Wild-type and H12A-mutant class II phospholipase D from L. intermedia venom were crystallized; the crystals diffracted to maximum resolutions of 1.95 and 1.60 Å, respectively.
Keywords: phospholipases D, Loxosceles intermedia, spider venoms
Abstract
Phospholipases D are the major dermonecrotic component of Loxosceles venom and catalyze the hydrolysis of phospholipids, resulting in the formation of lipid mediators such as ceramide-1-phosphate and lysophosphatidic acid which can induce pathological and biological responses. Phospholipases D can be classified into two classes depending on their catalytic efficiency and the presence of an additional disulfide bridge. In this work, both wild-type and H12A-mutant forms of the class II phospholipase D from L. intermedia venom were crystallized. Wild-type and H12A-mutant crystals were grown under very similar conditions using PEG 200 as a precipitant and belonged to space group P1211, with unit-cell parameters a = 50.1, b = 49.5, c = 56.5 Å, β = 105.9°. Wild-type and H12A-mutant crystals diffracted to maximum resolutions of 1.95 and 1.60 Å, respectively.
1. Introduction
Envenomation by members of the genus Loxosceles (brown spiders), considered to be the most dangerous form of arachnidism, is a serious public health problem in both North and South America (Santi Ferrara et al., 2009 ▶). Loxosceles venom can cause local dermonecrosis with gravitational spreading and systemic manifestations such as thrombocytopaenia, haemolysis and acute renal failure that can lead to death (Futrell, 1992 ▶; da Silva et al., 2004 ▶).
Several toxic proteins present in Loxosceles spp. venoms have been identified and biochemically characterized (da Silva et al., 2004 ▶; Gremski et al., 2010 ▶). Members of the phospholipase D family are abundant in the venoms of several Loxosceles spp. and contribute significantly to the typical response after envenomation (Kalapothakis et al., 2007 ▶; Senff-Ribeiro et al., 2008 ▶).
Phospholipases D (30–35 kDa), also referred to as dermonecrotic toxins, catalyze the hydrolysis of sphingomyelin and (lyso) glycerophospholipids, resulting in the formation of bioactive mediators such as ceramide-1-phosphate and lysophosphatidic acid which play a role in several pathological and biological responses (Van Meeteren et al., 2004 ▶; Moolenaar et al., 2004 ▶; Lee & Lynch, 2005 ▶). As proposed by Murakami et al. (2006 ▶), spider-venom phospholipases D can be classified into two classes. Members of class I possess a single disulfide bridge and contain an extended hydrophobic loop, whereas class II proteins contain an additional intra-chain disulfide bridge and display decreased catalytic activity towards phospholipids. To date, only the phospholipase D from L. laeta venom, a member of class I, has been structurally characterized (Murakami et al., 2005 ▶), despite the clinical importance of phospholipases D in loxoscelism. Based on its crystal structure, an acid–base catalytic mechanism was proposed in which His12 and His47 play key roles and are supported by a network of hydrogen bonds between Asp34, Asp52, Trp230, Asp233 and Asn252 (Murakami et al., 2005 ▶).
The recombinant dermonecrotic toxin (LiRecDT1) obtained from a cDNA library of the L. intermedia venom gland is able to directly induce renal injuries in mice and the haemolysis of human erythrocytes in vitro, suggesting that this protein is directly involved in the the nephrotoxicity and haematological disturbances evoked during envenomation by Loxosceles spiders (Chaim et al., 2006 ▶; Chaves-Moreira et al., 2009 ▶). Mutation of the catalytic residue His12 to Ala abolishes both the nephrotoxic effect of LiRecDT1 in mice and the haemolysis of human erythrocytes (Kusma et al., 2008 ▶; Chaves-Moreira et al., 2009 ▶).
The present report describes the crystallization and preliminary crystallographic analysis of recombinant wild-type (LiRecDT1) and mutant (LiRecDT1 H12A) dermonecrotic toxin from L. intermedia venom, which belongs to the class II phospholipases D. The structural characterization of LiRecDT1 will be essential to shed light on the structural determinants of the functional differentiation between members of the class I and class II phospholipases D.
