Abstract
We cloned the gene PA1361 (we designated the gene pmpM), which seemed to encode a multidrug efflux pump belonging to the MATE family, of Pseudomonas aeruginosa by the PCR method using the drug-hypersensitive Escherichia coli KAM32 strain as a host. Cells of E. coli possessing the pmpM gene showed elevated resistance to several antimicrobial agents. We observed energy-dependent efflux of ethidium from cells possessing the pmpM gene. We found that PmpM is an H+-drug antiporter, and this finding is the first reported case of an H+-coupled efflux pump in the MATE family. Disruption and reintroduction of the pmpM gene in P. aeruginosa revealed that PmpM is functional and that benzalkonium chloride, fluoroquinolones, ethidium bromide, acriflavine, and tetraphenylphosphonium chloride are substrates for PmpM in this microorganism.
Pseudomonas aeruginosa is an opportunistic pathogen and a leading cause of nosocomial infections. A major problem in the treatment of patients infected with P. aeruginosa is that this bacterium shows intrinsic and acquired resistance against many antibiotics and disinfectants, including most β-lactams, fluoroquinolones, tetracycline, chloramphenicol, erythromycin, and benzalkonium chloride (4, 7, 11, 12, 13, 15, 20, 27). Several mechanisms for drug resistance are known, such as (i) inactivation of drugs by degradation or modification, (ii) alteration of the target, (iii) emergence of an alternative pathway, and (iv) active efflux of the drug. Among these mechanisms, active efflux, especially multidrug efflux, has been recognized as a major mechanism for multidrug resistance. Once a bacterium acquires a gene for a certain multidrug efflux pump or if a silent or weak gene for a multidrug efflux pump is activated, the cell instantly becomes resistant to many antimicrobial agents because multidrug efflux pumps extrude many structurally unrelated antimicrobial agents from cells. Thus, multidrug efflux pumps play important roles in multidrug resistance in bacteria. Multidrug resistance in P. aeruginosa has been attributed mainly to the activity of several multidrug efflux pumps. MexAB-OprM (11, 15), MexCD-OprJ (20, 23), MexEF-OprN (7), MexXY-OprM (15, 16), MexJK-OprM (4), MexHI-OpmD (27), MexVW-OprM (13), and EmrE (12) have been characterized and are potent multidrug efflux pumps in P. aeruginosa. MexAB-OprM is the only constitutive Mex pump in wild-type P. aeruginosa (11, 15). MexCD-OprJ (20, 23) and MexXY-OprM (15, 16) are inducible pumps, and MexEF-OprN (7), MexJK-OprM (4), MexHI-OpmD (27), and MexVW-OprM (13) are silent pumps in the wild-type strain.
The genome sequence of P. aeruginosa (29; http://www.Pseudomonas.com) suggests the presence of about 34 multidrug efflux pumps in this microorganism. Twenty of these pumps belong to the major facilitator superfamily, 10 or 12 belong to the resistance nodulation cell division family, 6 belong to the small multidrug resistance (SMR) family (12), 2 or 3 belong to the multidrug and toxic compound extrusion (MATE) family, and 1 belongs to the ATP binding cassette superfamily. Multidrug efflux pumps of the major facilitator, resistance nodulation cell division, and SMR families utilize an electrochemical potential of H+ across the cytoplasmic membrane as the driving force for drug extrusion. Pumps of the ATP binding cassette superfamily utilize ATP as an energy source. The driving force in the MATE family is unique. Pumps of this family utilize an electrochemical potential of Na+ across the cytoplasmic membrane as the driving force (2, 19, 21, 22).
We are especially interested in multidrug efflux pumps belonging to the MATE family because of its unique energy coupling. So far, we have characterized several MATE family pumps, such as NorM (18) and VmrA (2) of Vibrio parahaemolyticus, YdhE of Escherichia coli (18), and VcmA (22) and VcrM (21) of Vibrio cholerae non-O1. We have shown that all of them utilize an electrochemical potential of Na+ across membranes as the driving force (2, 18, 21, 22). Here, we report that PmpM (a product of the PA1361 gene) (http://www.Pseudomonas.com) of P. aeruginosa is a unique multidrug efflux pump belonging to the MATE family that utilizes H+, but not Na+, as the coupling ion for drug extrusion.
