Piratoxin I, a noncatalytic and myotoxic Lys49-phospholipase A2 from B. pirajai venom, was cocrystallized with the inhibitor caffeic acid and a data set was collected to a resolution of 1.65 Å. The electron-density map unambiguously indicated that three inhibitor molecules interact with the C-terminus of the protein.
Keywords: Lys49-phospholipases A2, caffeic acid, snake venoms, piratoxin I
Abstract
Phospholipases A2 (PLA2s) are one of the main components of bothropic venoms; in addition to their phospholipid hydrolysis action, they are involved in a wide spectrum of pharmacological activities, including neurotoxicity, myotoxicity and cardiotoxicity. Caffeic acid is an inhibitor that is present in several plants and is employed for the treatment of ophidian envenomations in the folk medicine of many developing countries; as bothropic snake bites are not efficiently neutralized by conventional serum therapy, it may be useful as an antivenom. In this work, the cocrystallization and preliminary X-ray diffraction analysis of the Lys49-PLA2 piratoxin I from Bothrops pirajai venom in the presence of the inhibitor caffeic acid (CA) are reported. The crystals diffracted X-rays to 1.65 Å resolution and the structure was solved by molecular-replacement techniques. The electron-density map unambiguously indicated the presence of three CA molecules that interact with the C-terminus of the protein. This is the first time a ligand has been observed bound to this region and is in agreement with various experiments previously reported in the literature.
1. Introduction
Envenomation resulting from snake bites is an important public health problem in rural areas of tropical and subtropical countries in Asia, Africa, Oceania and Latin America and is considered as a neglected tropical disease by the World Health Organization. In spite of the majority of deaths from snake bites occurring in South and South East Asia and sub-Saharan Africa (Kasturiratne et al., 2008 ▶) these accidents are also an important health problem in Latin America as they may cause permanent tissue loss and amputation of the affected limb (Gutiérrez & Lomonte, 1995 ▶). Bites by snakes of the Bothrops genus are responsible for more than 85% of all ophidian accidents reported in Latin America (Fundação Nacional de Saúde, 2001 ▶; de Oliveira, 2009 ▶) and lead to drastic local tissue damage (Gutiérrez & Lomonte, 1995 ▶).
Phospholipases A2 (PLA2s) are one of the main components of the venom of these snakes (Fox & Serrano, 2008 ▶) and, in addition to their phospholipid hydrolysis action, are involved in a wide spectrum of pharmacological activities, including neurotoxicity, myotoxicity and cardiotoxicity (Bon et al., 1979 ▶; Gutiérrez et al., 1991 ▶; Fletcher et al., 1981 ▶). An important subgroup of PLA2s, the Lys49-PLA2s, which exhibit natural replacements of the Tyr28 and Asp49 residues by Asn28 and Lys49, respectively (Holland et al., 1990 ▶; Fernandes et al., 2010 ▶), are found in snakes of the Viperidae family. These substitutions hinder the binding of Ca2+ ion, an essential cofactor for PLA2 catalysis, which results in an inability of the Lys49-PLA2s to promote phospholipid hydrolysis (Arni & Ward, 1996 ▶). Despite their catalytic inactivity, Lys49-PLA2s play an important role in ophidic accidents, inducing drastic local myonecrosis by a Ca2+-independent mechanism (Gutiérrez & Lomonte, 1995 ▶). Synthetic peptides and site-directed mutagenesis experiments have shown that segment 115–129 of the C-terminal region is responsible for this myotoxic activity (Ward et al., 2002 ▶; Lomonte et al., 2003 ▶; Chioato et al., 2007 ▶). Recently, a myotoxic site of Lys49-PLA2s specific to snakes of the Bothrops genus that contains the C-terminal residues Lys115 and Arg118 and one residue from the N-terminal region (Lys20) has been proposed (dos Santos et al., 2009 ▶).
However, this pronounced local myotoxic effect is not efficiently neutralized by conventional serum therapy, the action of which is related to systemic mechanisms. Although a successful approach, in addition to this limited effectiveness in protecting against this rapid local tissue-damaging effect serum therapy presents other drawbacks such as (i) limited or lack of access to antivenoms in rural areas of developing countries, where most accidents occur, (ii) adverse reactions originating in patients owing to the infusion of animal proteins and (iii) significant variations in venom composition and antigenic reactivity owing to the geographic and taxonomic diversity of snakes (Soares et al., 2005 ▶). Therefore, an extensive search for and identification of new compounds, either synthetic or natural, that may be useful to complement antivenom treatment is extremely important.