2. Materials and methods
2.1. Expression and purification
DNA corresponding to the wild-type (LiRecDT1) and mutated (LiRecDT1 H12A) forms of the mature phospholipase D was cloned into pET-14b vector (Novagen, Madison, USA) as described by Chaim et al. (2006 ▶) and Kusma et al. (2008 ▶). Both recombinant constructs were expressed as fusion proteins with a 6×His tag at the N-terminus and a 13-amino-acid linker including a thrombin site between the 6×His tag and the mature protein. pET-14b/L. intermedia cDNA constructs were transformed into One Shot Escherichia coli BL21 (DE3) pLysS competent cells (Invitrogen) and plated on LB agar plates containing 100 mg ml−1 ampicillin and 34 mg ml−1 chloramphenicol. A single colony was inoculated into 50 ml LB broth (plus antibiotics) and allowed to grow overnight at 310 K. A 10 ml portion of this overnight culture was grown in 1 l LB broth/ampicillin/chloramphenicol at 310 K until the OD at 550 nm reached 0.5. IPTG (isopropyl β-d-1-thiogalactopyranoside) was added to a final concentration of 0.05 mM and the culture was induced by incubation for an additional 3.5 h at 303 K. Cells were harvested by centrifugation (400g, 7 min) and the pellet was frozen at 253 K overnight.
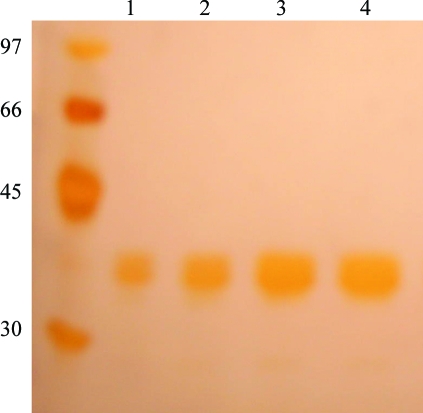
The cell suspensions were thawed and were additionally disrupted by six 10 s cycles of sonication at low intensity. The lysed materials were centrifuged (20 000g, 20 min) and the supernatants were incubated with 1 ml Ni2+–NTA agarose beads for 1 h at 277 K (with gentle agitation). The suspensions were loaded onto a column and the packed gel was exhaustively washed with 50 mM sodium phosphate pH 8.0, 500 mM NaCl, 20 mM imidazole until the OD at 280 nm reached 0.01. The recombinant proteins were eluted with 10 ml elution buffer (50 mM sodium phosphate pH 8.0, 500 mM NaCl, 250 mM imidazole) and 1 ml fractions were collected and analyzed by 12.5% SDS–PAGE under reducing conditions (Fig. 1 ▶). The fractions were pooled and dialyzed against phosphate-buffered saline (PBS). Site-directed mutagenesis did not alter the correct folding of the brown spider phospholipase D as assessed by circular-dichroism and fluorescence experiments (results not shown).
Figure 1.
Silver-stained SDS–PAGE (12%) of purified samples of wild-type and H12A-mutant dermonecrotic toxin from L. intermedia. Lane 1, molecular-weight markers (kDa); lanes 2 and 4, purified LiRecDT1 (18 and 34 µg, respectively); lanes 3 and 5, purified LiRecDT1 H12A (18 and 34 µg, respectively).
2.2. Crystallization
The wild-type and mutant proteins were initially crystallized by vapour diffusion in sitting drops using a Cartesian HoneyBee 963 system (Genomic Solutions) at 291 K. For initial screening, 1 µl protein solution at a concentration of 17 mg ml−1 for the wild type and of 9 mg ml−1 for the H12A mutant was mixed with 1 µl screening solution and equilibrated over a reservoir containing 100 µl of the latter solution. Small crystals of wild-type LiRecDT1 were observed in the condition 0.1 M Tris–HCl pH 8, 35%(v/v) PEG 200, which was refined by varying the PEG 200 concentration versus the pH using the hanging-drop method. The best wild-type LiRecDT1 crystals were observed in drops consisting of 2 µl protein solution (17 mg ml−1) and 2 µl reservoir solution equilibrated over 1 ml reservoir solution [0.1 M Tris–HCl pH 7.5, 40%(v/v) PEG 200] (Fig. 2 ▶ a). Crystals of the H12A mutant were grown in a very similar condition consisting of 0.1 M Tris–HCl pH 7.5 and 35%(v/v) PEG 200 (Fig. 2 ▶ b).
Figure 2.
