Abstract
Objective
Atherosclerosis encompasses a conspicuously maladaptive inflammatory response that might involve innate immunity. Here we compared the role of Toll-like receptor 4 (TLR4) vs. that of TLR2 in intimal foam cell accumulation and inflammation in apolipoprotein E (ApoE) knockout mice in vivo, and determined potential mechanisms of upstream activation and downstream action.
Methods and Results
We measured lipid accumulation and gene expression in the lesion-prone lesser curvature of the aortic arch (LCAA). TLR4 deficiency reduced intimal lipid by ~75% in ApoE KO mice, despite unaltered total serum cholesterol and triglyceride levels, while TLR2 deficiency reduced it by ~45%. TLR4 deficiency prevented the increased interleukin-1α (IL-1α) and monocyte chemoattractant protein-1 (MCP-1) mRNA levels seen within lesional tissue, and also lowered serum (IL-1α) levels. Smooth muscle cells (SMC) were present within the intima of the LCAA at this early lesion stage, and they enveloped and permeated nascent lesions, which consisted of focal clusters of foam cells. Cholesterol-enrichment of SMC in vitro stimulated acyl-CoA:cholesterol acyltransferase-1 mRNA expression, cytoplasmic cholesterol ester accumulation, and MCP-1 mRNA and protein expression in a TLR4-dependent manner.
Conclusions
TLR4 contributes to early-stage intimal foam cell accumulation at lesion-prone aortic sites in ApoE KO mice, as does TLR2 to a lesser extent. Intimal SMC surround and penetrate early lesions, where TLR4 signaling within them may influence lesion progression.
INTRODUCTION
Atherogenesis involves a strikingly maladaptive inflammatory response, initially to retained and modified lipoproteins 1, and later to apoptotic or necrotic cell debris that accumulates within the arterial wall 2. Toll-like receptors (TLRs) elicit innate immune responses and inflammation when activated by either exogenous microbial products or endogenous molecules with similar structural features, and are candidate mediators of atherogenic inflammation. TLR2 and TLR4 are expressed in arterial lesions of mice and humans 3, 4, and exogenous microbial TLR2 and TLR4 agonist ligands promote atherosclerosis in hypercholesterolemic mice 5, 6, implicating these receptors as candidate atherogenic mediators. In gene knockout mouse models, TLR2 deficiency reduced early atherogenic events and later aortic lesion development 7, 8. In contrast, deficiency of TLR4, the bacterial lipopolysaccharide (LPS) receptor, produced modest 9 or no effects 10 on aortic lesion burden, in studies limited to advanced disease stages. However, prolonged and severe hypercholesterolemia may generate distinct modified lipids or proteins that promote inflammation and atherogenesis via distinct, TLR-independent pathways. Therefore, here we tested the hypothesis that TLR4 promotes atherogenesis during early-stage disease, using a mouse model of shorter-term and less severe hypercholesterolemia, and compared its influence to that of TLR2.
TLR signaling strongly activates pro-inflammatory genes in multiple cell types found within early atherosclerotic lesions, but little is known about how this promotes atherogenesis. Such atherogenic TLR signaling may not be restricted to hematopoietic cells, because a proatherogenic influence of TLR2 in hypercholesterolemic mice was found to be conferred by non-hematopoietic cells 5, and TLR2-expressing endothelial cells were found at lesion-prone sites 7. Other candidate non-hematopoietic cell types include intimal SMC, which may express both TLR2 and TLR4 11, 12. In humans, several findings point to an important role of intimal SMC in early lesion formation. Intimal accumulation of SMC precedes human lesion development, and the earliest histologically-identifiable lesions contain extracellular lipids, thought to accumulate due to binding to proteoglycans released by SMC 13. Foam cells of myeloid and/or SMC origin then accumulate in the intima 14, and some evidence suggests that apoptotic death of SMC leads to the appearance of lipid pools 15. Thus, TLR2 and TLR4 within SMC could potentially contribute to early atherogenesis by multiple mechanisms, including early proteoglycan synthesis, foam cell accumulation, or release of macrophage-recruiting chemokines and pro-inflammatory cytokines 11, 12. A role of SMC in early atherogenesis has not previously been described in mouse models, but mouse aortic SMC are similar to human aortic SMC in their propensity to accumulate intracellular cholesteryl esters in vitro 16, 17. Therefore, in the present study we evaluated the potential roles of SMC in early intimal lipid accumulation, using a hypercholesterolemic mouse model of atherogenesis in concert with 3-D confocal analysis of SMC involvement in lesions in vivo. We also studied potential mechanisms of TLR action in SMC in vitro.
