Abstract
Our mechanistic understanding of the conversion of vitamin B12 into coenzyme B12 (a.k.a. adenosylcobalamin, AdoCbl) has been substantially advanced in recent years. Insights into the multiple roles played by ATP:Cob(I)alamin adenosyltransferase (ACA) enzymes have emerged through the crystallographic, spectroscopic, biochemical, and mutational analyses of wild-type and variant proteins. ACA enzymes circumvent the thermodynamic barrier posed by the very low redox potential associated with the reduction of cob(II)alamin to cob(I)alamin by generating a unique four-coordinate cob(II)alamin intermediate that is readily converted to cob(I)alamin by physiological reductants. ACA enzymes not only synthesize AdoCbl, they deliver it to the enzymes that use it, and, in some cases, enzymes whose function is needed to maintain the fidelity of the AdoCbl delivery process have been identified. Advances in our understanding of ACA enzyme function have provided valuable insights into the role of specific residues, and into why substitutions of these residues have profound negative effects on human health. From an applied science standpoint, a better understanding of the adenosylation reaction may lead to more efficient ways of synthesizing AdoCbl.
Keywords: Coenzyme B12, Adenosylcobalamin, Corrinoid adenosylation, Cyclic tetrapyrroles, Vitamin metabolism
General features of adenosylcobalamin
Coenzyme B12 (5′-deoxyadenosylcobalamin, AdoCbl) is a structurally complex molecule that is essential to animals, lower eukaryotes, and many prokaryotes (Escalante-Semerena & Warren 2008). This unique coenzyme is involved in catabolic and anabolic processes, including DNA synthesis. In mammals, AdoCbl is involved in the catabolism of amino acids, cholesterol and odd-chain fatty acids (Banerjee & Vlasie 2002; Banerjee & Chowdhury 1999; Rosenblatt & Fenton 1999). In humans, the inability to generate AdoCbl leads to methylmalonic aciduria, a fatal disorder associated with developmental retardation and infant mortality (Dobson et al. 2002; Fenton & Rosenberg 1978; Ciani et al. 2000; Cavicchi et al. 2006).
AdoCbl is one of the most complex coenzymes in nature (Fig. 1), and belongs to the family of cyclic tetrapyrroles, which include heme, chlorophyll, and factor F430 (Battersby 2000; Montforts & Glasenapp-Breiling 2002). In AdoCbl, the cobalt ion is held in place by four equatorial nitrogen ligands from the pyrrole moieties. This Co-containing cyclic tetrapyrole ring is known as the corrin ring. In nature, corrin rings are found with a variety of upper (Coβ) and lower (Coα) axial ligands. In the case of AdoCbl, the upper ligand is a 5′-deoxyadenosyl (Ado) moiety, and the lower ligand is a nitrogen atom from 5,6-dimethylbenzimidazole (DMB) (Lenhert 1968). The lower ligand base is tethered to the corrin ring via a structure known as the nucleotide loop, which is unique in several respects. Firstly, the N-glycosidic bond between DMB and the ribose is in the α configuration, rather than the β configuration usually found in nucleotides. Secondly, the phosphate group of the nucleotide is attached to the 3′ hydroxyl rather than the usual 5′ hydroxyl group of the ribose moiety. And thirdly, AdoCbl is one of only three coenzymes known to contain a phosphodiester bond; the other two are coenzyme F420 (Eirich et al. 1978), and methanopterin (van Beelen et al. 1984). AdoCbl-dependent enzymes bind the coenzyme in two different conformations known as the base-on (DMB-on) and base-off (DMB-off) conformation (Fig. 1).
Fig. 1.
Schematic representation of adenosylcobalamin (AdoCbl). The adenosyl moiety of AdoCbl is shown in red. The corrin ring, nucleotide loop, and the lower ligand DMB is known as Cbl
The lability of the organometallic (Co-C) bond between the Co ion and the Ado upper ligand lies at the heart of the reactivity of AdoCbl (Fig. 1). This Co-C bond is unique in nature because unlike other organometallic bonds it is relatively stable, but easy to break. The properties of this Co-C bond allows AdoCbl to facilitate intramolecular rearrangements (Banerjee & Chowdhury 1999; Buckel et al. 1999), deaminations (Bandarian & Reed 1999), dehydrations (Toraya 1999), reductions (Fontecave 1998; Fontecave & Mulliez 1999), and reductive dehalogenations (Wohlfarth & Diekert 1999) via radical intermediates.
Formation of the Co-C bond; the corrinoid adenosylation pathway
AdoCbl is the coenzymic form of vitamin B12 (cyanocobalamin, CNCbl). The cyano group of vitamin B12 is the result of the method used for the isolation of Cbl (Veer et al. 1950; Wijmenga et al. 1950; Spalla et al. 1989).
In humans, vitamin B12 is absorbed from our diet and transported by proteins with exceptionally high affinities for this vitamin. Once inside the cell, Cbl is activated into AdoCbl in the mitochondrion, or remains in the cytosol, where it participates as a methyl carrier in the conversion of homocysteine to methionine (Banerjee 2006).
The complete in vitro synthesis of vitamin B12 (>70 chemical reactions) was the culmination of a 12-year collaborative effort between the groups of R. B. Woodward (MIT, Cambridge, USA) and A. Eschenmoser (ETH, Zürich, Switzerland) (Woodward 1973; Eschenmoser & Wintner 1977). Although bacteria and archaea have trimmed the number of reactions needed to synthesize AdoCbl, they still dedicate a great deal of genetic information to the assembly of this complex molecule (Escalante-Semerena & Warren 2008).
