, and manipulation of DNA.
Plasmid DNA was prepared from crude lysates by the alkaline extraction method (8). Before lysis, cells of Gordonia, Rhodococcus, and Mycobacterium were incubated in the presence of lysozyme (2 g/liter) for 2 h at 37°C. Total DNA of Gordonia was prepared as described by Ausubel et al. (4), with modifications as follows: cells of 50-ml cultures were harvested by centrifugation and suspended in 8.5 ml of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and 1 ml of lysozyme solution (10 g of TE per liter). After incubation at 37°C for 2 h, 500 μl of a sodium dodecyl sulfate solution (100 g/liter) and 50 μl of a proteinase K solution (20 g of TE per liter) were added and mixed gently. After additional incubation at 37°C for 1 h, 5 ml of 5 M NaCl and 1.5 ml of a CTAB solution (100 g of cetyltrimethylammonium bromide per liter of 0.7 M NaCl) were added and the solution was incubated at 65°C for 20 min. DNA was digested with various restriction endonucleases (Gibco/BRL, Gaithersburg, Md.) under the conditions recommended by the manufacturer. All other genetic procedures and manipulations were conducted as described by Sambrook et al. (52).
Extrachromosomal DNA was detected by pulsed-field gel electrophoresis (PFGE). Preparation of total DNA embedded in low-melting-temperature agarose and linearization of circular plasmid DNA were done according to the methods of Barton et al. (5). Electrophoresis was performed in the CHEF-DR III system (Bio-Rad GmbH, Munich, Germany).
Plasmid curing.
For generation of plasmid-free mutants of G. westfalica Kb1, heat curing was performed (43). Fifty milliliters of St-I medium in 300-ml Erlenmeyer flasks was inoculated with cells of G. westfalica Kb1 and incubated at 42°C. Every 2 days, Erlenmeyer flasks with fresh St-I medium were inoculated with 1 ml of the grown culture and subsequently cultivated at 42°C. After 20 passages, the cells were diluted and spread on St-I agar plates. The colonies obtained were screened for the absence of plasmid pKB1 as described previously (27).
Cloning procedures.
The cadA gene from G. westfalica Kb1 was amplified by PCR from plasmid pKB1 with the primers P1 and P2 (listed in Table 2) and was cloned downstream of the tet promoter in plasmid pASK3 (IBA GmbH, Göttingen, Germany), leading to plasmid pECA34. The two insertion sequence (IS) elements, IS1 and IS3 (comprising ORF1/ORF2 and ORF53), were amplified from total DNA of wild-type G. westfalica Kb1 and the pKB1-free mutants with the primer sets P3 plus P4 and P5 plus P6 (listed in Table 2). The two IS elements, IS1 and IS3, with their contiguous regions, were then amplified with the two primer sets P3 plus P7 and P8 plus P6 (Table 2).
TABLE 2.
PCR and sequencing primers used in this study
| Oligonucleotide | Sequence (5′ → 3′) | Position in pKB1a | Function |
|---|---|---|---|
| P1 | AAA GAA TTC GCT GAC GCA TGC TGC GGA | 29829-29846 | 2,003-bp PCR product from cadA gene from G. westfalica (EcoRI and BamHI restriction sites used for cloning are underlined) |
| P2 | AAA GGA TCC GTG GCG TTC GCG ATG GGG | 31998-32015c | |
| P3 | TCG GGC AGC GTA CTC GGC CGG | 801-821 | 720-bp PCR product from IS1 (comprising ORF18) |
| P4 | TCG TCA ACT GCC GCA AGC GCA | 1501-1521c | |
| P5 | CGC TCA AGC GCG GAC GAG CAG | 50301-50321 | 720-bp PCR product from IS3 (comprising ORF50) |
| P6 | CGG CAA GCC GCT GTG CGC GGC | 51001-51021c | |
| P7 | TCG TGA TGG GAG CAG GCT GGC | 2801-2821c | With P3, 2,020-bp PCR product from IS1, with contiguous region (comprising ORF18) |
| P8 | GTC GAT GCA ATA CGA CCG CTC | 49001-49021 | With P6, 2,020-bp PCR product from IS3, with contiguous region (comprising ORF50) |
| P9 | GCC CTA TAC CTT GTC TGC CTC CCC G | 2520-2544b | 5,134-bp PCR product of the vector pBBR1MCS-2 and 4,758-bp PCR product of the vector pBBR1MCS-5 |
| P10 | GCT ACA GCC GAT AGT CTG GAA CAG C | 2510-2484cb | |
| P11 | AAG ACC ACG ATC CAG TCG GC | 5101-5120 | 2,332-bp PCR product of pKB1 comprising oriV of pKB1 |
| P12 | TTA ACT ATC GGG CGG AGT CG | 7414-7433c |
c, complementary strand.
Numbering is based on the vectors pBBR1MCS-2 and pBBR1MCS-5 (31).
Gene transfer.
Hybrid plasmids containing oriV of pKB1 were transferred to species of the genera Gordonia, Mycobacterium, and Rhodococcus by electroporation in a Model 2510 electroporator (Eppendorf-Netheler-Hinz, Hamburg, Germany). Preparation of the electrocompetent cells and the execution of electroporation were done as described recently (3, 28). For transformation of Streptomyces coelicolor strain A3(2) and Streptomyces lividans strain TK23, protoplasts of these strains were prepared as described by Okanishi et al. (47), Bibb et al. (7), and Thompson et al. (63). Transformations were done according to the rapid small procedure, as described by Bibb et al. (6), Thompson et al. (63), and Okanishi et al. (46). Conjugational plasmid transfer was carried out, applying a previously described protocol (18) employing E. coli S17-1 as donor and G. polyisoprenivorans as recipient.
DNA sequence analysis.
To obtain the complete sequence of megaplasmid pKB1, we constructed a shotgun library. Plasmid DNA was fragmented by hydro-shearing, cloned into pGEM-T vector DNA (Promega, Madison, Wis.), and sequenced by MWG Biotech (Ebersberg, Germany), resulting in fivefold sequence coverage. A few regions for which only uncertain sequences were obtained were sequenced by employing individual primers. Hybrid plasmids containing oriV-comprising DNA fragments were sequenced with IRD800-labeled universal and reverse primers, using the SequiTherm EXCEL II Long-Read L-C kit and a LI-COR 4200 sequencer (LI-COR Biosciences, Lincoln, Nebr.).
Open reading frames (ORFs) were identified by use of the program GeneMark (http://opal.biology.gatech.edu/GeneMark/) to indicate start codons, stop codons, and codon usage statistics for each reading frame (41). Database searches of the predicted protein sequences were performed with the BLAST program provided by EMBL/Heidelberg (1). Multiple sequence alignments were carried out with the program BioEdit (21). Protein sequences were also analyzed for functionally important motifs by use of SMART software (http://smart.embl-heidelberg.de/) (35, 55).
Nucleotide sequence accession number.
The DNA sequence of pKB1 has been deposited in the EMBL database under accession number AJ576039.
RESULTS AND DISCUSSION
Detection and characterization of the megaplasmid pKB1.
Since metabolic pathways involved in degradation of unusual compounds may be encoded by bacterial plasmids, the rubber-degrading bacteria G. polyisoprenivorans strains Kd2T, VH2, and Y2K and G. westfalica strain Kb1 were screened for the occurrence of plasmids. Cells were embedded in low-melting-temperature agarose and lysed, and the immobilized total DNA was treated with Aspergillus oryzae S1 nuclease to linearize possible circular plasmids. When the DNA samples were separated by PFGE, only those from G. westfalica Kb1 displayed a distinct band, which was visible only after S1 treatment and corresponded to a size of about 100 kbp (data not shown). Consequently, this strain contained a circular megaplasmid that we named pKB1. Plasmid pKB1 represents the first extrachromosomal DNA identified for the genus Gordonia.
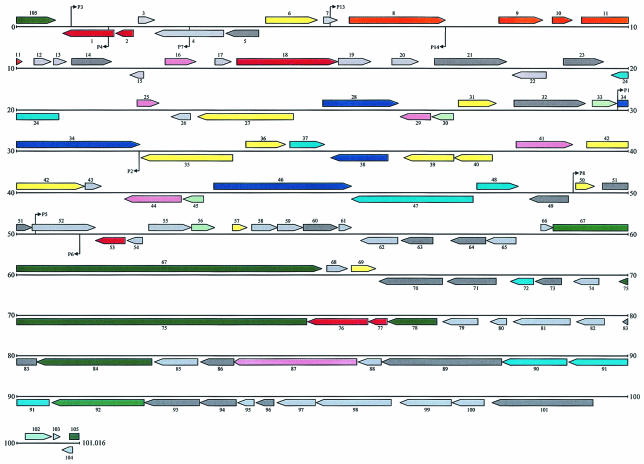
To investigate the possible metabolic function of pKB1, we completely sequenced it (Fig. 1). Plasmid pKB1 is a circular DNA molecule with a size of 101,016 bp, which confirmed the PFGE results. The G+C content was 66 mol%, as is expected for DNA from a bacterium of the CMN group. The plasmid carried 105 ORFs, 47 on one DNA strand and 58 on the other. The predicted products of 67 ORFs were related to proteins in the current databases, as indicated by a BLAST analysis, of which 46 were homologous to proteins with putative functions (Table 3). According to the predicted functions of their products, the genes located on plasmid pKB1 were clustered into three major regions that were all flanked by putative IS elements: a replication and partitioning region, a metabolic region, and a conjugational transfer region (Fig. 1). Investigations were carried out with ORF8 within the replication and partitioning region, which encodes a replication gene and mediates autonomous replication, and with ORF34, which encodes cadA, a cadmium P-type ATPase.