Cloning of pmpM.
We cloned the open reading frame PA1361 (http://www.Pseudomonas.com) by the PCR method using chromosomal DNA of P. aeruginosa PAO1 as a template. Chromosomal DNA was prepared by the procedure described by Chen (3). The primers used were forward primer 1 (F1), 5′-CTACGGAATTCCCCTGCCCAGACAAGGAC-3′ (containing an EcoRI site), and reverse primer 1 (R1), 5′-TCCTCGCCTCGGTCGACACTACCCTCAG-3′ (containing a SalI site). The primer F1 carries the Shine-Dalgarno sequence of the pmpM gene but not a putative promoter of the gene. We designated the gene pmpM (Pseudomonas MATE family efflux pump). The vectors used were pSTV28 and pUCP20T, and the resulting hybrid plasmids, pPBE2 and pUPBE2T, respectively, carry the pmpM gene but not its original promoter. The cloned pmpM gene is located downstream from the lactose promoter in the two plasmids. The addition of an inducer of the lactose operon (isopropyl β-d-thiogalactopyranoside) did not have a significant effect on the level of drug resistance when cells were grown in L broth, which may contain a natural inducer. Thus, we investigated drug resistance without the addition of an inducer.
Drug susceptibility.
To investigate the role of PmpM in drug resistance, plasmid pPBE2, carrying the pmpM gene, was introduced into cells of drug-hypersensitive E. coli KAM32 (ΔacrB ΔydhE Hsd−) (2). We compared the MICs of various antimicrobial agents for E. coli KAM32/pPBE2 and E. coli KAM32/pSTV28 (control) (Table 1). Elevated MICs of fluoroquinolones, fradiomycin, benzalkonium chloride, chlorhexidine gluconate, ethidium bromide, tetraphenylphosphonium chloride (TPPCl), and rhodamine 6G were observed for KAM32/pPBE2. Thus, the pmpM gene is responsible for conferring multidrug resistance. It seems that the disinfectant benzalkonium chloride is a good substrate for PmpM when expressed in E. coli cells.
TABLE 1.
MICs of various antimicrobial agents for E. coli KAM32/pSTV28 and E. coli KAM32/pPBE2
| Drug | MIC (μg/ml) for:
|
|
|---|---|---|
| E. coli KAM32/pSTV28 | E. coli KAM32/pPBE2 | |
| Norfloxacin | 0.015 | 0.06 |
| Ciprofloxacin | 0.002 | 0.015 |
| Ofloxacin | 0.008 | 0.03 |
| Fradiomycin | 0.5 | 2 |
| Streptomycin | 1 | 1 |
| Kanamycin | 0.5 | 0.5 |
| Erythromycin | 2 | 2 |
| Ampicillin | 1 | 1 |
| Tetracycline | 0.5 | 0.5 |
| Benzalkonium chloride | 1.2 | 37.5 |
| Chlorhexidine gluconate | 4.5 | 18 |
| Triclosan | 2 | 2 |
| Ethidium bromide | 2 | 16 |
| TPPCl | 4 | 128 |
| Rhodamine 6G | 4 | 64 |
| Acriflavine | 1 | 2 |
Drug transport via PmpM and cation coupling.
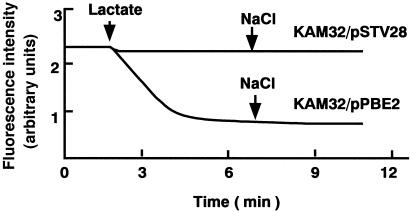
PmpM is a member of the MATE family of efflux pumps. Members of this family, such as NorM (18, 19) and VmrA (2) of V. parahaemolyticus and YdhE of E. coli (18), are Na+-coupled efflux pumps. Thus, it was anticipated that PmpM would also be a Na+-coupled efflux pump. If this were the case, then ethidium efflux would be very weak in the absence of Na+ and greatly enhanced by the addition of Na+ to the assay mixture. Thus, we tested the effect of NaCl on ethidium efflux. First, we prepared energy-starved and ethidium-loaded cells (17, 22). Then, lactate was added to energize the cells, and ethidium efflux was measured in the absence or presence of NaCl. Ethidium efflux energized by lactate was observed with cells of E. coli KAM32/pPBE2 but not with cells of KAM32/pSTV28 (Fig. 1). Addition of NaCl resulted in no significant change in the efflux. We added various concentrations of NaCl ranging from 1 to 100 mM and observed no significant effect (data not shown). Addition of NaCl prior to the addition of lactic acid gave no significant effect. We also tested the effect of LiCl, because Li+ can replace Na+ as a coupling cation in the case of NorM (18). However, no significant effect of LiCl was observed (data not shown).