The activation of vitamin B12 to AdoCbl involves the transfer of the adenosyl (Ado) moiety from ATP to the reduced Co1+ ion of cobalamin [cob(I)alamin]. This activation is catalyzed by ATP:Cob(I)alamin adenosyltransferase (ACA) enzymes. In this reaction, the Co1+ nucleophile attacks the 5′C of ATP generating the unique cobalt-carbon bond of AdoCbl. Recent detailed structural and functional characterization of these enzymes led to the discovery that ACA enzymes are multifunctional, since they also facilitate the generation of the corrinoid substrate, and play a role in the delivery of AdoCbl to the enzymes that use it. In this review, we discuss recently obtained insights into the mechanism of function by ACA enzymes.
Distribution of ACA enzymes
Cells from all domains of life synthesize ACA enzymes, which are of three types CobA, PduO, EutT. Surprisingly, CobA, EutT and PduO do not share sequence similarity at the nucleotide, amino acid, or tertiary structural level, suggesting separate lines of evolution. The Gram-negative enterobacterium Salmonella enterica synthesizes all three types (Suh & Escalante-Semerena 1995; Johnson et al. 2001; Buan & Escalante-Semerena 2006; Johnson et al. 2004), yet this bacterium uses each enzyme under different physiological conditions (Escalante-Semerena et al. 1990; Johnson et al. 2001; Buan et al. 2004; Sheppard et al. 2004).
The first ACA enzyme characterized was SeCobA, which is regarded as the housekeeping ACA enzyme in S. enterica (Escalante-Semerena & Warren 2008). SeCobA is involved in the de novo synthesis of Cbl, and in the scavenging of incomplete corrinoids (Escalante-Semerena et al. 1990). The activity of SeCobA is needed because several enzymes involved in the late steps AdoCbl biosynthesis require adenosylated corrinoid substrates (Escalante-Semerena & Warren 2008). Spectroscopic evidence suggests that SeCobA adenosylates incomplete corrinoids more efficiently than Cbl (Stich et al. 2005a).
S. enterica uses PduO- and EutT-type ACA enzymes for the catabolism of 1,2-propanediol and ethanolamine, respectively. The PduO-type is the most widely distributed ACA enzyme, and is the type of enzyme found in human cells (Leal et al. 2003). In humans, adenosylation of Cbl takes place in the mitochondrion (Banerjee & Chowdhury 1999). From a mechanistic standpoint, PduO-type ACA enzymes are best understood. In contrast, little is known about EutT. Initial biochemical characterization of EutT suggests that this type of ACA enzyme requires a metal ion for activity (Buan et al. 2004).
Structural characterization of ACA enzymes
Crystal structures of the CobA and PduO-type ACA enzymes have been solved at high resolution. Due to the difficulty purifying EutT, a model for the structure of this protein was not available at the time of this writing.
Structural characterizations of SeCobA, and several PduO-type enzymes from different sources, revealed that although these two enzymes catalyze the same reaction, they do not share tertiary nor quaternary structure homology (Fig. 2). Moreover, the SeCobA, the Lactobacillus reuteri (LrPduO), and the human adenosyltransferase (hATR) bind substrates in their active sites in different conformations (Bauer et al. 2001; St Maurice et al. 2008; St Maurice et al. 2007; Schubert & Hill 2006).
Fig. 2.
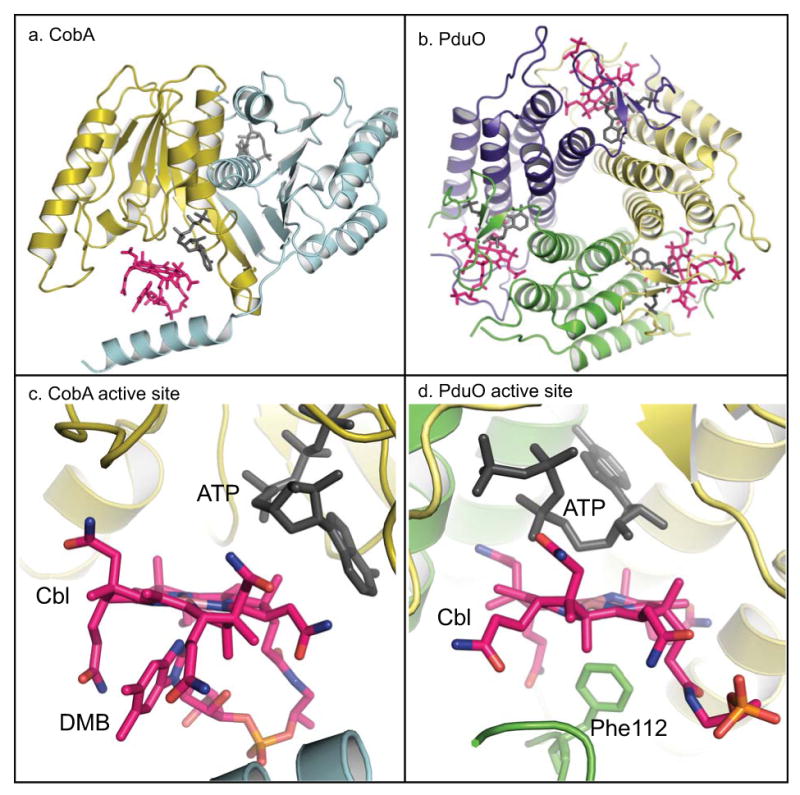
X-ray crystallography models of CobA and PduO ACA enzymes. a. The dimer of SeCobA is shown using ribbon representation with each subunit colored in cyan and gold; b. The trimer of LrPduO is shown with each subunit colored yellow, violet and green. c. SeCobA's active site. The location and conformation of ATP (black) and Cbl (pink) are shown using the stick format. Cbl is bound in the active site of CobA in the DMB-on conformation. d. LrPduO's active site. The substrates ATP (black) and Cbl (pink) are shown in the active site with Cbl bound in the DMB-off conformation. For simplicity, only one active site of CobA and PduO are shown with substrates bound. Figure was generated using the PyMOL program (http://www.pymol.org).