FIG. 1.
Schematic representation of the ORFs of the 101,016-bp plasmid pKB1. The predicted ORFs located on plasmid pKB1 are shown as arrows. The direction of the arrow indicates the DNA strand on which the ORF is located. The colors of the arrows group the assigned functions of the putative ORF products as follows: orange, ORFs related to replication and partitioning; yellow, ORFs with putative metabolic functions; blue, ORFs related to heavy-metal resistance; dark green, ORFs related to conjugation; red, ORFs related to IS elements and transposons; green, putative regulator proteins; pink, putative peptidases; light blue, putative membrane proteins; dark gray, conserved hypothetical ORFs; light gray, ORFs with no known function. The small black arrows indicate binding sites and orientations of pKB1-specific primers (Table 2).
TABLE 3.
Summary of ORFs identified by significant homology (BLAST search) or GENEMARK prediction
| ORF | No. of amino acids | Coding sequence position (start codon-stop codon)a | Gene or function of closest relative (source), identified protein domains | Data bank reference no. | No. of amino acids with identity/total (%) | E valueb |
|---|---|---|---|---|---|---|
| 1 | 278 | 759-1592c | Hypothetical protein (Rhodopseudomonas palustris), related to IS511, transposase OrfB (Caulobacter crescentus CB15), pfam00665 | ZP_00008656 | 111/265 (41%) | 7e−45 |
| AAK24707 | 103/271 (38%) | 1e−37 | ||||
| 2 | 97 | 1622-1912c | Hypothetical protein (Magnetospirillum magnetotacticum), related to IS1477 transposase (Xanthomonas campestris pv. campestris), pfam01527 | ZP_00053525 | 38/90 (42%) | 3e−09 |
| NP_637161 | 29/69 (42%) | 4e−04 | ||||
| 3 | 89 | 1992-2258 | GENEMARK prediction; no homology | |||
| 4 | 398 | 2241-3434c | GENEMARK prediction; no homology | |||
| 5 | 177 | 3431-3961c | Cinorf13 protein (Streptomyces cinnamoneus) | CAD60535 | 45/161 (27%) | 5e−06 |
| 6 | 282 | 4068-4913 | Epoxide hydrolase homolog YfhM (Bacillus subtilis), pfam00561 | BAA24479 | 91/277 (32%) | 1e−32 |
| 7 | 76 | 5017-5244 | GENEMARK prediction; no homology | |||
| 8 | 680 | 5404-7443 | GENEMARK prediction; no homology | |||
| 9 | 238 | 7882-8595 | Putative chromosome-partitioning ATPase-like protein (R. equi), COG1192 | BAB16660 | 144/232 (62%) | 2e−70 |
| 10 | 109 | 8754-9080 | Hypothetical protein (R. equi) | NP_858507 | 25/59 (42%) | 5e−04 |
| 11 | 293 | 9214-10092 | Putative mycobacteriophage excisionase (R. equi) | NP_066806 | 51/121 (42%) | 1e−10 |
| 12 | 109 | 10285-10611 | GENEMARK prediction; no homology | |||
| 13 | 69 | 10608-10814 | GENEMARK prediction; no homology | |||
| 14 | 217 | 10901-11551 | p24 (human immunodeficiency virus 1) | CAB87182 | 25/62 (40%) | 0.037 |
| 15 | 78 | 11867-12100c | GENEMARK prediction; no homology | |||
| 16 | 169 | 12431-12937 | gp8 (mycobacteriophage Bxb1), related to l-alanoyl-d-glutamate peptidase (bacteriophage A500) | NP_075275 | 44/132 (33%) | 2e−10 |
| CAA59365 | 25/71 (35%) | 4e−04 | ||||
| 17 | 90 | 13247-13516 | GENEMARK prediction; no homology | |||
| 18 | 547 | 13601-15241 | IS1554, transposase (M. tuberculosis CDC1551) | AAK45194 | 358/415 (86%) | 0.0 |
| 19 | 176 | 15242-15769 | GENEMARK prediction; no homology | |||
| 20 | 145 | 16137-16571 | GENEMARK prediction; no homology | |||
| 21 | 392 | 16831-18006 | Hypothetical protein (Nitrosomonas europaea ATCC 19718) | CAD84656 | 100/340 (29%) | 1e−27 |
| 22 | 186 | 18097-18654c | GENEMARK prediction; no homology | |||
| 23 | 220 | 18932-19591 | Predicted protein (Methanosarcina acetivorans C2A) | AAM07556 | 36/100 (36%) | 1e−05 |
| 24 | 323 | 19721-20689c | Hypothetical transmembrane protein (Bifidobacterium longum NCC2705), COG3021, related to iron deficiency-induced protein A (Synechococcus sp. PCC7942), pfam03372 | AAN25527 | 74/244 (30%) | 2e−18 |
| CAC40996 | 57/204 (27%) | 0.13 | ||||
| 25 | 121 | 21894-22256 | Conserved hypothetical protein (M. bovis subsp. bovis AF2122/97), related to membrane proteins related to metalloendopeptidases (Corynebacterium glutamicum ATCC 13032), pfam01551 | CAD93836 | 66/117 (56%) | 5e−35 |
| BAB98251 | 66/115 (57%) | 5e−32 | ||||
| 26 | 105 | 22532-22846c | GENEMARK prediction; no homology | |||
| 27 | 520 | 22970-24529c | Conserved hypothetical protein (M. tuberculosis CDC1551), related to ResB protein required for cytochrome c biosynthesis (C. glutamicum ATCC 13032), COG1333, pfam05140 | AAK44773 | 254/488 (52%) | e−134 |
| NP_599688 | 237/491 (48%) | e−117 | ||||
| 28 | 415 | 25001-26245 | Divalent cation-transport integral membrane protein MNTH (M. bovis subsp. bovis AF2122/97), pfam01566, COG1914 | CAD93809 | 233/391 (59%) | e−111 |
| 29 | 166 | 26272-26769c | Lipoprotein signal peptidase (Brucella melitensis 16M), pfam01252 | AAL52980 | 56/127 (44%) | 1e−10 |
| 30 | 118 | 26766-27119c | Hypothetical protein (Thermobifida fusca), related to transcriptional regulator (Nostoc sp. PCC 7120), smart00418, pfam01022 | ZP_00056748 | 67/97 (69%) | 3e−27 |
| BAB74465 | 27/67 (40%) | 1e−07 | ||||
| 31 | 207 | 27221-27841 | Hypothetical methyltransferase (Shewanella oneidensis MR-1), COG2226, pfam01209, related to menaquinone biosynthesis methyltransferase (2-heptaprenyl-1,4-naphthoquinone methyltransferase) (Methanosarcina acetivorans C2A) | NP_717595 | 70/189 (37%) | 1e−27 |
| NP_619210 | 35/116 (30%) | 3e−05 | ||||
| 32 | 391 | 28126-29298 | Probable conserved lipoprotein LppS (M. bovis subsp. bovis AF2122/97), pfam03734, COG1376 | CAD97408 | 174/386 (45%) | 6e−88 |
| 33 | 140 | 29414-29833 | cadC (Listeria monocytogenes), pfam01022 | AAA25276 | 36/98 (36%) | 4e−11 |
| 34 | 731 | 29826-32018 | Probable cation transport ATPase (M. tuberculosis), COG2217, pfam00122 | F70757 | 378/647 (58%) | e−151 |
| 35 | 501 | 32034-33536c | Putative polyprenol-phosphate-mannose synthase 2 (Ppm2) (M. smegmatis), COG0815, pfam00745 | CAC15462 | 205/483 (42%) | 5e−86 |
| 36 | 215 | 33754-34398 | Putative membrane protein (Corynebacterium efficiens), COG1651, related to thiol-disulfide oxidoreductase BdbD (B. cereus ATCC 14579), pfam01323 | BAC19796 | 95/209 (45%) | 8e−40 |
| NP_830362 | 49/217 (22%) | 6e−06 | ||||
| 37 | 191 | 34464-35036 | Hypothetical integral membrane protein (M. smegmatis), COG4243 | AAG30410 | 93/190 (48%) | 1e−41 |
| 38 | 314 | 35135-36076c | Hypothetical protein (Bifidobacterium longum DJO10A), related to cobalt-zinc-cadmium resistance protein (Xanthomonas axonopodis pv. citri 306), COG1230, pfam01545 | ZP_00120702 | 125/297 (42%) | 1e−49 |
| NP_641652 | 106/298 (35%) | 5e−38/PICK> | ||||
| 39 | 271 | 36335-37147c | Possible cytochrome c-type biogenesis protein CCDA (M. bovis subsp. bovis), related to a cytochrome c biogenesis protein (C. glutamicum ATCC 13032), pfam02683, COG0785 | CAD93402 | 120/233 (51%) | 4e−49 |
| NP_599687 | 116/254 (45%) | 1e−45 | ||||
| 40 | 209 | 37144-37770c | Hypothetical protein (M. leprae), COG0526, COG1225, related to thiol-disulfide isomerase and thioredoxins (C. glutamicum ATCC 13032) | S72901 | 94/179 (52%) | 4e−41 |
| BAB97832 | 73/174 (41%) | 1e−27 | ||||
| 41 | 309 | 38162-39088 | Hypothetical protein (M. leprae), COG0739, related to membrane proteins related to metalloendopeptidases (C. glutamicum ATCC 13032), pfam01551 | CAB36664 | 64/120 (53%) | 1e−29 |
| BAB98251 | 65/120 (54%) | 5e−27 | ||||
| 42 | 597 | 39321-41111 | Cytochrome c oxidase, subunit 1 (M. tuberculosis CDC1551), COG0843, pfam00115 | NP_337644 | 442/568 (77%) | 0.0 |
| 43 | 91 | 41108-41380 | GENEMARK prediction; no homology | |||