Our results reveal that early aortic lipid accumulation and expression of pro-inflammatory mediators are markedly dependent on TLR4, and to a lesser extent TLR2, at lesion-prone sites in apolipoprotein E-deficient mice. Also, intimal SMC showed integral involvement in the microarchitecture of some nascent lesions, enveloping and permeating early foam cell clusters, supporting their potential to modulate lipid accumulation. Furthermore, we found that SMC incubated in cholesterol-enriched media in vitro express MCP-1 and the cholesterol-esterifying enzyme acyl-CoA:cholesterol acyltransferase (ACAT1), and also accumulate intracellular cholesteryl ester in a TLR4-dependent fashion 18, raising the possibility that TLR4 expressed by SMC contributes to proinflammatory events and foam cell formation in early atherogenesis.
METHODS
A detailed description of the methods can be found online at http://atvb.ahajournals.org.
Mice and serum analysis
ApoE KO, TLR2 KO, and TLR4 KO mice were cross-bred to obtain double-knockout mice. Serum total cholesterol and triglyceride levels were assayed using enzymatic methods. Serum IL-1α, MCP-1, and soluble VCAM-1 levels were determined by ELISA, and serum endotoxin levels were determined after heat-inactivating serum proteins.
En face analysis of lesion microarchitecture
Neutral lipid was stained with Oil Red O or BODIPY. SMC were localized with antibody specific for smooth muscle α-actin (SMA), and nuclei stained with Topro3.
SMC foam cell formation in vitro
Mouse aortic SMC (passage 3) were incubated 68 h in serum-free media with or without cholesterol:methyl β cyclodextrin for 68 h, lipids extracted with ethanol, and analyzed for cholesterol ester by enzymatic methods. The cyclodextrin-cholesterol preparation was not a significant source of endotoxin.
RESULTS
Deficiency of TLR4 or TLR2 reduces early aortic lipid accumulation in the LCAA
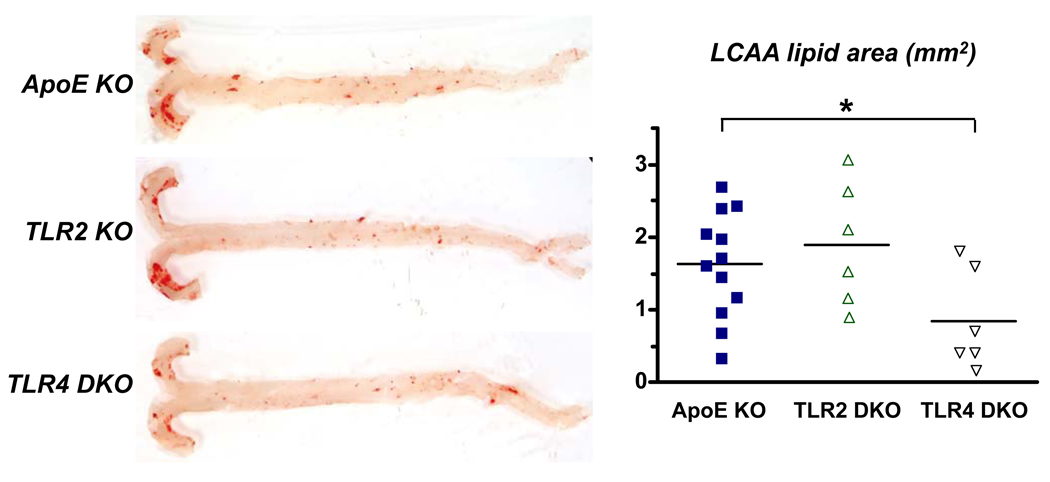
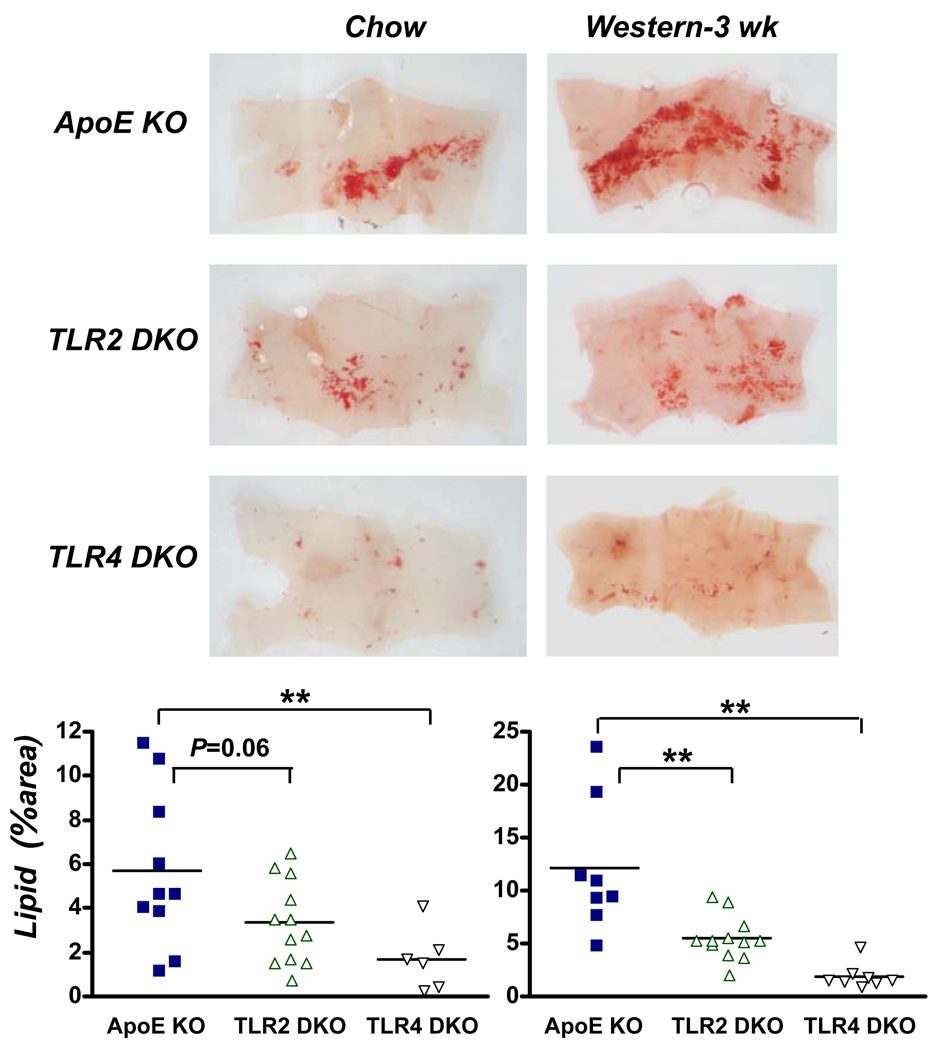