The SeCobA enzyme is a homodimer consisting of α/β structure with topology belonging to the P-loop containing family of nucleotide hydrolases (Bauer et al. 2001). Notably, the P-loop of CobA is one amino acid shorter than the consensus motif. In SeCobA, ATP is bound to the P-loop in a conformation that exposes the 5′C of ATP to the Co1+ nucleophile (Bauer et al. 2001). Such an orientation favors the transfer of the Ado moiety, rather than the γ phosphate group. In the crystal structure available in the database (PDB code 1G64), Cbl is bound to the active site with the lower ligand coordinated to the Co ion, a conformation known as the base-on conformation (Fig. 2). There are several unresolved questions regarding the SeCobA structure. First, the distance between the Co ion and the 5′C of ATP is too long for the nucleophilic attack to occur. Second, spectroscopic analyses revealed that adenosylation takes place via a four-coordinate cob(II)alamin DMB-off conformation intermediate (Stich et al. 2005a; Stich et al. 2004), however, as noted above, Cbl is bound in the active site in the base-on conformation, a five-coordinate species. A conformational change of CobA where the enzyme displaces the lower ligand of Cbl, and brings the Co ion in close proximity to the 5′C for the nucleophilic attack to take place would help explain these discrepancies.
In recent years, several groups reported structural characterizations of PduO-type ACA enzymes from several sources. An archaeal PduO-type enzyme with no substrates in the active site was reported to form a trimer with each subunit composed of five-helix bundles (Saridakis et al. 2004). The structural analysis of a bacterial and the human PduO-type enzymes showed that the active site of this class of ACA enzyme is formed by the N-terminus of one subunit and the C-terminus of the adjacent subunit (Schubert & Hill 2006; St Maurice et al. 2007). The structures of the proteins in complex with ATP uncovered a novel ATP binding motif (Thr-(Lys/Arg)-X-Gly-Asp-X-Gly-X-(Thr/Ser) that is unique to PduO-type ACA enzymes. The structure of LrPduO/ATP/Cbl ternary complex provided visual evidence of the existence of a base-off, four-coordinate cob(II)alamin intermediate (St Maurice et al. 2008) (Fig. 2).
How PduO-type ACA enzymes generate the Co1+ nucleophile
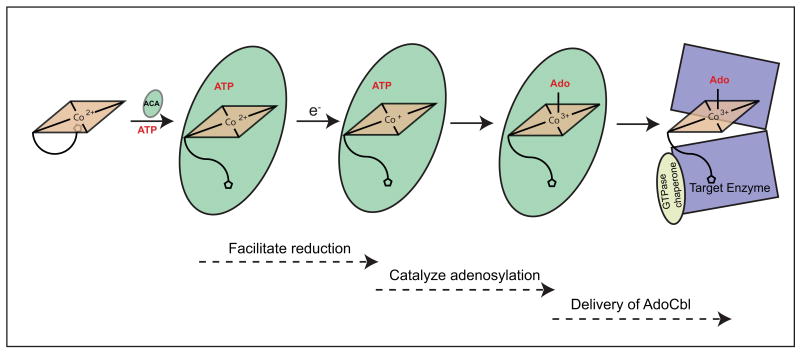
As mentioned above, the reduced Co1+ ion of cob(I)alamin is the nucleophile that attacks the 5′C of ATP (Fig. 3). Therefore the substrate of ACA enzymes is cob(I)alamin, a molecule with two important features. First, cob(I)alamin is very reactive, and, if left unprotected, it is rapidly oxidized to Co2+. Second, the midpoint redox potential of the Co2+/Co1+ couple [E°′ = -610 mV, (Lexa & Saveant 1976)] is too low for any known cellular reductant, thus the generation of cob(I)alamin is a challenging, thermodynamically unfavorable reaction. ACA enzymes solve both problems by preferentially binding Co2+ corrinoids, and generating a four-coordinate species that can be reduced by free or protein bound dihydroflavins (Mera & Escalante-Semerena 2010) (Fig. 3).
Fig. 3.
Schematic mechanism of the multiple functions performed by ACA enzymes. ACA enzymes (green oval) facilitate the reduction to cob(I)alamin by binding cob(II)alamin (rectangle) and generating a four-coordinate cob(II)alamin intermediate. After catalysis, the product AdoCbl is delivered to the AdoCbl-dependent enzyme (violet open-squares), a process that is facilitated by a GTPase chaperone.