| 44 | 311 | 41762-42694c | Hypothetical protein (T. fusca), COG0501, related to peptidase M48 (C. glutamicum) | ZP_00058818 | 47/117 (40%) | 3e−08 |
| AAL31539 | 37/111 (33%) | 3e−04 | ||||
| 45 | 113 | 42699-43037c | Hypothetical protein (T. fusca), COG3680, pfam03965, related to methicillin resistance regulatory protein (S. aureus subsp. aureus N315) | ZP_00056896 | 45/109 (41%) | 8e−13 |
| NP_373280 | 20/87 (22%) | 0.007 | ||||
| 46 | 730 | 43290-45479 | Cation-transporting ATPase (M. leprae), COG2216, COG2217, pfam00122 | NP_302350 | 337/724 (46%) | 3−148 |
| 47 | 663 | 45417-47405c | Possible membrane protein (M. leprae), related to CtaG (B. subtilis), COG3336 | NP_302349 | 212/598 (35%) | 8e−86 |
| NP_389376 | 50/234 (21%) | 0.004 | ||||
| 48 | 226 | 47520-48197 | Probable conserved integral membrane protein (M. bovis subsp. bovis AF2122/97) | CAD93856 | 50/183 (27%) | 3e−04 |
| 49 | 155 | 48266-48730c | Hypothetical protein (Cytophaga hutchinsonii) | ZP_00117563 | 29/79 (36%) | 2e−08 |
| 50 | 104 | 49143-49454 | Putative modification methylase (Streptomyces lividans) | AAO61179 | 22/64 (34%) | 0.19 |
| 51 | 223 | 49580-50248 | Hypothetical protein Rv1044 (M. tuberculosis H37Rv) | NP_215560 | 62/201 (30%) | 3e−05 |
| 52 | 344 | 50248-51279 | GENEMARK prediction; no homology | |||
| 53 | 164 | 51284-51775c | GENEMARK prediction; no homology | |||
| 54 | 85 | 51807-52061c | GENEMARK prediction; no homology | |||
| 55 | 231 | 52162-52854 | GENEMARK prediction; no homology | |||
| 56 | 125 | 52851-53225 | Helix-turn-helix protein (Pyrobaculum aerophilum IM2), related to SgraIC control protein (Streptomyces griseus), pfam01381 | AAL64755 | 29/61 (47%) | 1e−04 |
| AAG31560 | 24/60 (40%) | 0.002 | ||||
| 57 | 79 | 53531-53767 | Glutaredoxin electron transport component of NrdEF (M. leprae), COG0695, pfam00462 | NP_302197 | 60/77 (77%) | 4e−25 |
| 58 | 138 | 53846-54259 | GENEMARK prediction; no homology | |||
| 59 | 141 | 54256-54678 | GENEMARK prediction; no homology | |||
| 60 | 189 | 54682-55248 | gp82 (mycobacteriophage CJW1), GerE (Corynebacterium striatum, plasmid pTP10), COG0305, pfam00772 | AAN01696 | 41/147 (27%) | 0.008 |
| AAG03386 | 5/73 (34%) | 0.014 | ||||
| 61 | 69 | 55270-55476 | GENEMARK prediction; no homology | |||
| 62 | 180 | 55631-56170c | GENEMARK prediction; no homology | |||
| 63 | 171 | 56299-56811c | Hypothetical protein (Nostoc punctiforme) | ZP_00106356 | 34/95 (35%) | 5e−05 |
| 64 | 192 | 57097-57672c | Putative bacteriophage-related protein (Ralstonia solanacearum), related to gene 2.8 (enterobacteria phage T7) | NP_521357 | 33/95 (34%) | 5e−04 |
| NP_041971 | 30/97 (30%) | 0.010 | ||||
| 65 | 113 | 57690-58028c | GENEMARK prediction; no homology | |||
| 66 | 65 | 58573-58767 | GENEMARK prediction; no homology | |||
| 67 | 2,073 | 58773-64991 | TraA-like protein (R. equi), related to TraA (C. glutamicum), COG0507 | NP_066783 | 329/987 (33%) | e−105 |
| NP_776232 | 253/878 (28%) | 3e−56 | ||||
| 68 | 113 | 65072-65410 | GENEMARK prediction; no homology | |||
| 69 | 133 | 65473-65871 | Putative glycosyl transferase (Streptomyces nogalater) | AAF01809 | 28/82 (34%) | 0.032 |
| 70 | 345 | 65928-66962c | Hypothetical protein (Microbulbifer degradans 2-40) | ZP_00068299 | 71/150 (47%) | 2e−27 |
| 71 | 275 | 67047-67871c | Conserved hypothetical protein (C. efficiens YS-314) | NP_736653 | 48/181 (26%) | 1e−09 |
| 72 | 128 | 68071-68454c | Putative membrane protein (Streptomyces avermitilis MA-4680) | NP_825985 | 29/82 (35%) | 0.002 |
| 73 | 144 | 68480-68911c | WD40-repeat-containing protein (Methanosarcina acetivorans C2A) | NP_617428 | 22/70 (31%) | 0.30 |
| 74 | 139 | 69109-69525c | GENEMARK prediction; no homology | |||
| 75 | 1,631 | 69852-74744c | Putative methylase (or helicase) (R. equi), related to helicase SNF2 family (Agrobacterium tumefaciens C58), COG4646 | BAB16635 | 489/1302 (37%) | 0.0 |
| AAL46337 | 399/1325 (30%) | e−125 | ||||
| 76 | 331 | 74754-75746c | Putative transposase (C. efficiens YS-314), related to IS1601-D (Mycobacterium avium), pfam00665, COG2801 | NP_739229 | 186/284 (65%) | 7e−96 |
| AAD44200 | 7e−55 | |||||
| 77 | 104 | 75743-76054c | Putative transposase (C. efficiens YS-314), related to transposase IS911 helix-turn-helix and LZ region (N. europaea ATCC 19718), pfam01527 | NP_739228 | 64/103 (62%) | 1e−23 |
| 78 | 266 | 76073-76870c | Putative methylase (or helicase) (R. equi), related to DNA meth- ylase (Listeria innocua), COG2263 | BAB16635 | 110/264 (41%) | 6e−41 |
| NP_569161 | 65/182 (35%) | 5e−27 | ||||
| 79 | 193 | 76999-77577c | GENEMARK prediction; no homology | |||
| 80 | 90 | 77862-78131c | GENEMARK prediction; no homology | |||
| 81 | 312 | 78251-79186c | GENEMARK prediction; no homology | |||
| 82 | 154 | 79275-79736c | GENEMARK prediction; no homology | |||
| 83 | 136 | 79916-80323c | Hypothetical protein (Arthrobacter nicotinovorans) | CAD47981 | 30/68 (44%) | 2e−08 |
| 84 | 630 | 80323-82212c | Conjugative transfer gene complex protein-like protein (R. equi), related to transfer complex protein TrsK (S. aureus), pfam02534, COG3505 | NP_066787 | 187/531 (35%) | 9e−77 |
| C56976 | 28/78 (35%) | 8e−07 | ||||
| 85 | 238 | 82251-82964c | GENEMARK prediction; no homology | |||
| 86 | 183 | 83008-83556c | Hypothetical protein (R. equi) | NP_066788 | 40/161 (24%) | 0.037 |
| 87 | 675 | 83553-85577c | Putative peptidase (R. equi), related to peptidase M23/M37 family (Bacillus anthracis Ames), pfam01551, COG0739 | NP_066789 | 150/318 (47%) | 4e−59 |
| NP_844314 | 38/104 (36%) | 1e−11 | ||||
| 88 | 133 | 85570-85968c | GENEMARK prediction; no homology | |||
| 89 | 653 | 85965-87923c | Hypothetical protein (R. equi), related to ATP binding protein-like protein (R. equi) | NP_858493 | 192/560 (34%) | 1e−70 |
| NP_066791 | 192/560 (34%) | 1e−70 | ||||
| 90 | 353 | 87943-89001c | Integral membrane protein-like protein (R. equi) | NP_066792 | 71/266 (26%) | 3e−06 |
| 91 | 499 | 89029-90525c | Integral membrane protein-like protein (R. equi) | NP_066792 | 174/473 (36%) | 2e−68 |
| 92 | 505 | 90570-92084c | Putative transfer gene complex protein-like protein (A. nicotinovorans) | CAD47985 | 27/72 (37%) | 2e−05 |
| 93 | 301 | 92078-92980c | Hypothetical protein (R. equi) | NP_066795 | 76/258 (29%) | 2e−10 |
| 94 | 198 | 92985-93578c | Hypothetical protein (R. equi) | NP_066796 | 65/191 (34%) | 3e−18 |
| 95 | 90 | 93617-93886c | GENEMARK prediction; no homology | |||
| 96 | 97 | 93912-94202c | Hypothetical protein (Haemophilus somnus 129PT), related to preprotein translocase SecY subunit (Haemophilus influenzae Rd) | ZP_00123677 | 29/80 (36%) | 0.16 |
| NP_438957 | 27/75 (36%) | 0.31 | ||||
| 97 | 211 | 94249-94881c | GENEMARK prediction; no homology | |||
| 98 | 413 | 94887-96125c | GENEMARK prediction; no homology | |||
| 99 | 279 | 96272-97108c | GENEMARK prediction; no homology | |||
| 100 | 109 | 97105-97431c | GENEMARK prediction; no homology | |||
| 101 | 551 | 97778-99430c | Hypothetical protein (R. equi) | NP_066802 | 151/332 (45%) | 3e−58 |
| 102 | 144 | 100135-100566 | Putative repressor protein (R. equi), related to transcriptional repressor (B. cereus ATCC 14579), pfam1381, COG1476 | NP_066803 | 49/141 (34%) | 6e−16 |
| NP_830818 | 20/61 (32%) | 5e−05 | ||||
| 103 | 37 | 100591-100701 | GENEMARK prediction; no homology | |||
| 104 | 59 | 100804-100980c | GENEMARK prediction; no homology | |||
| 105 | 227 | 100858-522 | gp48 (mycobacteriophage Che8), related to methyltransferase-helicase polyprotein (grapevine rootstock stem lesion-associated virus) | NP_817386 | 36/91 (39%) | 4e−08 |
| AAN63466 | 27/92 (29%) | 0.052 |
c, complementary strand.