To test whether TLR4 and TLR2 promote lipid accumulation in the aortic intima, we studied ApoE KO mice fed chow diet. Accumulation of lipid within the intima was minimal in the descending aorta of 36 week-old ApoE KO mice (Fig. 1). In contrast, substantial lipid accumulation occurred within the intima of the lesser curvature of the aortic arch (LCAA), which is lesion-prone due to non-laminar blood flow 19. Intimal lipid areas in the LCAA were significantly reduced (48%) by TLR4 deficiency, but not by TLR2 deficiency. Lipid accumulation also occurred in the LCAA of younger (20 week-old) ApoE KO mice (Fig. 2), and TLR4 deficiency reduced lipid area markedly, by 71% (P<0.01), while TLR2 deficiency produced an apparent reduction of 41%, which did not achieve statistical significance (P=0.06). Furthermore, in 18 week-old mice fed Western diet for 3 weeks, TLR4 deficiency also markedly reduced lipid accumulation in the LCAA (by 84%), as did TLR2 to lesser extent (55%) (Fig. 2). Western diet-fed ApoE KO mice thus showed the most dramatic effect of TLR4 deficiency, despite a tripling of their serum cholesterol levels (Online Table I) and doubling of LCAA lesion area (Fig. 2) vs. those seen in their chow-fed controls. In contrast with the marked reduction seen in intimal lipid accumulation, total serum cholesterol or triglyceride levels were unaffected by TLR4 or TLR2 deficiency at any age or diet condition (Online Tables I and II).
Figure 1.
TLR4 deficiency reduced intimal lipid accumulation in the lesser curvature of the aortic arch (LCAA) of 36 week-old chow-fed male mice. Representative photographs depict en face analysis of oil Red O-stained ascending and descending aorta, from its origin to the iliac bifurcation. Mean and individual LCAA lesion areas in mm2 are shown in the chart. Bars indicate means; symbols indicate individual values. Minimal lipid accumulation was seen in the descending aorta. Number of mice per group: ApoE (12), TLR2 DKO (6), and TLR4 DKO (6). *P < 0.05 vs. ApoE KO.