Elegant spectroscopic studies by T. Brunold and co-workers provided insights into the strategy used by SeCobA and LrPduO/hATR to overcome the thermodynamic barrier posed by the reduction of cob(II)alamin (Stich et al. 2005a; Stich et al. 2005b; Park et al. 2008). To solve this problem, ACA enzymes raise the reduction potential of the Co2+ ion to within physiological range by displacing the lower ligand of Cbl, generating a four-coordinate cob(II)alamin intermediate. Detailed structural and kinetic analyses of LrPduO revealed the critical role of a conserved bulky residue (phenylalanine, F112 in LrPduO), which displaces the lower ligand (Mera et al. 2009).
The source of the electron used to reduce the ACA enzyme-bound cob(II)alamin to cob(I)alamin remains an unresolved question. Although several putative electron sources have been suggested for CobA- and PduO-type enzymes, there is no in vivo evidence in support of these ideas. In vitro, SeCobA was shown to interact with flavodoxin A (FldA), a well-studied flavin mononucleotide (FMN)-dependent electron transfer protein, whose interactions with the Cbl-dependent methionine synthase (MetH) enzyme of E. coli have been elegantly documented by Rowena Matthews and co-workers (Hoover et al. 1997; Hall et al. 2000; Hall et al. 2001). Reduced FldA was shown to efficiently drive the adenosylation of cob(II)alamin (Fonseca & Escalante-Semerena 2001; Buan & Escalante-Semerena 2005). In Brucella melitensis and in S. enterica, the CobR and PduS flavoproteins, were respectively shown in vitro to reduce cob(III)alamin in two consecutive one-electron reductions to cob(I)alamin (Lawrence et al. 2008; Sampson et al. 2005). Recently, we found that free reduced flavins, and non-specific flavoproteins can reduce cob(II)alamin bound in the active site of ACA enzymes (Mera & Escalante-Semerena 2010). These results prompted us to consider the questions of whether there is the need of a protein involved in this reduction, and if there is, is such a protein an electron transfer protein, or an enzyme. Based on data available at this point, we have suggested that bona fide cobalamin reductases have not been identified (Mera & Escalante-Semerena 2010).
Additional details about the adenosylation reaction
ACA enzymes follow an ordered sequential binding scheme, where ATP binds first (Stich et al. 2005a; Mera et al. 2007; St Maurice et al. 2007; Suh & Escalante-Semerena 1995). ACA enzymes can bind and transfer the nitrogenous base from different nucleoside triphosphates to cob(I)alamin, albeit not with the same efficiency (Fonseca et al. 2002; Johnson et al. 2004; St Maurice et al. 2007; Mera et al. 2007; Buan & Escalante-Semerena 2006). In the case of LrPduO, two functional groups of ATP explain the reduced ability of the enzyme to use alternate nucleotides, i) the amino group at the C-6 position, which contributes to the affinity of the enzyme, and ii) the nitrogen at the N-7 position, which is involved in catalysis (Mera et al. 2007). Unlike PduO and EutT, SeCobA requires the 2′OH group of the ribosyl moiety of ATP for activity (Fonseca et al. 2002).
Unlike most of ATP-dependent enzymes, PduO and CobA eliminate the tripolyphosphate group of ATP, whereas Pi and PP i were shown to be the byproducts of the EutT reaction (Fonseca et al. 2002; Johnson et al. 2004; St Maurice et al. 2007; Buan & Escalante-Semerena 2006).
Critical residues of the PduO-type ACA enzymes have been studied more closely, specifically because mutations in the human adenosyltransferase (hATR) have been found in patients with methylmalonic aciduria (Zhang et al. 2006). Detailed genetic, structural, and kinetic analyses have unveiled why these substitutions are deleterious to the function of the enzyme. One key residue that has been characterized by several groups is the conserved arginine 186 (position in hATR), whose change to a tryptophan is the most common substitution found in hATR (Lerner-Ellis et al. 2006). Arg186 is critical for catalysis, even though it does not directly interact with ATP (Schubert & Hill 2006; St Maurice et al. 2007; Mera et al. 2007; Fan & Bobik 2008; Zhang et al. 2009). From studies performed with the bacterial LrPduO, we now understand that Arginine 186 (Arg128 in LrPduO) forms a salt bridge with a conserved aspartate from the adjacent subunit (St Maurice et al. 2007; Mera et al. 2007). Structural and kinetic analyses of LrPduO revealed that the salt bridge affects the rate at which the enzyme/ATP complex forms. In other words, changes in the ability to form this salt bridge affects the catalytic efficiency of the enzyme (Mera et al. 2007). Furthermore, the structure of LrPduO/ATP/Cbl complex unveiled a direct contact between the side chain Arg186 (Arg128 in LrPduO) and one of the corrin ring amide (St Maurice et al. 2008). In LrPduO, a conserved substitution R128K resulted in a 9-fold drop in the affinity of the enzyme for cob(I)alamin. Intriguingly, the mutation of this residue to a tryptophan in hATR was reported by Gravel and co-workers to have a defect in binding AdoCbl but not a defect in binding cob(I)alamin. Additional kinetic and structural analyses of this tryptophan variant in LrPduO or hATR are needed to clearly understand the function of the interaction between this conserved arginine residue and the corrin ring. Certainly, affinity is not the only problem generated by the change at that position since a hATRR186W variant is inactive (Saridakis et al. 2004; Zhang et al. 2006).