In folk medicine, especially in developing countries, several vegetal species are employed for the treatment of ophidian envenomations in communities that lack prompt access to serum therapy (Soares et al., 2005 ▶; Samy et al., 2008 ▶). In recent years, a large number of studies have investigated the effects of several plants on snakebites, including the isolation and characterization of their active constituents and the elucidation of their possible mechanisms of action (Mors et al., 2000 ▶; Soares et al., 2005 ▶; Cintra-Francischinelli et al., 2008 ▶; Ticli et al., 2005 ▶). Caffeic acid (CA) is a cinnamic acid derivative with exceptional biochemical reactivity that is present in several plants with anti-snake venom properties such as Prestonia coalita, Strychnos nux-vomica, Taraxacum officinale and Vernonia condensate (Mors et al., 2000 ▶; Soares et al., 2005 ▶). Furthermore, crystalline caffeic acid derivatives have been demonstrated to be antidotes for snake venoms by oral or parenteral administration (Agoro, 1978 ▶).
In this work, we report the crystallization, collection of X-ray diffraction data and molecular-replacement solution of piratoxin I (PrTX-I), a basic noncatalytic and myotoxic Lys49-PLA2 from B. pirajai venom, complexed with caffeic acid. The final crystallographic model of this complex may provide insight into the mechanisms that lead to inhibition of the myotoxicity of snake-venom PLA2s.
2. Materials and methods
2.1. Protein purification and crystallization
PrTX-I was isolated from B. pirajai snake venom by gel-filtration and ion-exchange chromatography as described previously (Soares et al., 2001 ▶). A lyophilized sample of PrTX-I was dissolved in ultrapure water to a concentration of 15 mg ml−1. CA was purchased from Sigma–Aldrich and was dissolved in 50% ethanol to give an 8:1 molar ratio of inhibitor:protein. Crystals were obtained by the hanging-drop vapour-diffusion method (McPherson, 2003 ▶); the drops consisted of 1 µl protein solution, 0.2 µl CA solution and 0.8 µl reservoir solution and were equilibrated against 500 µl of the same reservoir solution. The best crystals were obtained after an optimization process from the native protein crystallization conditions (dos Santos et al., 2009 ▶); the reservoir solution consisted of 30% polyethylene glycol 4000, 100 mM Tris–HCl pH 8.1 and 200 mM lithium sulfate and crystals were obtained after one month at 291 K (Fig. 1 ▶).
Figure 1.
Crystals of PrTX-I complexed with CA.
2.2. X-ray data collection and processing
X-ray diffraction data were collected from a single PrTX-I–CA crystal at a wavelength of 1.4586 Å (at 100 K) using a synchrotron-radiation source [MX2 station, Laboratório Nacional de Luz Síncroton (LNLS), Campinas, Brazil] and a MAR CCD imaging-plate detector (MAR Research). A crystal was mounted in a nylon loop and flash-cooled in a stream of nitrogen at 100 K without using any cryoprotectant. The crystal-to-detector distance was 85 mm and an oscillation range of 1° was used, resulting in the collection of a total of 141 images. Data processing was carried out at 1.65 Å resolution using the HKL program suite (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
The data-collection statistics are shown in Table 1 ▶. The crystals belonged to the orthorhombic space group P212121, with unit-cell parameters a = 39.2, b = 72.0, c = 44.6 Å, β = 102.8°. The data set was 93.8% complete at 1.65 Å resolution, with an R merge of 6.5%. Calculations based on the protein molecular weight indicated the presence of two molecules in the asymmetric unit. This corresponds to a Matthews coefficient (Matthews, 1968 ▶) V M of 2.20 Å3 Da−1 and a calculated solvent content of 44.2%. These values are within the typical range for protein crystals, assuming a value of 0.74 cm3 g−1 for the protein partial specific volume. The crystal structure of PrTX-I–CA was determined by molecular-replacement techniques implemented in the program MOLREP (Vagin & Teplyakov, 1997 ▶) from the CCP4i program package (Potterton et al., 2003 ▶) using the coordinates of bothropstoxin I complexed with polyethylene glycol 4000 (PDB code 3iq3; Fernandes et al., 2010 ▶) as a search model and confirmed the presence of a dimer in the asymmetric unit.
Table 1. X-ray diffraction data-collection and processing statistics.
Values in parentheses are for the highest resolution shell. Data were processed using the HKL suite (Otwinowski & Minor, 1997 ▶).
| Unit-cell parameters (Å, °) | a = 39.2, b = 72.0, c = 44.6, β = 102.8 |
| Space group | P21 |
| Resolution (Å) | 40–1.65 (1.73–1.65) |
| Unique reflections | 27856 (3444) |
| Completeness (%) | 94.6 (93.8) |
| Rmerge (%)† | 6.5 (39.5) |
| Radiation source | MX2 station, LNLS |
| Data-collection temperature (K) | 100 |
| Average I/σ(I) | 27.4 (2.34) |
| Multiplicity | 2.8 (2.7) |
| Matthews coefficient VM (Å3 Da−1) | 2.20 |
| Molecules in the asymmetric unit | 2 |
| Solvent content (%) | 44.2 |
R
merge = 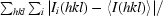
 , where Ii(hkl) is the intensity of an individual measurement of the reflection with Miller indices hkl and 〈I(hkl)〉 is the mean intensity of that reflection. Calculated using reflections with I > −3σ(I).