An E value of >0.4 indicates no homology.
Identification of oriV of plasmid pKB1.
The replication and partitioning region of plasmid pKB1 was further characterized, with the intention of constructing E. coli-Gordonia shuttle vectors. For cloning and identification of the origin of replication, oriV, of plasmid pKB1, a plasmid library of pKB1 was generated by use of the mobilizable vector pBBR1MCS-2, which is not able to replicate in Gordonia strains (unpublished data). The DNA of pKB1 was partially digested with XhoI and ligated to XhoI-linearized pBBR1MCS-2 plasmid DNA. Since plasmid transfer into G. westfalica yielded no transformants, the DNA mixture was electroporated into G. polyisoprenivorans strain VH2. The cells were screened for 4 days at 30°C for kanamycin resistance, which is encoded by plasmid pBBR1MCS-2.
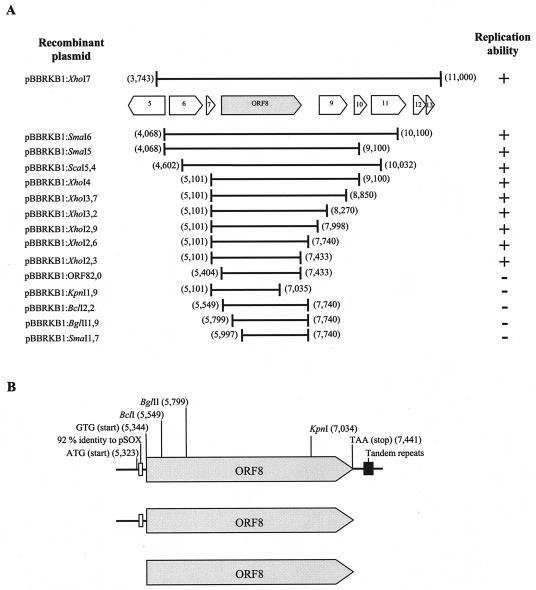
The resulting kanamycin-resistant colonies of G. polyisoprenivorans strain VH2 contained hybrid plasmids that were composed of at least three XhoI fragments: plasmid pBBR1MCS-2 DNA plus two additional fragments, of 2,331 and 4,927 bp (data not shown). Neither fragment alone was able to mediate replication in G. polyisoprenivorans VH2 (data not shown), indicating that essential elements were located on both fragments, which together correspond to the 7,258-bp region from nucleotides 3,743 to 11,000 on the sequence map of plasmid pKB1. This region contains nine ORFs (ORF 6 to ORF 14) (Fig. 2A) and five tandem repeats, with a period size of 16 bp, downstream of ORF8 (nucleotides 7,558 to 7,637) (Fig. 2B).
FIG. 2.
Mapping of the oriV region of plasmid pKB1. (A) The ability of DNA fragments of plasmid pKB1 to confer autonomous replication in Gordonia to a suicide plasmid was tested in G. polyisoprenivorans. Successfully replicating hybrid plasmids conferred kanamycin resistance to the host, as indicated on the right. Initially, a 7-kbp dual XhoI fragment of plasmid pKB1 was identified in a gene bank of this plasmid. A hybrid plasmid containing this 7-kbp XhoI region (pBBRKB1:XhoI7) and the ORFs carried by it is diagrammed at the top. Below, the sizes and locations of derivatives of the 7-kbp DNA fragment are given. The numbers at the right and left margins of the fragments indicate the exact positions of these fragments in the map of plasmid pKB1. (B) To identify the smallest DNA region of plasmid pKB1 that is essential to confer stable replication, three fragments were amplified by PCR: pBBRKB1:XhoI2,6 (top), pBBRKB1:XhoI2,3 (middle), and pBBRKB1:ORF82,0 (bottom). The gray arrows indicate the size and position of ORF8, which encodes a protein with unknown function. The ORF8 product may be translated by two possible start codons, ATG (at position 5,323 on the pKB1 map) and GTG (at position 5,344). The stop codon, TAA, of ORF8 is at position 7,441. Moreover, the positions of two possible cis-acting sites are given, one a 39-bp sequence upstream of ORF8 with 92% identity to a region of plasmid pSOX from Rhodococcus sp. strain X309 (nucleotides 5,327 to 5,365) and the other downstream tandem repeats (nucleotides 7,558 to 7,637). Single restriction sites for BclI (5,549), BglII (5,799), and KpnI (7,034) are also shown.
To identify the genes and cis-acting elements essential for replication and partitioning, we deleted the 7,258-bp gene region from both ends by exonuclease III and endonuclease treatment. The autonomous replication ability of the resulting fragments was tested (Fig. 2A). The smallest of the fragments able to mediate stable plasmid replication in G. polyisoprenivorans strain VH2 had a size of 2,332 bp and harbored only the predicted ORF8 plus a sequence of 39 bp (nucleotides 5,327 to 5,365) (Fig. 2B). Further reduction of the 2,332-bp fragment by use of restriction enzymes (BclI, BglII, and KpnI) (Fig. 2B) or by amplification of ORF8 by PCR did not generate fragments that were able to maintain stable plasmid replication. The ORF8 product had a predicted size of 680 amino acid (aa) residues. Neither the sequence of this fragment nor the predicted amino acid sequence of ORF8 showed similarities to DNA elements or proteins known to be involved in plasmid replication. The only element associated with replication function was the 39-bp sequence. Thus, the 39-bp sequence was also required for replication. This sequence was 92% identical at the nucleotide level to a region of plasmid pSOX from Rhodococcus sp. which is located between the replication genes of that plasmid (14). The tandem repeats downstream of ORF8 were not essential for autonomous replication.
ORF9 may encode an ATPase (238 aa) involved in chromosome partitioning. The predicted protein, containing a parA domain, was 62% identical to a hypothetical protein of a virulence plasmid from Rhodococcus equi (60). ORF10 encodes a predicted protein (109 aa) comprising a helix-turn-helix-like motif of the CopG family, which is involved in dimerization of RepA proteins but not in DNA binding. Homologies were obtained to hypothetical proteins of the virulence plasmids p33701 and p103 of R. equi strains ATCC 33701 and 103, respectively. These are plasmids with an origin of replication (oriV) that may belong to a novel type (60). The predicted ORF11 product (293 aa) was 42% identical to a putative prophage excisionase, which again is harbored by the virulence plasmids p33701 and p103 as part of a cluster of replication genes (60). The functions of the other ORFs in this region remained unknown or could be not related to replication.
Host range of pKB1 replicon.
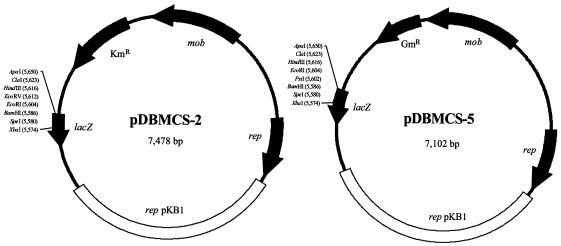
The broad-host-range cloning vectors pBBR1MCS-2 (Kmr) and pBBR1MCS-5 (Gmr), mediating resistance to kanamycin and gentamicin, respectively, were chosen for construction of E. coli-Gordonia shuttle vectors. Both plasmids and the 2,332-bp oriV-containing fragment of plasmid pKB1 were amplified by PCR (primer pairs P9-P10 and P11-P12, respectively) (Table 2), ligated, and tested for replication in E. coli and Gordonia, leading to the shuttle vectors pDBMCS-2 and pDBMCS-5 (Fig. 3). Both new vector plasmids possess an extended multiple cloning site (MCS), allow for blue-white selection in E. coli via alpha complementation, are compatible with IncP, IncQ, IncW, ColE1, and P15a group plasmids, and can be mobilized if the RK2 transfer functions are provided in trans. We also constructed derivatives of plasmids pBBR1MCS (Cmr), pBBR1MCS-3 (Tcr), and pBBR1MCS-4 (Apr), containing the 2,332-bp fragment comprising oriV cloned into the MCS, which did not mediate resistance to the respective antibiotics in G. polyisoprenivorans.