Microphotograph of crystals of (a) wild-type and (b) mutant dermonecrotic toxin from L. intermedia.
2.3. X-ray diffraction analysis
LiRecDT1 and LiRecDT1 H12A crystals were directly flash-cooled in a 100 K nitrogen-gas stream. X-ray diffraction data were collected on the W01B-MX2 beamline at the Brazilian Synchrotron Light Laboratory (Campinas, Brazil). The LiRecDT1 crystal was exposed for 60 s per 2° rotation in ϕ with the crystal-to-detector distance set to 100 mm. The LiRecDT1 H12A crystal was exposed for 20 s per 1° rotation in ϕ with the crystal-to-detector set to 69 mm. A total of 180 and 300 images were collected from the LiRecDT1 and the LiRecDT1 H12A crystals, respectively. The data were indexed, integrated and scaled using the DENZO and SCALEPACK programs from the HKL-2000 package (Otwinowski & Minor, 1997 ▶). Data-processing statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics.
Values in parentheses are for the last resolution shell.
| LiRecDT1 | LiRecDT1 H12A | |
|---|---|---|
| Data collection | ||
| Temperature (K) | 100 | 100 |
| Radiation source | Brazilian Synchrotron Light Laboratory | Brazilian Synchrotron Light Laboratory |
| Beamline | W01B-MX2 | W01B-MX2 |
| Wavelength (Å) | 1.458 | 1.458 |
| Detector | MAR Mosaic 225 mm | MAR Mosaic 225 mm |
| Space group | P1211 | P1211 |
| Unit-cell parameters (Å, °) | a = 50.08, b = 49.43, c = 56.59, β = 105.88 | a = 49.58, b = 49.46, c = 56.40, β = 105.56 |
| Resolution range (Å) | 30.0–2.0 (2.07–2.00) | 30.0–1.60 (1.66–1.60) |
| Rmerge (%)† | 12.1 (49.4) | 7.3 (37.0) |
| 〈I/σ(I)〉 | 9.3 (2.4) | 19.5 (2.9) |
| Data completeness (%) | 99.5 (98.1) | 98.9 (92.4) |
| No. of unique reflections | 18148 (1765) | 34632 (3241) |
| Multiplicity | 3.1 (2.7) | 5.1 (3.3) |
| Data analysis | ||
| VM (Å3 Da−1) | 2.25 | 2.22 |
| Solvent content (%) | 45.25 | 44.63 |
| Molecules per asymmetric unit | 1 | 1 |
R
merge = 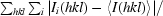
 , where Ii(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations I of reflection hkl.
, where Ii(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations I of reflection hkl.
3. Results and discussion
LiRecDT1 and LiRecDT1 H12A crystals diffracted to resolutions of 1.95 and 1.60 Å, respectively. Although the mutant protein crystal diffracted better than the native protein crystal, the H12A mutation did not alter the crystal packing or the unit-cell symmetry and parameters. The LiRecDT1 and LiRecDT1 H12A data sets were indexed in the monoclinic crystal system. The presence of systematic absences indicated that the crystals belonged to space group P1211. Both crystals possessed highly similar unit-cell parameters (Table 1 ▶). The Matthews coefficient calculated for the LiRecDT1 crystal was 2.25 Å3 Da−1, corresponding to a solvent content of 45% (Matthews, 1968 ▶). Considering the molecular weight of 30 000 Da, one molecule is present in the asymmetric unit of both crystals. Data-processing statistics for both data sets are presented in Table 1 ▶.
The atomic coordinates of the phospholipase D from L. laeta venom (PDB code 1xx1; Murakami et al., 2005 ▶), which displays a sequence identity of 58% with LiRecDT1, were used to generate a search model and molecular-replacement calculations were carried out using the program MOLREP in the resolution range 15.0–3.0 Å (Vagin & Teplyakov, 2010 ▶). A solution was obtained for one molecule in the asymmetric unit in space group P1211. Analysis of the packing contacts and steric clashes clearly showed that this was the correct solution. REFMAC5 (Murshudov et al., 1997 ▶) was used for rigid-body refinement of this solution in the resolution range 30.0–1.95 Å (excluding 5% of reflections for R free calculations), resulting in a correlation coefficient of 49.2, a score of 0.675 (the score of the next highest peak was 0.338) and an R factor of 45.6% (R free = 49.1%). Structure refinement and analysis are currently in progress. Determination of the LiRecDT1 H12A crystal structure will be performed using the final model of wild-type LiRecDT1.