FIG. 1.
Ethidium transport assays. Cells (E. coli KAM32/pSTV28 and KAM32/pPBE2) were grown in L broth (10) under aerobic conditions at 37°C. The cells were harvested, washed twice with 0.1 M MOPS (morpholinepropanesulfonic acid)-tetramethylammonium hydroxide (pH 7.0) containing 2 mM MgSO4, and suspended in the same medium containing 25 μM ethidium bromide and 40 μM carbonyl cyanide m-chlorophenylhydrazone (16, 21). The cells were incubated at 37°C for 1 h to starve cellular energy and to load with ethidium (16, 21). The cells were washed three times with the same medium (no carbonyl cyanide m-chlorophenylhydrazone) and resuspended in the same medium. This cell suspension was preincubated at 37°C for 5 min, and lactic acid (the pH was adjusted to 7.0 with tetramethylammonium hydroxide) was added to the assay mixture at 40 mM. The change in the fluorescence intensity was measured. Where indicated, NaCl was added at 10 mM to test the effect of NaCl.
If the coupling ion is Na+, we should be able to observe Na+ efflux from cells elicited by influx of substrate, as observed with NorM (18, 19) and VmrA (2). We measured Na+ flux by using a Na+ electrode. No Na+ flux was detected when substrates of the PmpM pump were added to a suspension of Na+-loaded cells of E. coli KAM32/pPBE2, although Na+ efflux was observed with cells of E. coli KAM32/pMVP36 (data not shown). pMVP36 carries norM, the gene for a Na+-coupled multidrug efflux pump, NorM, belonging to the MATE family (18, 19). Thus, it is highly likely that H+ instead of Na+ is the coupling cation for PmpM.
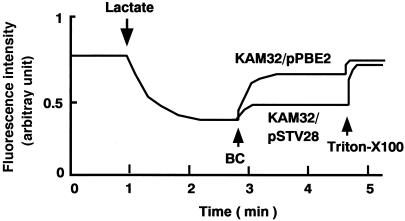
If the coupling cation in PmpM is an H+, then it must be an H+-substrate antiporter. One convenient method to test this possibility is to measure the flux of H+ caused by a substrate of the pump involved. A previous study has reported evidence for H+-chloramphenicol antiport in a multidrug efflux pump, Cmr (MdfA), by measuring fluorescence quenching of quinacrine (17). Therefore, we measured fluorescence quenching in everted membrane vesicles prepared from cells of E. coli KAM32/pSTV28 and KAM32/pPBE2 (Fig. 2). Fluorescence quenching due to inwardly directed H+ transport by the respiratory chain was elicited by the addition of lactate in both types of everted membrane vesicles. We chose benzalkonium chloride as the substrate for the assay because some other substrates of PmpM had some unfavorable effects on the fluorescence of quinacrine. Addition of benzalkonium chloride to the assay mixture caused efflux of H+, indicating that H+-benzalkonium chloride antiport took place. Thus, we conclude that the coupling ion in PmpM is H+ but not Na+.
FIG. 2.
Benzalkonium chloride-H+ antiport activity in membrane vesicles. Cells (E. coli KAM32 and KAM32/pPBE2) were grown in L broth supplemented with 20 mM potassium lactate, and everted membrane vesicles were prepared by passing cells through a French press (9). Benzalkonium chloride-H+ antiport was measured by quinacrine fluorescence quenching (the final concentration of quinacrine was 0.5 μM). At the time point indicated by the arrow, potassium lactate (5 mM) was added to initiate respiration. After the fluorescence quenching reached a steady state, benzalkonium chloride (BC; the final concentration was 0.0004%) was added to the assay mixture. Finally, at the time point indicated by the arrow, Triton X-100 was added at a concentration of 0.0125% to collapse the H+ gradient.