After facilitating the generation of substrate and catalyzing the formation of product, ACA enzymes ensure that this valuable coenzyme gets to the right target enzyme (Fig. 3). The concentrations of AdoCbl inside the cell are very low making simple diffusion the unlikely mechanism by which AdoCbl-dependent enzymes get AdoCbl (Banerjee 2006). In humans, methylmalonyl-CoA mutase (MCM) is the AdoCbl-dependent enzyme that isomerizes methylmalonyl-CoA into succinyl-CoA inside the mitochondrion (Mancia et al. 1996). Ruma Banerjee and co-workers have shown that the PduO enzyme from M. extorquens (MePduO) transfers AdoCbl directly to the methylmalonyl-CoA mutase (MCM) (Padovani et al. 2008). MCM binds AdoCbl in the base-off/His-on conformation, and it is this histidine residue that plays a critical role in the transfer of the coenzyme from MePduO to MCM (Padovani et al. 2008; Mancia et al. 1996). Results from kinetic and thermodynamic analyses of the MePduO enzyme support a rotary mechanism in which, at any given time, only two of its active sites are used for AdoCbl synthesis (Padovani & Banerjee 2009b). In this proposed mechanism, the binding of ATP to the vacant active site of PduO induces the release/transfer of product to the acceptor enzyme.
In addition, the same group has assigned the GTPase chaperone protein MeaB a key role in the fidelity of the AdoCbl loading process (Padovani & Banerjee 2009a). The data obtained by these authors is supported by the finding of a substitution in the human orthologue of MeaB in a patient with methylmalonic aciduria; the mutant MeaB fails to discriminate between active and non-active cofactors (Padovani & Banerjee 2009a).
A role for ACA enzymes in the delivery of cancer-fighting drugs
In the fight against cancer, researchers would like to advantage of the fact that Cbl is an essential nutrient of human cells, and that tumor cells have a higher requirement for Cbl. The idea of using Cbl as a means to deliver tumor-fighting drugs to a target has been explored in the past (Bagnato et al. 2004), and a recent report showed that ACA enzymes effciently use cisplatin-like conjugates of Cbl as substrates, with the concomitant production of AdoCbl, and the release of the drug (Ruiz-Sanchez et al. 2008). The use of Cbl conjugates to fight cancer seems promising.
AdoCbl production
The structural complexity of AdoCbl makes the chemical synthesis of this molecule too expensive for industrial production. In fact, the need to mass produce vitamin B12 drove the isolation of proteins involved in the biosynthesis of Cbl by bacteria (Warren et al. 2002). At present, more than 80% of the world's production of vitamin B12 is synthesized by the fermentation using genetically engineered Pseudomonas dentrificans strains (Martens et al. 2002).
Bacteria efficiently synthesize AdoCbl, however, the sensitivity of the cobalt-carbon bond of AdoCbl to light, heat, and reagents such as cyanide, complicates its purification. Stable, and readily purifiable ACA enzymes offer a feasible alternative to AdoCbl production. Recent advances in our understanding of how the cob(I)alamin substrate is generated, further make the use of ACA enzymes for AdoCbl production a viable alternative.
Acknowledgments
This work was supported by PHS grant R37 GM40313 (to J.C.E.-S.).
References
- Bagnato JD, Eilers AL, Horton RA, Grissom CB. Synthesis and characterization of a cobalamin-colchicine conjugate as a novel tumor-targeted cytotoxin. J Org Chem. 2004;69:8987–8996. doi: 10.1021/jo049953w. [DOI] [PubMed] [Google Scholar]
- Bandarian V, Reed GH. Ethanolamine ammonia-lyase. In: Banerjee R, editor. Chemistry ad Biochemistry of B12. New York: John Wiley & Sons, Ic.; 1999. pp. 811–833. [Google Scholar]
- Banerjee R. B12 trafficking in mammals: A for coenzyme escort service. ACS Chem Biol. 2006;1:149–159. doi: 10.1021/cb6001174. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Chowdhury S. Methylmalonyl-CoA mutase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons, Inc.; 1999. pp. 707–729. [Google Scholar]
- Banerjee R, Vlasie M. Controlling the reactivity of radical intermediates by coenzyme B(12)-dependent methylmalonyl-CoA mutase. Biochem Soc Trans. 2002;30:621–624. doi: 10.1042/bst0300621. [DOI] [PubMed] [Google Scholar]
- Battersby AR. Tetrapyrroles: the pigments of life. Nat Prod Rep. 2000;17:507–526. doi: 10.1039/b002635m. [DOI] [PubMed] [Google Scholar]
- Bauer CB, Fonseca MV, Holden HM, Thoden JB, Thompson TB, Escalante-Semerena JC, Rayment I. Three-dimensional structure of ATP:corrinoid adenosyltransferase from Salmonella typhimurium in its free state, complexed with MgATP, or complexed with hydroxycobalamin and MgATP. Biochemistry. 2001;40:361–374. doi: 10.1021/bi002145o. [DOI] [PubMed] [Google Scholar]
- Buan NR, Escalante-Semerena JC. Computer-assisted docking of flavodoxin with the ATP:Co(I)rrinoid adenosyltransferase (CobA) enzyme reveals residues critical for protein-protein interactions but not for catalysis. J Biol Chem. 2005;280:40948–40956. doi: 10.1074/jbc.M506713200. [DOI] [PubMed] [Google Scholar]