Figure 2.
TLR4 and TLR2 deficiency reduce early intimal lipid accumulation in the LCAA of younger ApoE KO mice fed either chow (20 week-old female), or Western diet for 3 weeks before lesion analysis (18 week-old male). Representative Oil Red O-stained aortic arch segments from mice of the indicated genotypes and diet are shown in A. Charts in B show % of LCAA surface area staining positively for neutral lipid. Number of mice per group (chow and Western diet): ApoE (10 and 8), TLR2 DKO (12 and 12), and TLR4 DKO (6 and 8). **P < 0.01 vs. ApoE KO.
To determine whether the prototypical TLR4 agonist LPS might contribute to TLR4-dependent intimal lipid accumulation in ApoE-deficient mice, we measured serum LPS levels in 12 week-old female chow-fed mice. LPS was consistently detectable in serum of ApoE KO mice at low levels (~100 pg/ml), similar to those seen in wild-type and TLR4 DKO mice, while LPS levels in TLR2 DKO mice were somewhat reduced (~50 pg/ml, Online Table III). Together, the results indicate an important role of TLR4 in early-stage arterial lipid accumulation in ApoE KO mice, exceeding that of TLR2 under the conditions studied, independent of any changes in serum lipid levels, and associated with low but detectable levels of serum LPS.
TLR4 deficiency reduces lesional IL-1α gene expression and serum IL-1α
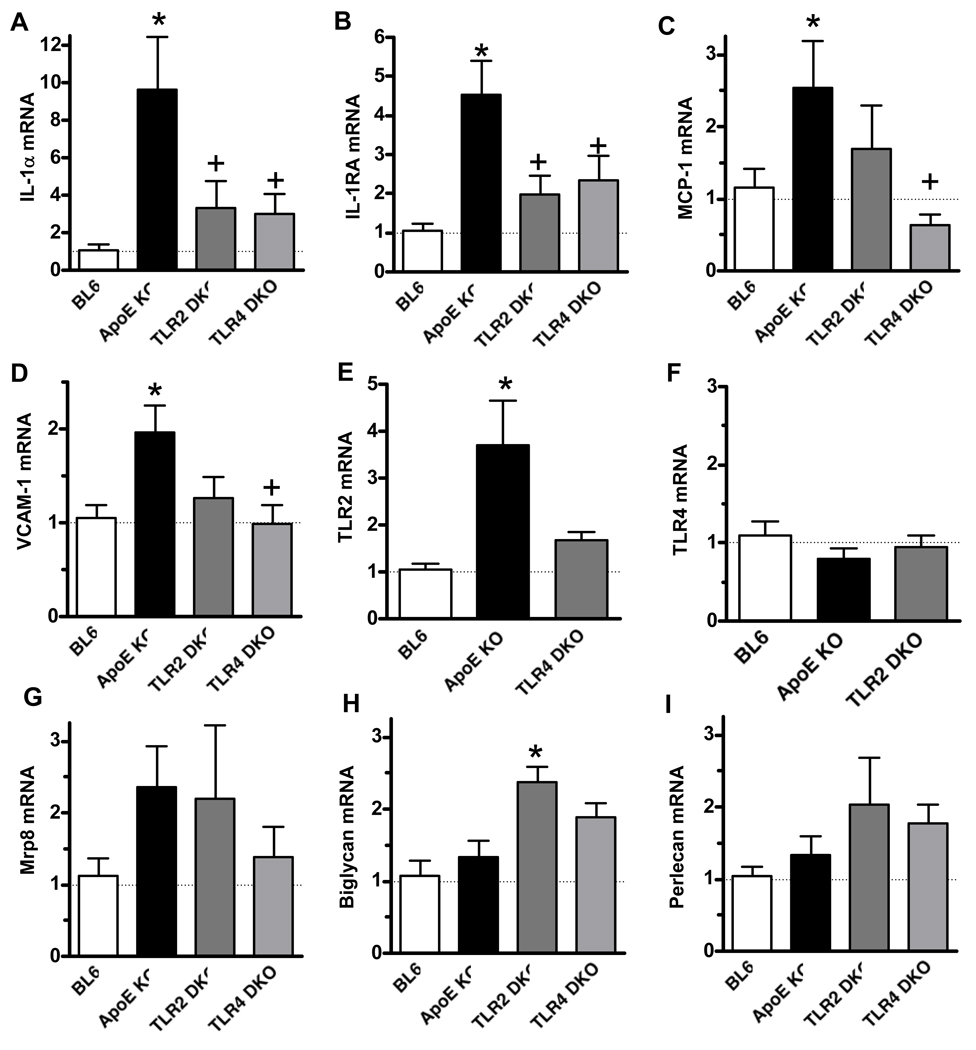
To elucidate the mechanisms whereby TLR4 and TLR2 promote lipid accumulation in the LCAA, we used real-time RT-PCR to analyze local expression of mRNAs encoding mediators relevant to early atherogenesis. IL-1 receptor signaling has been implicated in lesion development in ApoE KO mice 20. Therefore, we tested whether TLR4 contributes to local IL-1α gene expression in the lesion-prone LCAA. IL-1α mRNA levels at this site were increased 9-fold in ApoE KO vs. those in wild-type mice, and this effect was markedly reduced in both TLR4 DKO and TLR2 DKO mice (Fig. 3). TLR4-deficiency also reduced serum IL-1α levels in ApoE KO mice (Table 1), further supporting an important role of IL-1α in the proatherogenic effects of TLR4. IL-1 receptor antagonist (IL-1RA) mRNA was also increased in ApoE KO mice relative to wild-type mice (4.5-fold), and this increase was similarly reduced in both TLR4 DKO and TLR2 DKO mice (Fig. 3). Because IL-1RA is strongly inducible by IL-1, and thus may be indicative of ongoing IL-1 signaling 21, 22, the results suggest that both TLR4 and TLR2 may promote IL-1 signaling within aortic lesions.