, where Ii(hkl) is the intensity of an individual measurement of the reflection with Miller indices hkl and 〈I(hkl)〉 is the mean intensity of that reflection. Calculated using reflections with I > −3σ(I).
It has been demonstrated for the structures of dimeric Lys49-PLA2s that apo structures belong to space group P3121, while complexed forms belong to space groups P21 or P212121 (dos Santos et al., 2009 ▶). The space-group change arises from conformational changes when a ligand is bound to Lys49-PLA2s (dos Santos et al., 2009 ▶). Since space group P212121 is observed for the PrTX-I–CA complex, it is possible to suggest that inhibitor binding has led to changes in the PrTX-I quaternary structure.
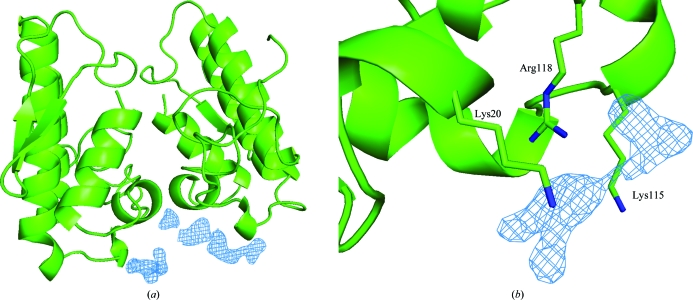
Observation of the electron-density map unambiguously indicated the presence of three CA molecules in the C-terminal region (Fig. 2 ▶). As proposed previously, this indicates the presence of a myotoxic site specific to the Lys49-PLA2s of snakes from the Bothrops genus that is formed by two residues from the C-terminal region (Lys115 and Arg118) and one residue from the N-terminal region (Lys20) (dos Santos et al., 2009 ▶). Here, this myotoxic site is in the neighbourhood of a molecule of caffeic acid in one of the monomers. Thus, for the first time, the structure of a Lys49-PLA2–inhibitor complex supports the hypothesis of a myotoxic site in its C-terminus in addition to the previously demonstrated classic binding site (known as the active site; Marchi-Salvador et al., 2009 ▶).
Figure 2.
(a) Electron-density difference map contoured at 1.0 standard deviation of the C-terminal region of PrTX-I–CA where electron density that corresponds to caffeic acid molecules was found. (b) Detailed view of the myotoxic site of the Bothrops genus including a difference electron-density map that corresponds to a caffeic acid molecule. The caffeic acid molecules were not considered in the calculation of the electron-density maps. This figure was drawn using PyMOL (DeLano, 2002 ▶).
The crystallization and X-ray diffraction analysis of PrTX-I complexed with rosmarinic acid (RA; dos Santos et al., 2010 ▶), an ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid, has recently been reported. RA is found in several plants that have antivenom properties such as Cordia verbenacea and several species of the genera Echinacea and Perilla (Soares et al., 2005 ▶; Mors et al., 2000 ▶). Interestingly, RA binds in a different region to the CA molecule. Detailed comparative structural studies of PrTX-I–CA and PrTX-I–RA may thus provide new and important details of how these vegetal molecules lead to toxin inhibition.
In conclusion, a systematic study of PrTX-I complexed with different ligands may lead to the development of effective inhibitors that can be used in biotechnological applications, as helpful supplemental treatments to serum therapy and as important models for the synthesis of new drugs.
Acknowledgments
The authors gratefully acknowledge financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Laboratório Nacional de Luz Síncrotron (LNLS, Campinas-SP).
References
- Agoro, J. W. (1978). US Patent 4124724.
- Arni, R. K. & Ward, R. J. (1996). Toxicon, 34, 827–841. [DOI] [PubMed]
- Bon, C., Changeux, J.-P., Jeng, T.-W. & Fraenkel-Conrat, H. (1979). Eur. J. Biochem. 99, 471–481. [DOI] [PubMed]
- Chioato, L., Aragão, E. A., Lopes Ferreira, T., Medeiros, A. I., Faccioli, L. H. & Ward, R. J. (2007). Biochim. Biophys. Acta, 1768, 1247–1257. [DOI] [PubMed]
- Cintra-Francischinelli, M., Silva, M. G., Andréo-Filho, N., Gerenutti, M., Cintra, A. C., Giglio, J. R., Leite, G. B., Cruz-Höfling, M. A., Rodrigues-Simioni, L. & Oshima-Franco, Y. (2008). Phytother. Res. 22, 784–790. [DOI] [PubMed]
- DeLano, W. L. (2002). PyMOL http://www.pymol.org.