- Buan NR, Escalante-Semerena JC. Purification and initial biochemical characterization of ATP:Cob(I)alamin adenosyltransferase (EutT) enzyme of Salmonella enterica. J Biol Chem. 2006;281:16971–16977. doi: 10.1074/jbc.M603069200. [DOI] [PubMed] [Google Scholar]
- Buan NR, Suh SJ, Escalante-Semerena JC. The eutT gene of Salmonella enterica encodes an oxygen-labile, metal-containing ATP:corrinoid adenosyltransferase enzyme. J Bacteriol. 2004;186:5708–5714. doi: 10.1128/JB.186.17.5708-5714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel W, Bröker G, Bothe H, Pierik A. Glutamate mutase and 2-Methylglutarate mutase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons, Inc.; 1999. pp. 757–782. [Google Scholar]
- Cavicchi C, Donati MA, Funghini S, la Marca G, Malvagia S, Ciani F, Poggi GM, Pasquini E, Zammarchi E, Morrone A. Genetic and biochemical approach to early prenatal diagnosis in a family with mut methylmalonic aciduria. Clin Genet. 2006;69:72–76. doi: 10.1111/j.1399-0004.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- Ciani F, Donati MA, Tulli G, Poggi GM, Pasquini E, Rosenblatt DS, Zammarchi E. Lethal late onset cblB methylmalonic aciduria. Crit Care Med. 2000;28:2119–2121. doi: 10.1097/00003246-200006000-00078. [DOI] [PubMed] [Google Scholar]
- Dobson CM, Wai T, Leclerc D, Kadir H, Narang M, Lerner-Ellis JP, Hudson TJ, Rosenblatt DS, Gravel RA. Identification of the gene responsible for the cblB complementation group of vitamin B12-dependent methylmalonic aciduria. Hum Mol Genet. 2002;11:3361–3369. doi: 10.1093/hmg/11.26.3361. [DOI] [PubMed] [Google Scholar]
- Eirich LD, Vogels GD, Wolfe RS. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry. 1978;17:4583–4593. doi: 10.1021/bi00615a002. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena JC, Suh SJ, Roth JR. cobA function is required for both de novo cobalamin biosynthesis and assimilation of exogenous corrinoids in Salmonella typhimurium. J Bacteriol. 1990;172:273–280. doi: 10.1128/jb.172.1.273-280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena JC, Warren MJ. Biosynthesis and Use of Cobalamin (B12) In: Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, editors. EcoSal - Escherichia coli and Salmonella: cellular and molecular biology. Washington, D. C.: ASM Press; 2008. [Google Scholar]
- Eschenmoser A, Wintner CE. Natural product synthesis and vitamin B12. Science. 1977;196:1410–1420. doi: 10.1126/science.867037. [DOI] [PubMed] [Google Scholar]
- Fan C, Bobik TA. Functional characterization and mutation analysis of human ATP:Cob(I)alamin adenosyltransferase. Biochemistry. 2008;47:2806–2813. doi: 10.1021/bi800084a. [DOI] [PubMed] [Google Scholar]
- Fenton WA, Rosenberg LE. Mitochondrial metabolism of hydroxocobalamin: synthesis of adenosylcobalamin by intact rat liver mitochondria. Arch Biochem Biophys. 1978;189:441–447. doi: 10.1016/0003-9861(78)90232-1. [DOI] [PubMed] [Google Scholar]
- Fonseca MV, Buan NR, Horswill AR, Rayment I, Escalante-Semerena JC. The ATP:co(I)rrinoid adenosyltransferase (CobA) enzyme of Salmonella enterica requires the 2′-OH Group of ATP for function and yields inorganic triphosphate as its reaction byproduct. J Biol Chem. 2002;277:33127–33131. doi: 10.1074/jbc.M203893200. [DOI] [PubMed] [Google Scholar]
- Fonseca MV, Escalante-Semerena JC. An in vitro reducing system for the enzymic conversion of cobalamin to adenosylcobalamin. J Biol Chem. 2001;276:32101–32108. doi: 10.1074/jbc.M102510200. [DOI] [PubMed] [Google Scholar]
- Fontecave M. Ribonucleotide reductases and radical reactions. Cell Mol Life Sci. 1998;54:684–695. doi: 10.1007/s000180050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M, Mulliez E. Ribonucleotide Reductases. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons, Inc.; 1999. pp. 731–756. [Google Scholar]
- Hall DA, Jordan-Starck TC, Loo RO, Ludwig ML, Matthews RG. Interaction of flavodoxin with cobalamin-dependent methionine synthase. Biochemistry. 2000;39:10711–10719. doi: 10.1021/bi001096c. [DOI] [PubMed] [Google Scholar]
- Hall DA, Vander Kooi CW, Stasik CN, Stevens SY, Zuiderweg ER, Matthews RG. Mapping the interactions between flavodoxin and its physiological partners flavodoxin reductase and cobalamin-dependent methionine synthase. Proc Natl Acad Sci USA. 2001;98:9521–9526. doi: 10.1073/pnas.171168898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DM, Jarrett JT, Sands RH, Dunham WR, Ludwig ML, Matthews RG. Interaction of Escherichia coli cobalamin-dependent methionine synthase and its physiological partner flavodoxin: binding of flavodoxin leads to axial ligand dissociation from the cobalamin cofactor. Biochemistry. 1997;36:127–138. doi: 10.1021/bi961693s. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Buszko ML, Bobik TA. Purification and initial characterization of the Salmonella enterica PduO ATP:Cob(I)alamin adenosyltransferase. J Bacteriol. 2004;186:7881–7887. doi: 10.1128/JB.186.23.7881-7887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Pechonick E, Park SD, Havemann GD, Leal NA, Bobik TA. Functional genomic, biochemical, and genetic characterization of the Salmonella pduO gene, an ATP:cob(I)alamin adenosyltransferase gene. J Bacteriol. 2001;183:1577–1584. doi: 10.1128/JB.183.5.1577-1584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Deery E, McLean KJ, Munro AW, Pickersgill RW, Rigby SE, Warren MJ. Identification, characterization, and structure/function analysis of a corrin reductase involved in adenosylcobalamin biosynthesis. J Biol Chem. 2008;283:10813–10821. doi: 10.1074/jbc.M710431200. [DOI] [PubMed] [Google Scholar]
- Leal NA, Park SD, Kima PE, Bobik TA. Identification of the human and bovine ATP: cob(I)alamin adenosyltransferase cDNAs based on complementation of a bacterial mutant. J Biol Chem. 2003;278:9227–9234. doi: 10.1074/jbc.M212739200. [DOI] [PubMed] [Google Scholar]
- Lenhert PG. The structure of vitamin B12 VII. The X-ray analysis of the vitamin B12 coenzyme. Proc R Soc London, Ser A. 1968;303:45–84. [Google Scholar]
- Lerner-Ellis JP, Gradinger AB, Watkins D, Tirone JC, Villeneuve A, Dobson CM, Montpetit A, Lepage P, Gravel RA, Rosenblatt DS. Mutation and biochemical analysis of patients belonging to the cblB complementation class of vitamin B12-dependent methylmalonic aciduria. Mol Genet Metab. 2006;87:219–225. doi: 10.1016/j.ymgme.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Lexa D, Saveant JM. Electrochemistry of vitamin B12. I. Role of the base-on/base-off reaction in the oxidoreduction mechanism of the B12r-B12s system. J Am Chem Soc. 1976;98:2652–2658. doi: 10.1021/ja00425a039. [DOI] [PubMed] [Google Scholar]
- Mancia F, Keep NH, Nakagawa A, Leadlay PF, McSweeney S, Rasmussen B, Bosecke P, Diat O, Evans PR. How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2Å resolution. Structure. 1996;4:339–350. doi: 10.1016/s0969-2126(96)00037-8. [DOI] [PubMed] [Google Scholar]
- Martens JH, Barg H, Warren MJ, Jahn D. Microbial production of vitamin B12. Appl Microbiol Biotechnol. 2002;58:275–285. doi: 10.1007/s00253-001-0902-7. [DOI] [PubMed] [Google Scholar]
- Mera PE, Escalante-Semerena JC. Dihydroflavin-driven adenosylation of 4-coordinate Co(II) corrinoids: are cobalamin reductases enzymes or electron transfer proteins? J Biol Chem. 2010;285:2911–2917. doi: 10.1074/jbc.M109.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera PE, Maurice MS, Rayment I, Escalante-Semerena JC. Structural and functional analyses of the human-type corrinoid adenosyltransferase (PduO) from Lactobacillus reuteri. Biochemistry. 2007;46:13829–13836. doi: 10.1021/bi701622j. [DOI] [PubMed] [Google Scholar]
- Mera PE, St Maurice M, Rayment I, Escalante-Semerena JC. Residue Phe112 of the human-type corrinoid adenosyltransferase (PduO) enzyme of Lactobacillus reuteri is critical to the formation of the four-coordinate Co(II) corrinoid substrate and to the activity of the enzyme. Biochemistry. 2009;48:3138–3145. doi: 10.1021/bi9000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montforts FP, Glasenapp-Breiling M. Naturally occurring cyclic tetrapyrroles. Fortschr Chem Org Naturst. 2002;84:1–51. doi: 10.1007/978-3-7091-6160-9_1. [DOI] [PubMed] [Google Scholar]
- Padovani D, Banerjee R. A G-protein editor gates coenzyme B12 loading and is corrupted in methylmalonic aciduria. Proc Natl Acad Sci U S A. 2009a;106:21567–21572. doi: 10.1073/pnas.0908106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovani D, Banerjee R. A rotary mechanism for coenzyme B(12) synthesis by adenosyltransferase. Biochemistry. 2009b;48:5350–5357. doi: 10.1021/bi900454s. [DOI] [PubMed] [Google Scholar]
- Padovani D, Labunska T, Palfey BA, Ballou DP, Banerjee R. Adenosyltransferase tailors and delivers coenzyme B12. Nat Chem Biol. 2008;4:194–196. doi: 10.1038/nchembio.67. [DOI] [PubMed] [Google Scholar]
- Park K, Mera PE, Escalante-Semerena JC, Brunold TC. Kinetic and spectroscopic studies of the ATP:corrinoid adenosyltransferase PduO from Lactobacillus reuteri: substrate specificity and insights into the mechanism of Co(II)corrinoid reduction. Biochemistry. 2008;47:9007–9015. doi: 10.1021/bi800419e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt DS, Fenton WA. Inborn errors of metabolism. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons; 1999. pp. 367–384. [Google Scholar]
- Ruiz-Sanchez P, Mundwiler S, Spingler B, Buan NR, Escalante-Semerena JC, Alberto R. Syntheses and characterization of vitamin B(12)-Pt(II) conjugates and their adenosylation in an enzymatic assay. J Biol Inorg Chem. 2008;13:335–347. doi: 10.1007/s00775-007-0329-4. [DOI] [PubMed] [Google Scholar]
- Sampson EM, Johnson CL, Bobik TA. Biochemical evidence that the pduS gene encodes a bifunctional cobalamin reductase. Microbiology. 2005;151:1169–1177. doi: 10.1099/mic.0.27755-0. [DOI] [PubMed] [Google Scholar]