We compared the amino acid sequences of Na+-coupled pumps (NorM, YdhE, VmrA, VcmA, and VcrM) and H+-coupled PmpM and tried to find residues or regions important for recognition of ions. Unfortunately, we have not succeeded in locating such residues or regions, so far. It would be interesting to isolate or construct mutant-type PmpMs (or NorMs) which show differences in ion recognition in order to gain an insight into the mechanism of ion recognition. Previously, other studies identified residues important for ion recognition in the melibiose transport protein by a similar strategy (5, 8).
We prepared a dendrogram using representatives of the MATE family of efflux pumps or putative pumps (data not shown). There are several subfamilies within the MATE family. NorM of V. parahaemolyticus (18), YdhE of E. coli (19), and VcmA of V. cholerae (22) belong to one subfamily. It seems that PmpM belongs to this subfamily but is a little apart from these three. VmrA of V. parahaemolyticus (2) and VcrM of V. cholerae (21) belong to another subfamily. All of the MATE family pumps so far characterized are Na+-coupled efflux pumps. Since PmpM is an H+-coupled pump, it is likely that there are other H+-coupled pumps in the MATE family. It seems that PA5294 of P. aeruginosa is a member of the first subfamily, although PA5294 has not previously been recognized as a drug efflux pump. Thus, PA5294 may be a multidrug efflux pump and may be an H+-coupled pump because it is apart from the Na+-coupled NorM, YdhE, and VcmA pumps in the dendrogram.
Deletion of the pmpM gene and role of the PmpM pump in P. aeruginosa.
So far, several P. aeruginosa mutants lacking genes for Mex multidrug efflux pumps have been constructed (20, 27). P. aeruginosa YM64 lacks four major Mex pumps, MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY (20). Recently, a mutant PMX52 lacking an additional pump, MeHI-OpmD, was constructed from YM64 (27). The pmpM gene from the chromosome of PMX52 was deleted.
Chromosomal DNA from P. aeruginosa PAO1 was used as a template for PCR. We cloned a longer DNA region containing the pmpM gene for disruption of the gene, because longer fringe regions are better for recombination to take place. The primers used were forward primer 2 (F2), 5′-AGATAATTCACCGGGCTCTTCG-3′ (containing an EcoRI site), and reverse primer 2 (R2), 5′-CCTTGCCCGGTACCCTGGAAATGG-3′ (containing a KpnI site). The PCR product with the length of 2.2-kbp was digested with EcoRI and KpnI, the EcoRI-KpnI fragment containing the pmpM gene was ligated into vector pSTV29 (TaKaRa Co., Kyoto, Japan), and the resulting pPBEA29 plasmid was obtained. The pPBEA29 plasmid was digested with StuI, and a 1.9-kbp SmaI-SmaI fragment from pPS858 (6) containing a gentamicin-resistance marker sandwiched by two FRT sites was ligated to the StuI-StuI sites, which are present in the pmpM gene of pPBEA29. The resulting recombinant plasmid was designated pPBEA29G. Plasmid pPBEA29G contains a disrupted pmpM gene. The length of the deleted StuI fragment is 796 bp. Finally, a 4.7-kbp SspI-SspI fragment from plasmid pPBEA29G was ligated to the SmaI site of pEX100T (30), which contains a sacB gene (6), to construct plasmid pPBEA29GS. The pmpM gene was removed from the chromosomal DNA of strain PMX52 by replacing the pmpM region with the corresponding deleted region of pPBEA29GS by a Flp-FRT recombination system, as previously reported (1, 20, 24, 25, 26, 28), to obtain strain PMX6. Disruption of the pmpM gene in PMX6 was confirmed by PCR methods.
We compared the MICs of various antimicrobial agents for P. aeruginosa PMX52, PMX6, and PMX6/pUPBE2T in order to evaluate the role of the PmpM pump in P. aeruginosa (Table 2). Deletion of the pmpM gene from the chromosome of PMX52 resulted in a decrease in the MICs of benzalkonium chloride, ethidium bromide, acriflavine, and TPPCl. Thus, we conclude that PmpM is functional in the parental cell PMX52. Since PMX52 is a deletion derivative of wild-type PAO1, it seems that PmpM is also functional in wild-type PAO1.