FIG. 3.
Physical maps of two E. coli-Gordonia shuttle vectors. The 2,332-bp fragment of plasmid pKB1 that is the smallest essential part necessary to confer stable replication in Gordonia was amplified by PCR (open white bar) and cloned into suicide plasmids pBBR1MCS-2 and pBBR1MCS-5 (31), leading to the new vector plasmids pDBMCS-2 and pDBMCS-5, respectively. The genes located on the suicide plasmids are indicated by black arrows. Unique restriction sites present in the MCS of each plasmid are shown.
To test the host range of oriV from plasmid pKB1, the oriV-carrying hybrid plasmid pBBRKB1:XhoI7 was transferred to different species of the genera Gordonia, Rhodococcus, and Mycobacterium by electroporation as well as to two species of the genus Streptomyces by transformation of protoplasts (Table 4). Kanamycin-resistant derivatives of Mycobacterium smegmatis mc2155, Rhodococcus opacus PD630, G. polyisoprenivorans VH2, and G. polyisoprenivorans Y2K were obtained, indicating functional expression of the pDBMCS-2-encoded kanamycin resistance gene and stable propagation of the vector plasmid. For all other strains listed in Table 4, no kanamycin-resistant colonies could be obtained. This indicates a rather narrow host range of the oriV from plasmid pKB1 that is limited to Gordonia strains and closely related bacteria. In addition to the E. coli-Rhodococcus shuttle vector systems based on pNC903, which were previously described as functional replicons for G. polyisoprenivorans Y2K and VH2 (3), the two E. coli-Gordonia shuttle vectors constructed in this study represent a second functional replication system.
TABLE 4.
Transfer of pKb1 oria
| Strain | Method of DNA transfer | Autonomous replicationb |
|---|---|---|
| G. alkanivorans HKI 0136 | Electroporation | − |
| G. desulfuricans 213E | Electroporation | − |
| G. polyisoprenivorans Kd2 | Electroporation | − |
| G. polyisoprenivorans VH2 | Electroporation | + |
| G. polyisoprenivorans Y2K | Electroporation | + |
| G. westfalica Kb1 | Electroporation | − |
| G. westfalica Kb2 | Electroporation | − |
| M. smegmatis mc2155 | Electroporation | + |
| R. opacus PD630 | Electroporation | + |
| R. rhodochrous RNMS1 | Electroporation | − |
| R. ruber | Electroporation | − |
| S. coelicolor A3(2) | Protoplast transformation | − |
| S. lividans TK23 | Protoplast transformation | − |
The oriV-containing hybrid plasmid pBBRKB1:XhoI7 (Fig. 2A) was transferred to different members of the genera Gordonia, Rhodococcus, and Mycobacterium by electroporation and of Streptomyces by protoplast transformation. Resistant colonies were screened for the presence of pBBRKB1:XhoI7 by plating on St-I agar plates with 50 mg of kanamycin per liter.
+, positive DNA transfer; −, negative DNA transfer.
Generation of a plasmid-free mutant strain of G. westfalica Kb1.
Thirty-four ORFs were localized within the metabolic region of pKB1. Three ORFs represented putative regulators, four represented putative membrane proteins, four represented putative peptidases, eight represented putative metabolic functions, and four were putatively involved in heavy-metal resistance. No function could be assigned to 11 ORFs. For identification of the functions encoded by plasmid pKB1, a plasmid-cured derivative strain of G. westfalica Kb1 was generated by heat curing (43). This method was used because the bacterium is very sensitive to mitomycin C. The curing procedure was successful, and 14 of 50 tested mutant strains did not contain plasmid pKB1 DNA (data not shown). The plasmid-free G. westfalica strains Kb1-K38 and Kb1-K43 were further characterized.
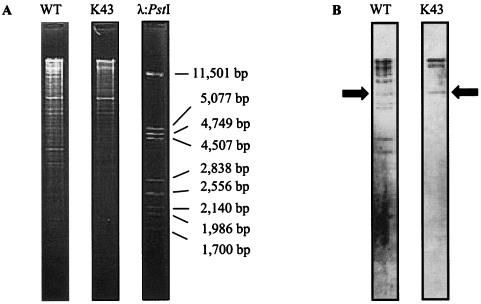
Since plasmid pKB1 contains many putative IS elements, total DNA isolated from the plasmid-free strain Kb1-K43 was analyzed for possible chromosomal insertions of parts of pKB1. Southern hybridization experiments were performed with the complete plasmid pKB1 as probe and total DNA from the plasmid-containing wild-type strain G. westfalica Kb1 serving as a positive control, and all 11 EcoRI fragments of plasmid pKB1 were visible (Fig. 4). An additional fragment (8,000 bp) for the positive control was observed which could not be derived from plasmid pKB1. When total DNA from the plasmid-free derivative strain Kb1-K43 was used instead of wild-type DNA, signals corresponding to the two largest EcoRI fragments (16,247 and 24,349 bp) of plasmid pKB1 and the signal corresponding to the 8,000-bp fragment were still obtained. This indicated the presence of DNA that was highly homologous to parts of plasmid pKB1 in the chromosome of the cured derivative strain Kb1-K43.
FIG. 4.
Southern hybridization analysis for detection of pKB1 in total DNA of pKB1-deficient mutant Kb1-K43 and G. westfalica Kb1. The isolated total DNA of the G. westfalica Kb1 wild type and of the pKB1-deficient mutant Kb1-K43 was digested with EcoRI, separated by agarose gel electrophoresis, and stained with ethidium bromide (A), and the fragments were transferred to a nylon membrane for Southern blotting (B). The DNA of complete plasmid pKB1 was used as a digoxigenin-labeled probe. λ:PstI, λ DNA digested with PstI; WT, total DNA of G. westfalica Kb1 wild type; K43, total DNA of G. westfalica K43.
The two IS elements that are putatively responsible for integration, comprising ORF1/ORF2 and ORF53 (only weak homologies to tnpA from Arthrobacter nicotinivorans, GenBank CAA65743), respectively, coding for putative transposases, were identified by PCR (Fig. 1). The PCR products generated with the primer sets P3-P4 and P5-P6 (Table 2; Fig. 1), containing only the sequences of the putative IS elements (ORF1/ORF2 and ORF53), could be amplified from both the wild type and the mutant Kb1-K43, whereas the PCR products generated with the primer sets P3-P7 and P8-P6 (Table 2; Fig. 1), containing the complete sequences of the two putative IS elements and parts of the adjacent regions, which were expected to be absent from the mutant Kb1-K43 (Fig. 4), could indeed only be amplified from the genome of the wild type and not from that of the mutant Kb1-K43. Therefore, an insertion into the chromosome of the two IS elements harboring ORF1/ORF2 and ORF53 and of the 51,527-bp region between these two IS elements must have already occurred in the wild-type strain Kb1 of G. westfalica. The insertion was then maintained in the chromosomes of the pKB1-free mutant; however, this insertion contained the complete conjugation region only. This demonstrates that the metabolic and heavy-metal resistance genes were eliminated during plasmid curing and that insertion of the conjugation region may have occurred early in the history of G. westfalica.
In conclusion, the metabolic region may constitute a catabolic transposon, which is a widespread occurrence among eubacteria and is found in gram-negative as well as gram-positive bacteria. Composite transposons are flanked by related, but not necessarily identical, IS elements and may be very large, exceeding 50 kbp (62).
The plasmid-free mutant strain of G. westfalica was not able to grow on cis-1,4-polyisoprene as sole carbon source.
Since several examples for plasmid-encoded degradation pathways are known (62), the possible involvement of plasmid pKB1 in rubber degradation was tested. The G. westfalica strain Kb1 wild type and plasmid-free mutant derivatives were cultivated for 40 days in the presence of cis-1,4-polyisoprene as the sole carbon source. While the turbidity at 600 nm of the wild-type culture increased from 0.15 to 0.75 during incubation, that of the mutant strains did not. This indicated that the mutant strains were not able to use cis-1,4-polyisoprene as a sole carbon source and that, therefore, at least some genes of plasmid pKB1 of G. westfalica must be involved in rubber degradation.
Genes essential for rubber biodegradation are probably encoded by the 49,489-bp region that is definitively missing from the cured mutants (Fig. 4). Since little is known about the biodegradation of rubber (25, 36) and proteins involved in this biochemical process have not been identified, it is not surprising that many ORFs with a hitherto unknown function are located in this metabolic region. These are strong candidates for rubber degradation genes. Electron transport proteins are often involved in catabolic and anabolic reactions and may also be involved in the initiation of biodegradation of rubber. Three ORFs (ORF27, ORF39, and ORF42) encode putative proteins that may be involved in cytochrome c biosynthesis. ORF27 encodes a protein of 520 aa, exhibiting 52% identity to a conserved hypothetical protein from Mycobacterium tuberculosis, which is putatively involved in cytochrome c biosynthesis. ORF39 encodes a protein of 271 aa, whose predicted protein comprises a DsbD (cytochrome c biogenesis) domain, and exhibits 51% identity to CCDA from Mycobacterium bovis. ORF42 encodes a protein of 597 aa which contains a COX1 domain and exhibits 77% identity to the cytochrome c oxidase subunit I isolated from M. tuberculosis. Cytochromes are involved in various electron transport systems and do not function only in aerobic or anaerobic respiration (17), e.g., cytochrome c catalyses peroxidase-like reactions in the presence of an electron acceptor like hydrogen peroxide (68). During rubber biodegradation, cytochrome c may catalyze epoxidation of the cis-1,4-polyisoprene molecule.