- Saridakis V, Yakunin A, Xu X, Anandakumar P, Pennycooke M, Gu J, Cheung F, Lew JM, Sanishvili R, Joachimiak A, Arrowsmith CH, Christendat D, Edwards AM. The structural basis for methylmalonic aciduria. The crystal structure of archaeal ATP:cobalamin adenosyltransferase. J Biol Chem. 2004;279:23646–23653. doi: 10.1074/jbc.M401395200. [DOI] [PubMed] [Google Scholar]
- Schubert HL, Hill CP. Structure of ATP-bound human ATP:cobalamin adenosyltransferase. Biochemistry. 2006;45:15188–15196. doi: 10.1021/bi061396f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DE, Penrod JT, Bobik T, Kofoid E, Roth JR. Evidence that a B12-adenosyl transferase is encoded within the ethanolamine operon of Salmonella enterica. J Bacteriol. 2004;186:7635–7644. doi: 10.1128/JB.186.22.7635-7644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalla C, Grein A, Garofano L, Ferni G. Microbial Production of Vitamin B12. In: Vandamme EJ, editor. Biotechnology of Vitamins, Pigments, and Growth Factors. London: 1989. pp. 257–284. [Google Scholar]
- St Maurice M, Mera P, Park K, Brunold TC, Escalante-Semerena JC, Rayment I. Structural characterization of a human-type corrinoid adenosyltransferase confirms that coenzyme B12 is synthesized through a four-coordinate intermediate. Biochemistry. 2008;47:5755–5766. doi: 10.1021/bi800132d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Maurice M, Mera PE, Taranto MP, Sesma F, Escalante-Semerena JC, Rayment I. Structural characterization of the active site of the PduO-type ATP:Co(I)rrinoid adenosyltransferase from Lactobacillus reuteri. J Biol Chem. 2007;282:2596–2605. doi: 10.1074/jbc.M609557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich TA, Buan NR, Brunold TC. Spectroscopic and computational studies of Co(2+)corrinoids: spectral and electronic properties of the biologically relevant base-on and base-off forms of Co(2+)cobalamin. J Am Chem Soc. 2004;126:9735–9749. doi: 10.1021/ja0481631. [DOI] [PubMed] [Google Scholar]
- Stich TA, Buan NR, Escalante-Semerena JC, Brunold TC. Spectroscopic and computational studies of the ATP:Corrinoid adenosyltransferase (CobA) from Salmonella enterica: Insights into the mechanism of adenosylcobalamin biosynthesis. J Am Chem Soc. 2005a;127:8710–8719. doi: 10.1021/ja042142p. [DOI] [PubMed] [Google Scholar]
- Stich TA, Yamanishi M, Banerjee R, Brunold TC. Spectroscopic evidence for the formation of a four-coordinate Co(2+)cobalamin species upon binding to the human ATP:Cobalamin adenosyltransferase. J Am Chem Soc. 2005b;127:7660–7661. doi: 10.1021/ja050546r. [DOI] [PubMed] [Google Scholar]
- Suh S, Escalante-Semerena JC. Purification and initial characterization of the ATP:corrinoid adenosyltransferase encoded by the cobA gene of Salmonella typhimurium. J Bacteriol. 1995;177:921–925. doi: 10.1128/jb.177.4.921-925.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T. Diol dehydratase and glycerol dehydratase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons, Inc.; 1999. pp. 783–809. [Google Scholar]
- van Beelen P, Stassen AP, Bosch JW, Vogels GD, Guijt W, Haasnoot CA. Elucidation of the structure of methanopterin, a coenzyme from Methanobacterium thermoautotrophicum, using two-dimensional nuclear-magnetic-resonance techniques. Eur J Biochem. 1984;138:563–571. doi: 10.1111/j.1432-1033.1984.tb07951.x. [DOI] [PubMed] [Google Scholar]
- Veer WL, Edelhausen JH, Wijmenga HG, Lens J. Vitamin B12. I. The relation between vitamins B12 and B12b. Biochim Biophys Acta. 1950;6:225–228. doi: 10.1016/0006-3002(50)90095-3. [DOI] [PubMed] [Google Scholar]
- Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. The biosynthesis of adenosylcobalamin (vitamin B12) Nat Prod Rep. 2002;19:390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- Wijmenga HG, Veer WL, Lens J. II. The influence of HCN on some factors of the vitamin B12 group. Biochim Biophys Acta. 1950;6:229–236. doi: 10.1016/0006-3002(50)90096-5. [DOI] [PubMed] [Google Scholar]
- Wohlfarth G, Diekert G. Reductive dehalogenases. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons, Inc.; 1999. pp. 871–893. [Google Scholar]
- Woodward RB. The total synthesis of vitamin B12. Pure Appl Chem. 1973;33:145–177. doi: 10.1351/pac197333010145. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dobson CM, Wu X, Lerner-Ellis J, Rosenblatt DS, Gravel RA. Impact of cblB mutations on the function of ATP:cob(I)alamin adenosyltransferase in disorders of vitamin B12 metabolism. Mol Genet Metab. 2006;87:315–322. doi: 10.1016/j.ymgme.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu X, Padovani D, Schubert HL, Gravel RA. Ligand-binding by catalytically inactive mutants of the cblB complementation group defective in human ATP:cob(I)alamin adenosyltransferase. Mol Genet Metab. 2009;98:278–284. doi: 10.1016/j.ymgme.2009.06.014. [DOI] [PubMed] [Google Scholar]