Acknowledgments
This work was supported by grants from Secretaria de Estado de Ciência, Tecnologia e Ensino Superior (SETI) do Paraná, Fundação Araucária-PR, TWAS, FAPESP, CNPq and CAPES-Brazil.
References
- Chaim, O. M., Sade, Y. B., da Silveira, R. B., Toma, L., Kalapothakis, E., Chávez-Olórtegui, C., Mangili, O. C., Gremski, W., von Dietrich, C. P., Nader, H. B. & Veiga, S. S. (2006). Toxicol. Appl. Pharmacol. 211, 64–77. [DOI] [PubMed]
- Chaves-Moreira, D., Chaim, O. M., Sade, Y. B., Paludo, K. C., Gremski, L. H., Donatti, L., de Moura, J., Mangili, O. C., Gremski, W., da Silveira, R. B., Senff-Ribeiro, A. & Veiga, S. S. (2009). J. Cell. Biochem. 107, 655–666. [DOI] [PubMed]
- da Silva, P. H., da Silveira, R. B., Appel, M. H., Mangili, O. C., Grewski, W. & Veiga, S. S. (2004). Toxicon, 44, 693–709. [DOI] [PubMed]
- Futrell, J. M. (1992). Am. J. Med. Sci. 304, 261–267. [DOI] [PubMed]
- Gremski, L. H., da Silveira, R. B., Chaim, O. M., Probst, C. M., Ferrer, V. P., Nowatzki, J., Weinschutz, H. C., Madeira, H. M., Gremski, W., Nader, H. B., Senff-Ribeiro, A. & Veiga, S. S. (2010). Mol. Biosyst. 6, 2403–2416. [DOI] [PubMed]
- Kalapothakis, E., Chatzaki, M., Gonçalves-Dornelas, H., de Castro, C. S., Silvestre, F. G., Laborne, F. V., de Moura, J. F., Veiga, S. S., Chávez-Olórtegui, C., Granier, C. & Barbaro, K. C. (2007). Toxicon, 50, 938–946. [DOI] [PubMed]
- Kusma, J., Chaim, O. M., Wille, A. C., Ferrer, V. P., Sade, Y. B., Donatti, L., Gremski, W., Mangili, O. C. & Veiga, S. S. (2008). Biochimie, 90, 1722–1736. [DOI] [PubMed]
- Lee, S. & Lynch, K. R. (2005). Biochem. J. 391, 317–323. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Meeteren, L. A. van, Frederiks, F., Giepmans, B. N., Pedrosa, M. F., Billington, S. J., Jost, B. H., Tambourgi, D. V. & Moolenaar, W. H. (2004). J. Biol. Chem. 279, 10833–10836. [DOI] [PubMed]
- Moolenaar, W. H., van Meeteren, L. A. & Giepmans, B. N. (2004). Bioessays, 26, 870–881. [DOI] [PubMed]
- Murakami, M. T., Fernandes-Pedrosa, M. F., de Andrade, S. A., Gabdoulkhakov, A., Betzel, C., Tambourgi, D. V. & Arni, R. K. (2006). Biochem. Biophys. Res. Commun. 342, 323–329. [DOI] [PubMed]
- Murakami, M. T., Fernandes-Pedrosa, M. F., Tambourgi, D. V. & Arni, R. K. (2005). J. Biol. Chem. 280, 13658–13664. [DOI] [PubMed]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Santi Ferrara, G. I. de, Fernande-Pedrosa, M. F., Junqueira-de-Azevedo, I. L. M., Gonçalves-de-Andrade, R. M., Portaro, F. C. V., Manzoni-de-Almeida, D. M., Murakami, M. T., Arni, R. K., van den Berg, C. W., Ho, P. L. & Tambourg, D. V. (2009). Toxicon, 53, 743–753. [DOI] [PubMed]
- Senff-Ribeiro, A., Henrique da Silva, P., Chaim, O. M., Gremski, L. H., Paludo, K. S., Bertoni da Silveira, R., Gremski, W., Mangili, O. C. & Veiga, S. S. (2008). Biotechnol. Adv. 26, 210–218. [DOI] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]