A putative epoxide hydrolase encoded by ORF6, which is localized about 10 kbp downstream of the metabolic region, may subsequently catalyze hydrolysis of the epoxide to the corresponding diol as has been shown for other epoxide hydrolases (69). A similar initiation of isoprene degradation was described by Johan et al. (26) for Rhodococcus sp. strain AD45. Such a sequence of reactions would in principal be consistent with the occurrence of cleavage products identified during biodegradation of natural rubber or related compounds (9, 10, 16, 53, 64). In addition, ORF28 and ORF40 encode proteins which are putatively necessary for the transport of electrons. The predicted ORF40 product (209 aa) contains a thioredoxin domain and exhibits 52% identity to a hypothetical protein isolated from Mycobacterium leprae.
Since biodegradation of rubber must occur outside of the cell or at the cell surface, membrane and transport proteins most probably perform a crucial function in rubber biodegradation. Furthermore, special proteins or other biopolymers may be required to establish a tight contact of the cells with the rather hydrophobic rubber molecules, in particular for those bacteria exhibiting adhesive growth as a biofilm on natural rubber, such as all species of the genus Gordonia (36). The protein encoded by ORF4, which is located 10 kbp downstream of the metabolic region, exhibited weak homology to TmtpC from M. smegmatis, which is involved in the transport of glycopeptidolipids through the cytoplasm membrane. Glycopeptidolipids are especially necessary for the formation of biofilms on polyvinylchloride (48). Finally, the probably complex biochemical process of rubber biodegradation and of other functions encoded by pKB1 will most probably be strongly regulated, thus explaining the occurrence of several genes for putative regulator proteins on pKB1. Thus, plasmid pKB1 encodes numerous candidate proteins that may be involved in the initial attack on natural rubber and/or in facilitating physical contact between the degrading bacterium and its substrate.
Furthermore, the metabolic region harbors a putative methyltransferase (ORF31, isolated from Shewanella oneidensis; 37% identity), a putative polyprenol-phosphate-mannose synthase 2 (ORF35, isolated from M. smegmatis; 42% identity), and a putative oxidoreductase (ORF36, isolated from Bacillus cereus; 22% identity).
Cadmium sensitivity of the wild type and the plasmid-free mutant.
In addition to genes encoding putative degradation pathways, plasmid pKB1 contains several genes that might be involved in heavy-metal homeostasis (ORFs 27, 29, 32, 33, 37, and 45) (Table 3). Moreover, G. westfalica was able to grow on St-I agar plates in the presence of 800 μM Cd(II) (Table 5). The bacterial species G. amicalis, G. bronchialis 43341, and G. nitida exhibited similar cadmium resistance, while the tolerance of cadmium under the same conditions of other related bacteria, G. alkanivorans 44369 (growth up to 600 μM cadmium), G. desulfuricans (growth up to 300 μM cadmium), G. polyisoprenivorans, G. sputi, M. smegmatis mc155, R. opacus PD630, Rhodococcus rhodochrous (growth up to 200 μM cadmium), G. terrae (growth up to 100 μM cadmium), was significantly lower. Thus, G. westfalica was more resistant to cadmium than 8 of 12 bacteria tested.
TABLE 5.
Determination of cadmium tolerance for different strains of Gordonia, Rhodococcus, and Mycobacteriuma
| Strain | Growth in the presence of CdCl2 (μM)b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 150 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | |
| G. alkanivorans | ++ | ++ | ++ | ++ | + | + | +/− | +/−− | +/−− | +/−− |
| G. amicalis | ++ | ++ | ++ | ++ | ++ | ++ | + | +/− | +/− | +/− |
| G. bronchialis | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| G. desulfuricans | ++ | ++ | ++ | + | +/−− | − | − | − | − | − |
| G. nitida | ++ | ++ | ++ | ++ | ++ | ++ | + | + | + | + |
| G. polyisoprenivorans VH2 | ++ | ++ | + | − | − | − | − | − | − | − |
| G. polyisoprenivorans Y2K | ++ | ++ | + | − | − | − | − | − | − | − |
| G. sputi | ++ | +/− | +/−− | − | − | − | − | − | − | − |
| G. terrae | ++ | − | − | − | − | − | − | − | − | − |
| G. westfalica K43 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| G. westfalica Kb1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + |
| M. smegmatis mc2155 | ++ | +/− | − | − | − | − | − | − | − | − |
| R. opacus PD630 | ++ | + | − | − | − | − | − | − | − | − |
| R. rhodochrous | ++ | +/− | − | − | − | − | − | − | − | − |
The strains were cultivated on St-I agar plates containing the indicated concentration of cadmium. After an incubation period of 3 days at 30 °C, growth was evaluated.
++, good growth; +, growth; +/−, limited growth; +/−−, very limited growth; −, no growth.
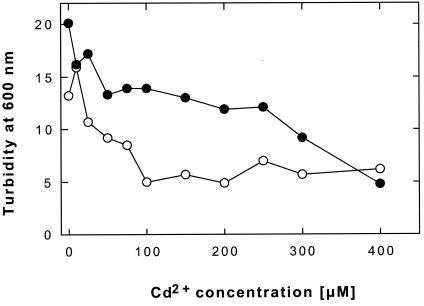
To investigate whether plasmid pKB1 mediates cadmium resistance in this bacterium, we compared growth of G. westfalica and the plasmid-free mutant strain Kb1-K43 in liquid medium in the presence of increasing cadmium concentrations (Fig. 5). A concentration of about 300 μM cadmium was required to decrease growth of the G. westfalica wild type to 50%, while about 40 μM cadmium was sufficient to reduce growth of the plasmid-free strain to half. Thus, the absence of plasmid pKB1 diminished cadmium resistance of G. westfalica by a factor of about 7, indicating that plasmid pKB1 is involved in cadmium resistance.
FIG. 5.
Effect of Cd2+ on growth of G. westfalica. Dose-response curves for cadmium were done with the G. westfalica Kb1(pKB1) wild type (•) and the plasmid-cured derivative strain K43 (○).
The ORF34 gene product CadA may contribute to pKB1-mediated cadmium resistance.
ORF34 from G. westfalica Kb1 plasmid pKB1 encodes a putative Cd2+/Zn2+-transporting P-type ATPase (Table 3). The predicted protein exhibits the closest similarity (58% identity) to a cation-transporting P-type ATPase G (CtpG; NP_216508) of M. tuberculosis, another gram-positive bacterium with high GC content, and to other P-type ATPases, e.g., CadA from Staphylococcus aureus, the first described example of a heavy-metal effluxing P-type or CPx-type ATPase (45).
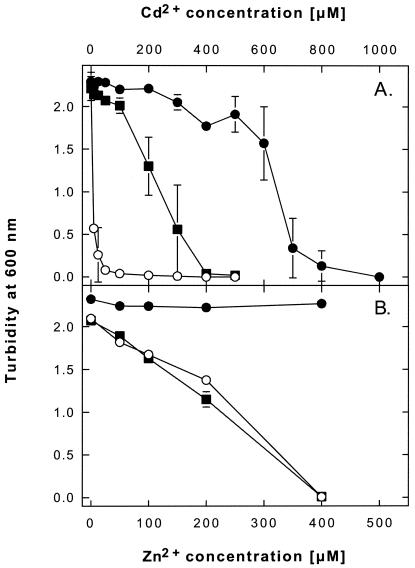
ORF34 was amplified by PCR from plasmid DNA of G. westfalica Kb1 and the gene was cloned into plasmid pASK3. The resulting plasmid, pECA34, was transferred into E. coli strain RW3110 (ΔzntA), a metal-sensitive strain with a deletion in the native Zn2+/Cd2+-effluxing P-type ATPase ZntA of this bacterium (50). The G. westfalica Kb1 gene corresponding to ORF34 conferred cadmium resistance to E. coli RW3110, but not zinc resistance (Fig. 6). It was therefore designated cadA and its product was designated GoCadA.
FIG. 6.
Cadmium and zinc resistance of E. coli strains expressing a P-type ATPase from G. westfalica Kb1, GoCadA. Dose-response curves for cadmium (A) and zinc (B) are shown for E. coli strain RW3110 (ΔzntA) complemented in trans with the cadA gene of G. westfalica Kb1 cloned into plasmid pASK3 (▪). The negative control strain is RW3110(pASK3) (○), and the positive control is the wild-type strain W3110(pASK3) (•). The mean results of three (cadmium) or two (zinc) independent experiments are shown, with standard deviation bars.