TABLE 2.
MICs of various antimicrobial agents for P. aeruginosa PMX52, PMX6, and PMX6/pUPBE2T
| Drug | MIC (μg/ml) for:
|
||
|---|---|---|---|
| PMX52 | PMX6 | PMX6/pUPBE2T | |
| Norfloxacin | 0.03 | 0.03 | 0.12 |
| Ciprofloxacin | 0.06 | 0.06 | 0.24 |
| Ofloxacin | 0.06 | 0.06 | 0.12 |
| Kanamycin | 16 | 16 | 16 |
| Fradiomycin | 4 | 4 | 4 |
| Tetracycline | 0.25 | 0.25 | 0.25 |
| Benzalkonium chloride | 20 | 5 | 40 |
| Chlorhexidine gluconate | 5 | 5 | 5 |
| Ethidium bromide | 8 | 1 | 16 |
| Acriflavine | 2 | 0.25 | 2 |
| TPPCl | 32 | 8 | 64 |
| Rhodamine 6G | 16 | 16 | 16 |
Seven Mex multidrug efflux pumps (MexAB-OprM, MexCD-OprJ, MexEF-OprN, MexXY-OprM, MexJK-OprM, MexHI-OpmD, and MexVW-OprM) (4, 7, 11, 12, 13, 15, 16, 20, 23, 27) and one SMR pump (12) have been characterized in P. aeruginosa so far. Benzalkonium chloride is an inducer and substrate of MexCD-OprJ (20). It has been reported that cells of P. aeruginosa that were adapted to benzalkonium chloride showed resistance to other membrane-active agents (14). One reason for this adaptive resistance might be that the MexCD-OprJ multidrug efflux pump is induced by benzalkonium chloride. It is also possible that another membrane-related mechanism(s) is involved in this adaptive resistance (14). The PmpM pump is also responsible for resistance to benzalkonium chloride. The MIC of benzalkonium chloride for P. aeruginosa PAO1 was 64 μg/ml. Deletion of the mexCD-oprJ operon reduced the MIC to 20 μg/ml. Further deletion of the pmpM gene reduced the value to 5 μg/ml (Table 2). It seems that these two pumps are major systems for extrusion of benzalkonium chloride in P. aeruginosa.
The MICs of fluoroquinolones, fradiomycin, chlorhexidine, and rhodamine 6G, which are thought to be substrates of PmpM in E. coli cells (Table 1), were not changed by pmpM gene disruption in P. aeruginosa. On the other hand, introduction of the pmpM gene into the pmpM-deleted PMX6 cell resulted in an increase in the MICs not only of benzalkonium chloride, ethidium bromide, acriflavine, and TPPCl but also of fluoroquinolones (norfloxacin, ciprofloxacin, and ofloxacin). The observed MICs of benzalkonium chloride, ethidium bromide, and TPPCl for PMX6/pUPBE2T were about twofold higher than those for PMX52, perhaps due to a gene dosage effect. The increase in the MICs of fluoroquinolones for PMX6/pUPBE2T may also be due to the gene dosage effect.
Acknowledgments
We thank M. Varela of Eastern New Mexico University for critical reading of the manuscript prior to submission.