- dos Santos, J. I., Santos-Filho, N. A., Soares, A. M. & Fontes, M. R. M. (2010). Acta Cryst. F66, 699–701. [DOI] [PMC free article] [PubMed]
- dos Santos, J. I., Soares, A. M. & Fontes, M. R. (2009). J. Struct. Biol. 167, 106–116. [DOI] [PubMed]
- Fernandes, C. A., Marchi-Salvador, D. P., Salvador, G. M., Silva, M. C., Costa, T. R., Soares, A. M. & Fontes, M. R. (2010). J. Struct. Biol. 171, 31–43. [DOI] [PubMed]
- Fletcher, J. E., Rapuano, B. E., Condrea, E., Yang, C. C. & Rosenberg, P. (1981). Toxicol. Appl. Pharmacol. 59, 375–388. [DOI] [PubMed]
- Fox, J. W. & Serrano, S. M. (2008). Proteomics, 8, 909–920. [DOI] [PubMed]
- Fundação Nacional de Saúde (2001). Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos Brasilia: Fundação Nacional de Saúde
- Gutiérrez, J. M. & Lomonte, B. (1995). Toxicon, 33, 1405–1424. [DOI] [PubMed]
- Gutiérrez, J. M., Núñez, J., Díaz, C., Cintra, A. C., Homsi-Brandeburgo, M. I. & Giglio, J. R. (1991). Exp. Mol. Pathol. 55, 217–229. [DOI] [PubMed]
- Holland, D. R., Clancy, L. L., Muchmore, S. W., Ryde, T. J., Einspahr, H. M., Finzel, B. C., Heinrikson, R. L. & Watenpaugh, K. D. (1990). J. Biol. Chem. 265, 17649–17656. [DOI] [PubMed]
- Kasturiratne, A., Wickremasinghe, A. R., de Silva, N., Gunawardena, N. K., Pathmeswaran, A., Premaratna, R., Savioli, L., Lalloo, D. G. & de Silva, H. J. (2008). PLoS Med. 5, e218. [DOI] [PMC free article] [PubMed]
- Lomonte, B., Angulo, Y. & Santamaria, C. (2003). Toxicon, 42, 307–312. [DOI] [PubMed]
- Marchi-Salvador, D. P., Fernandes, C. A., Silveira, L. B., Soares, A. M. & Fontes, M. R. (2009). Biochim. Biophys. Acta, 1794, 1583–1590. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- McPherson, A. (2003). Introduction to Macromolecular Crystallography. Hoboken: Wiley.
- Mors, W. B., Nascimento, M. C., Pereira, B. M. & Pereira, N. A. (2000). Phytochemistry, 55, 627–642. [DOI] [PubMed]
- Oliveira, R. C. W. de (2009). Animais Peçonhentos do Brasil: Biologia, Clínica e Terapêutica dos Envenenamentos São Paulo: Sarvier.
- Otwinoswki, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Potterton, E., Briggs, P., Turkenburg, M. & Dodson, E. (2003). Acta Cryst. D59, 1131–1137. [DOI] [PubMed]
- Samy, R. P., Thwin, M. M., Gopalakrishnakone, P. & Ignacimuthu, S. (2008). J. Ethnopharmacol. 115, 302–312. [DOI] [PubMed]
- Soares, A. M., Andrião-Escarso, S. H., Bortoleto, R. K., Rodrigues-Simioni, L., Arni, R. K., Ward, R. J., Gutiérrez, J. M. & Giglio, J. R. (2001). Arch. Biochem. Biophys. 387, 188–196. [DOI] [PubMed]
- Soares, A. M., Ticli, F. K., Marcussi, S., Lourenco, M. V., Januario, A. H., Sampaio, S. V., Giglio, J. R., Lomonte, B. & Pereira, P. S. (2005). Curr. Med. Chem. 12, 2625–2641. [DOI] [PubMed]
- Ticli, F. K., Hage, L. I., Cambraia, R. S., Pereira, P. S., Magro, A. J., Fontes, M. R., Stabeli, R. G., Giglio, J. R., Franca, S. C., Soares, A. M. & Sampaio, S. V. (2005). Toxicon, 46, 318–327. [DOI] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Ward, R. J., Chioato, L., de Oliveira, A. H., Ruller, R. & Sá, J. M. (2002). Biochem. J. 362, 89–96. [DOI] [PMC free article] [PubMed]