Half-maximal inhibition of E. coli strain RW3110 (ΔzntA) and wild-type strain W3110 occurred at about 5 and 650 μM Cd2+, respectively (Fig. 6). Complementation in trans with cadA from G. westfalica Kb1 led to half-maximal inhibition at about 250 μM Cd2+. Thus, GoCadA was able to protect E. coli cells against cadmium about half as efficiently as the native efflux system ZntA.
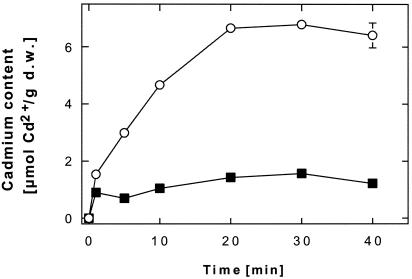
The metal cation uptake into cells of E. coli strain RW3110 expressing GoCadA was examined (Fig. 7). Cells of metal-sensitive E. coli strain RW3110 accumulated 6.6 μmol of 109Cd2+ per g of cell dry weight within 20 min in an assay buffer containing 10 μM cadmium. In contrast, cells containing GoCadA accumulated only 1.4 μmol of cadmium per g of cell dry weight, only 20% of the amount bound to metal-sensitive control cells. Thus, GoCadA was functionally expressed in E. coli and decreased the accumulation of cadmium by E. coli cells, probably by cadmium efflux. These data indicate that GoCadA may contribute to plasmid pKB1-mediated cadmium resistance in this bacterium.
FIG. 7.
Presence of P-type ATPase GoCadA from G. westfalica Kb1 diminishes accumulation of Cd2+ in E. coli strain RW3110. E. coli strain RW3110 (ΔzntA) was complemented in trans with the gene encoding GoCadA (▪). The negative control (○) contained only the vector plasmid, pASK3. The mean values of two independent experiments with 10 μM 109Cd2+ are shown. d.w., dry weight.
Plasmid pKB1 harbored putative heavy-metal resistance genes in addition to cadA, which is a notable situation. ORF28 encodes a protein of 415 aa containing a domain of the Nramp family and exhibiting 59% identity to a divalent cation transporter integral membrane protein isolated from M. bovis. ORF38 encodes a predicted protein (314 aa) comprising a domain of the cation efflux family, and 35% identity was observed to a cobalt-zinc-cadmium resistance protein from Xanthomonas axonopodis. ORF46 encodes a protein of 730 aa and exhibits highest identity (46%) to a cation-transporting ATPase of M. leprae.
Thus, plasmid pKB1 seems to harbor two genes for P-type ATPases. Bacteria that contain more than one CPx-type ATPase of the Zn/Cd/Pb group of proteins may exhibit differentiation of the functions of these proteins, while in bacteria that contain only one of these proteins, the substrate specificity of the single transporter should be broader. In E. coli, ZntA is responsible for detoxification of Zn2+, Cd2+, and Pb2+ (49-51). In contrast, in the gram-negative bacterium Ralstonia metallidurans, the three CPx-type ATPases of the Zn/Cd/Pb group mainly concentrate on Zn2+ (ZntA), Cd2+ (CadA), or Pb2+ (PbrA) with respect to the regulation of their expression and their substrate specificities (11, 34). CadA from Bacillus subtilis confers resistance to cadmium, zinc, and cobalt (19), and Bxa1 from Oscillatoria brevis confers resistance to zinc and cadmium (40) in E. coli (34).
Conjugation region.
The third region of the plasmid appears to be concerned with conjugation, a complex process which involves many genes (39). This region is divided into two parts by the presence of an IS element comprising ORF76 and ORF77. This conjugation region contains 49 detected ORFs and the putative transcriptional products of 6 ORFs exhibiting homologies to proteins putatively involved in conjugational processes. ORF67 presumably encodes a TraA-like protein with 33% identity to a protein from R. equi that contains an ATP/GTP binding motive. The transcriptional product predicted for ORF75 represents a protein of 1,631 aa, comprising a DEAD-like helicase superfamily domain, and 37% identity was observed to a putative methylase from R. equi. Further similarities were observed in particular to the plasmid-encoded conjugative transfer gene complexes (trs) of Lactococcus lactis DPC3147 (15) and S. aureus (42). This provides evidence of possible pKB1-encoded conjugative transfer and should be further investigated.
Acknowledgments
We are grateful for financial support provided by the Deutsche Bundesstiftung Umwelt (Osnabrück, Germany) to A.S. (AZ. 13072 within the ICBIO project), by the Deutsche Forschungsgemeinschaft to D.H.N. (Ni262/3-3), and by the Fonds der Chemischen Industrie to A.S. and D.H.N.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenskötter, M., D. Baumeister, M. M. Berekaa, G. Pötter, R. M. Kroppenstedt, A. Linos, and A. Steinbüchel. 2001. Taxonomic characterization of two rubber degrading bacteria belonging to the species Gordonia polyisoprenivorans and analysis of hyper variable regions of 16S rDNA sequences. FEMS Microbiol. Lett. 205:277-282. [DOI] [PubMed] [Google Scholar]
- 3.Arenskötter, M., D. Baumeister, R. Kalscheuer, and A. Steinbüchel. 2003. Identification and application of plasmids suitable for transfer of foreign DNA to members of the genus Gordonia. Appl. Environ. Microbiol. 69:4971-4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struni. 1987. Current protocols in molecular biology, vol. 1, 1st ed. John Wiley and Sons, New York, N.Y.
- 5.Barton, B. M., G. P. Harding, and A. J. Zuccarelli. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235-240. [DOI] [PubMed] [Google Scholar]
- 6.Bibb, M. J., J. L. Schottel, and S. N. Cohen. 1980. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature 284:526-531. [DOI] [PubMed] [Google Scholar]
- 7.Bibb, M. J., J. M. Ward, and D. A. Hopwood. 1978. Transformation of plasmid DNA into Streptomyces at high frequency. Nature 274:398-400. [DOI] [PubMed] [Google Scholar]
- 8.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode, H. B., A. Zeeck, K. Pluckhahn, and D. Jendrossek. 2000. Physiological and chemical investigations into microbial degradation of synthetic poly(cis-1,4-isoprene). Appl. Environ. Microbiol. 66:3680-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode, H. B., K. Kerkhoff, and D. Jendrossek. 2001. Bacterial degradation of natural and synthetic rubber. Biomacromolecules 2:295-303. [DOI] [PubMed] [Google Scholar]
- 11.Borremans, B., J. L. Hobman, A. Provoost, N. L. Brown, and D. Van der Lelie. 2001. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 183:5651-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullock, W. O., J. M. Fernandez, and J. M. Stuart. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 13.Chun, J., S. O. Kang, Y. C. Hah, and M. Goodfellow. 1996. Phylogeny of mycolic acid-containing actinomycetes. J. Ind. Microbiol. 17:205-213. [Google Scholar]
- 14.Denis-Larose, C., H. Bergeron, D. Labbé, C. W. Greer, J. Hawari, M. J. Grossman, B. M. Sankey, and P. C. K. Lau. 1998. Characterization of the basic replicon of Rhodococcus plasmid pSOX and development of a Rhodococcus-Escherichia coli shuttle vector. Appl. Environ. Microbiol. 64:4363-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty, B. A., C. Hill, J. F. Weidman, D. R. Richardson, J. C. Venter, and R. P. Ross. 1998. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol. Microbiol. 29:1029-1038. [DOI] [PubMed] [Google Scholar]
- 16.Enoki, M., Y. Doi, and T. Iwata. 2003. Oxidative degradation of cis- and trans-1,4-polyisoprenes and vulcanized natural rubber with enzyme-mediator systems. Biomacromolecules 4:314-320. [DOI] [PubMed] [Google Scholar]
- 17.Freedman, J. A., and S. H. Chan. 1984. Interactions in cytochrome oxidase: functions and structure. J. Bioenerg. Biomembr. 16:75-100. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich, B., C. Hogrefe, and H. G. Schlegel. 1981. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J. Bacteriol. 147:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaballa, A., and J. D. Helmann. 2003. Bacillus subtilis CPx-type ATPases: characterization of Cd, Zn, Co and Cu efflux systems. Biometals 16:497-505. [DOI] [PubMed] [Google Scholar]
- 20.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 183:4664-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 22.Haywood, G., A. J. Anderson, D. Williams, E. A. Dawes, and D. Ewing. 1991. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus ruber NCIMB 40126. Int. J. Biol. Macromol. 13:83-88. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Perez, G., F. Fayolle, and J.-P. Vandecasteele. 2001. Biodegradation of ethyl t-butyl ether (ETBE), methyl t-butyl ether (MTBE) and t-amyl methyl ether (TAME) by Gordonia terrae. Appl. Microbiol. Biotechnol. 55:117-121. [DOI] [PubMed] [Google Scholar]
- 24.Hopwood, D. A., M. J. Bibb, K. F. Chater, T. Kieser, C. J. Bruton, H. M. Kieser, D. J. Lydiate, C. P. Smith, J. M. Ward, and H. Schrempf. 1985. Genetic manipulation of Streptomyces. A laboratory manual. John Innes Foundation, Norwich, Conn.