This work was supported by a grant-in-aid for Scientific Research on Priority Areas (C) “Genome Biology” from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Barekzi, N., K. Beinlich, T. T. Hoang, X.-Q. Pham, R. Karkhoff-Schweizer, and H. P. Schweizer. 2000. High-frequency Flp recombinase-mediated inversions of the oriC-containing region of the Pseudomonas aeruginosa genome. J. Bacteriol. 182:7070-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, J., Y. Morita, M. Nazmul Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2002. VmrA, a member of a novel class of Na+-coupled multidrug efflux pumps from Vibrio parahaemolyticus. J. Bacteriol. 184:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, W. P., and T. T. Kuo. 1993. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 21:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuanchuen, R., C. T. Narasaki, and H. P. Schweizer. 2002. The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J. Bacteriol. 184:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding, P. Z., and T. H. Wilson. 2001. The effect of modifications of the charged residues in the transmembrane helices on the transport activity of the melibiose carrier of Escherichia coli. Biochem. Biophys. Res. Commun. 285:348-354. [DOI] [PubMed] [Google Scholar]
- 6.Hoang, T. T., R. R. Karkhoff-Schwiezer, A. J. Kutchman, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 7.Kohler, T., M. Michea-Hamzehpour, U. Henze, N. Gotoh, L. K. Curty, and J. C. Pechere. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:345-354. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda, M., T. H. Wilson, and T. Tsuchiya. 2001. Regulation of galactoside transport by the PTS. J. Mol. Microbiol. Biotechnol. 3:381-384. [PubMed] [Google Scholar]
- 9.Kuroda, T., T. Shimamoto, K. Inaba, M. Tsuda, and T. Tsuchiya. 1994. Properties and sequences of the NhaA Na+/H+ antiporter of Vibrio parahaemolyticus. J. Biochem. 116:1030-1038. [DOI] [PubMed] [Google Scholar]
- 10.Lennox, E. S. 1995. Transduction of linked genetic characters of host by bacteriophage P1. Virology 1:190-206. [DOI] [PubMed] [Google Scholar]
- 11.Li, X. Z., H. Nikaido, and K. Poole. 1995. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1948-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, X. Z., K. Poole, and H. Nikaido. 2003. Contributions of MexAB-OprM and an EmrE homolog to intrinsic resistance of Pseudomonas aeruginosa to aminoglycosides and dyes. Antimicrob. Agents Chemother. 47:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, Y., T. Mima, Y. Komori, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. A new member of the tripartite multidrug efflux pumps, MexVW-OprM, in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 52:572-575. [DOI] [PubMed] [Google Scholar]
- 14.Loughlin, M. F., M. V. Jones, and P. A. Lambert. 2002. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J. Antimicrob. Chemother. 49:631-639. [DOI] [PubMed] [Google Scholar]
- 15.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1999. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:415-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. Evidence for chloramphenicol/H+ antiport in Cmr (MdfA) system of Escherichia coli and properties of the antiporter. J. Biochem. 124:187-193. [DOI] [PubMed] [Google Scholar]
- 18.Morita, Y., A. Kataoka, S. Shiota, T. Mizushima, and T. Tsuchiya. 2000. NorM of Vibrio parahaemolyticus is an Na+-driven multidrug efflux pump. J. Bacteriol. 182:6694-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita, Y., K. Kodama, S. Shiota, T. Mine, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 42:1778-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita, Y., Y. Komori, T. Mima, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2001. Construction of a series of mutants lacking all of the four major mex operons for multidrug efflux pumps or possessing each one of the operons from Pseudomonas aeruginosa PAO1: MexCD-OprJ is an inducible pump. FEMS Microbiol. Lett. 202:139-143. [DOI] [PubMed] [Google Scholar]
- 21.Nazmul Huda, M., J. Chen, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Gene cloning and characterization of VcrM, a Na+-coupled multidrug efflux pump, from Vibrio cholerae non-O1. Microbiol. Immunol. 47:419-427. [DOI] [PubMed] [Google Scholar]
- 22.Nazmul Huda, M., Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2001. Na+-driven multidrug efflux pump VcmA from Vibrio cholera non-O1, a non-halophilic bacterium. FEMS Microbiol. Lett. 203:235-239. [DOI] [PubMed] [Google Scholar]
- 23.Poole, K., K. Tetro, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X. Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-0prJ efflux operon in nfxB-type multidrug resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 25.Schweizer, H. P. 1994. A method for construction of bacterial hosts for lac-based cloning and expression vector: alpha-complementation and regulated expression. BioTechniques 17:452-456. [PubMed] [Google Scholar]
- 26.Schweizer, H. P. 1991. Improved broad-host-range lac-based plasmid vectors for the isolation and characterization of protein fusions in Pseudomonas aeruginosa. Gene 103:87-92. [DOI] [PubMed] [Google Scholar]
- 27.Sekiya, H., T. Mima, Y. Morita, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Functional cloning and characterization of a multidrug efflux pump, MexHI-OpmD, from a mutant of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:2990-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 29.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickyey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saler, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 30.West, S. E. H., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 128:81-86. [DOI] [PubMed] [Google Scholar]