- 25.Jendrossek, D., G. Tomasi, and R. M. Kroppenstedt. 1997. Bacterial degradation of natural rubber: a privilege of actinomycetes? FEMS Microbiol. Lett. 150:179-188. [DOI] [PubMed] [Google Scholar]
- 26.Johan, E. T., H. van Vlieg, H. Leemhuis, J. H. L. Spielberg, and D. B. Janssen. 2000. Characterization of the gene cluster involved in isoprene metabolism in Rhodococcus sp. strain AD45. J. Bacteriol. 182:1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kado, C. I., and S. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalscheuer, R., M. Arenskötter, and A. Steinbüchel. 1999. Establishment of a gene transfer system for Rhodococcus opacus PD630 based on electroporation and its application for recombinant biosynthesis of poly(3-hydroxyalkanoic acids). Appl. Microbiol. Biotechnol. 52:508-515. [DOI] [PubMed] [Google Scholar]
- 29.Kim, S. B., R. Brown, C. Oldfield, S. C. Gilbert, and M. Goodfellow. 1999. Gordonia desulfuricans sp. nov., a benzothiophene-desulphurizing actinomycete. Int. J. Syst. Bacteriol. 49:1845-1851. [DOI] [PubMed] [Google Scholar]
- 30.Kim, S. B., R. Brown, C. Oldfield, S. C. Gilbert, S. Iliarionov, and M. Goodfellow. 2000. Gordonia amicalis sp. nov., a novel dibenzothiophene-desulphurizing actinomycete. Int. J. Syst. Evol. Microbiol. 50:2031-2036. [DOI] [PubMed] [Google Scholar]
- 31.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad host range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 32.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. Bio/Technology 16:800-802. [PubMed] [Google Scholar]
- 33.Kummer, C., P. Schumann, and E. Stackebrandt. 1999. Gordonia alkanivorans sp. nov., isolated from tar-contaminated soil. Int. J. Syst. Bacteriol. 49:1513-1522. [DOI] [PubMed] [Google Scholar]
- 34.Legatzki, A., A. Anton, G. Grass, C. Rensing, and D. H. Nies. 2003. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J. Bacteriol. 185:4354-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letunic, I., L. Goodstadt, N. J. Dickens, T. Doerks, J. Schultz, R. Mott, F. Ciccarelli, R. R. Copley, C. P. Ponting, and P. Bork. 2002. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res. 30:242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linos, A., and A. Steinbüchel. 2001. Biodegradation of natural and synthetic rubbers, p. 321-359. In T. Koyama and A. Steinbüchel (ed.), Biopolymers, vol. 2, 1st ed. Wiley-VCH, Weinheim, Germany.
- 37.Linos, A., A. Steinbüchel, C. Spröer, and R. M. Kroppenstedt. 1999. Gordonia polyisoprenivorans sp. nov., a rubber degrading actinomycete isolated from automobile tire. Int. J. Syst. Bacteriol. 49:1785-1791. [DOI] [PubMed] [Google Scholar]
- 38.Linos, A., M. M. Berekaa, A. Steinbüchel, K. K. Kim, C. Spröer, and R. M. Kroppenstedt. 2002. Gordonia westfalica sp. nov., a novel rubber-degrading actinomycete. Int. J. Syst. Evol. Microbiol. 52:1133-1139. [DOI] [PubMed] [Google Scholar]
- 39.Liosa, M., F. X. Gomis-Rüth, M. Coll, and F. de la Cruz. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 40.Liu, T., S. Nakashima, K. Hirose, Y. Uemura, M. Shibasaka, M. Katsuhara, and K. Kasamo. 2003. A metallothionein and CPx-ATPase handle heavy-metal tolerance in the filamentous cyanobacterium Oscillatoria brevis. FEBS Lett. 542:159-163. [DOI] [PubMed] [Google Scholar]
- 41.Lukashin, A. V., and M. Borodovsky. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morton, T. M., D. M. Eaton, J. L. Johnston, and G. L. Archer. 1993. DNA sequence and units of transcription of the conjugative transfer gene complex (trs) of Staphylococcus aureus plasmid pGO1. J. Bacteriol. 175:4436-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nester, E. W. 1984. Genetics: gene mutation, p. 222-242. In P. Gerhardt (ed.), Manual of methods for general bacteriology, 3rd ed. American Society for Microbiology, Washington, D.C.
- 44.Nies, D. H., and S. Silver. 1989. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J. Bacteriol. 171:896-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nucifora, G., L. Chu, T. K. Misra, and S. Silver. 1989. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proc. Natl. Acad. Sci. USA 86:3544-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okanishi, M., K. Katagiri, T. Furumai, K. Takeda, K. Kawaguchi, M. Saitoh, and S. Nabeshima. 1983. Basic techniques for DNA cloning and conditions required for streptomycetes as host. J. Antibiot. 36:99-108. [DOI] [PubMed] [Google Scholar]
- 47.Okanishi, M., K. Suzuki, and H. Umezawa. 1974. Formation and reversion of streptomycete protoplasts: cultural condition and morphological study. J. Gen. Microbiol. 80:389-400. [DOI] [PubMed] [Google Scholar]
- 48.Recht, J., A. Martinez, S. Torello, and R. Kolter. 1999. Genetic analysis of sliding motility in Mycobacterium smegmatis. J. Bacteriol. 182:4348-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rensing, C., M. Ghosh, and B. P. Rosen. 1999. Families of soft-metal-ion-transporting ATPases. J. Bacteriol. 181:5891-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rensing, C., B. Mitra, and B. P. Rosen. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 94:14326-14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rensing, C., Y. Sun, B. Mitra, and B. P. Rosen. 1998. Pb(II)-translocating P-type ATPases. J. Biol. Chem. 273:32614-32617. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 53.Sato, S., Y. Honda, M. Kuwahara, and T. Watanabe. 2003. Degradation of vulcanized and nonvulcanized polyisoprene rubbers by lipid peroxidation catalyzed by oxidative enzymes and transition metals. Biomacromolecules 4:321-329. [DOI] [PubMed] [Google Scholar]
- 54.Schlegel, H. G., H. Kaltwasser, and G. Gottschalk. 1961. Ein submersverfahren zur kultur wasserstoffoxidierender bakterien: wachstumsphysiologische untersuchungen. Arch. Mikrobiol. 38:209-222. [PubMed] [Google Scholar]
- 55.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 26:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sermonti, F., A. Mancinelli, and I. Spada-Sermonti. 1960. Heterogenous clones (heteroclones) in Streptomyces coelicolor A3 (2). Genetics 45:669-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram-negative bacteria. Biol. Technol. 1:784-791. [Google Scholar]
- 58.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 59.Stackebrandt, E., A. F. Rainey, and N. L. Ward-Rainey. 1997. Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int. J. Syst. Bacteriol. 47:479-491. [Google Scholar]
- 60.Takai, S., S. A. Hines, T. Sekizaki, V. M. Nicholson, D. A. Alperin, M. Osaki, D. Takamatsu, M. Nakamura, K. Suzuki, N. Ogino, T. Kakuda, H. Dan, and J. F. Prescott. 2000. DNA sequence and comparison of virulence plasmids from Rhodococcus equi ATCC 33701 and 103. Infect. Immun. 68:6840-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takaichi, S., Y. Tamura, K. Azegami, Y. Yamamoto, and J. Ishidsu. 1997. Carotenoid glucoside mycolic acid esters from the nocardioform actinomycetes, Rhodococcus rhodochrous. Phytochemistry 45:505-508. [Google Scholar]
- 62.Tan, H.-M. 1999. Bacterial catabolic transposons. Appl. Microbiol. Biotechnol. 51:1-12. [DOI] [PubMed] [Google Scholar]
- 63.Thompson, C. J., J. M. Ward, and D. A. Hopwood. 1982. Physical analysis of antibiotic-resistant genes from Streptomyces and their use in vector construction. Gene 20:51-62. [DOI] [PubMed] [Google Scholar]
- 64.Tsuchii, A., T. Suzuki, and K. Takeda. 1985. Microbial degradation of natural rubber vulcanizates. Appl. Environ. Microbiol. 50:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsukamura, M. 1971. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J. Gen. Microbiol. 68:15-26. [DOI] [PubMed] [Google Scholar]
- 66.Tsukamura, M. 1978. Numerical classification of Rhodococcus (formerly Gordona) organisms recently isolated from sputa of patients: description of Rhodococcus sputi Tsukamura sp. nov. Int. J. Syst. Bacteriol. 28:169-181. [Google Scholar]
- 67.Tsukamura, M. 1982. Numerical analysis of the taxonomy of nocardiae and rhodococci. Microbiol. Immunol. 26:1101-1119. [DOI] [PubMed] [Google Scholar]
- 68.Vazquez-Duhalt, R. 1999. Cytochrome c as a biocatalyst. J. Mol. Cat. B 7:241-249. [Google Scholar]
- 69.Yamada, T., C. Morisseau, J. E. Maxwell, M. A. Argiriadi, D. W. Christianson, and B. D. Hammock. 2000. Biochemical evidence for the involvement of tyrosine in epoxide activation during the catalytic cycle of epoxide hydrolase. J. Biol. Chem. 275:23082-23088. [DOI] [PubMed] [Google Scholar]
- 70.Yoon, J.-H., J. J. Lee, S.-S. Kang, M. Takeuchi, Y. K. Shin, S. T. Lee, K. H. Kang, and Y. H. Park. 2000. Gordonia nitida sp. nov., a bacterium that degrades 3-ethylpyridine and 3-methylpyridine. Int. J. Syst. Evol. Microbiol. 50:1203-1210. [DOI] [PubMed] [Google Scholar]