Figure 3.
IL-1α, IL-1RA, MCP-1, VCAM-1, and TLR2 mRNAs are increased in a TLR4-dependent manner within the LCAA of ApoE KO mice. Transcript levels were analyzed by real-time RT-PCR of RNA prepared from aortic arch tissue of chow-fed male18 week-old (A–G) or 12 week-old (H,I) mice, normalized to β-actin mRNA levels, and then expressed relative to the values observed in LCAA tissue from wild-type C57BL6/J (BL6) mice. (18wk: n=7/group, except BL6, n=6. 12 wk: n=5/group, except ApoE KO, n=4.). * P < 0.01 vs. BL6; + P < 0.01 vs. ApoE KO.
Table 1.
TLR4 deficiency reduces serum IL-1α (pg/ml)
| Age | 18 weeks | 36 weeks |
|---|---|---|
| BL6 | 13 (9,16) (6) | |
| ApoE KO | 16 (10,41) (7) | 3 (1,11) (7) |
| TLR2 DKO | 9 (0,22) (5) | |
| TLR4 DKO | 1 (0,4) (4)* | 0 (0, 4) (6)* |
Values are median (25th percentile, 75th percentile) (n), for male mice fed a standard chow diet.
P<0.05 vs. ApoE KO
TLR4 deficiency reduces lesional VCAM-1, MCP-1, and TLR2 gene expression
Vascular cell adhesion molecule-1 (VCAM-1) is a key mediator of monocyte adhesion to endothelium during early atherogenesis 23–25, while monocyte chemoattractant protein-1 (MCP-1) released into the subendothelial space promotes monocyte diapedesis and migration into the intima 26, 27. VCAM-1 mRNA levels were not affected at age 12 wk (data not shown) but were doubled in aortic arch of 18 week-old ApoE KO mice vs. those in wild-type controls (Fig. 3), and this increase was abolished in TLR4-deficient mice. MCP-1 mRNA expression increased 2.5-fold in the LCAA of ApoE KO mice vs. those seen in wild-type mice, and this increase was also abolished in TLR4 DKO mice (Fig. 3). VCAM-1 and MCP-1 mRNA levels appeared lower in TLR2 DKO mice, but the differences were not statistically significant. Serum levels of soluble VCAM-1 (Online Table IV) and MCP-1 (data not shown) were unchanged by TLR2 or TLR4 deficiency.
Because TLR2 and TLR4 expression are increased in human atherosclerotic lesions 3, 4, we also tested whether altered TLR2 or TLR4 mRNA expression occurred during early lipid accumulation. TLR2 mRNA levels increased more than 3-fold in LCAA of ApoE KO mice vs. those in wild-type mice (Fig. 3), but were unaltered in TLR4 DKO mice, indicating that TLR2 upregulation was likewise TLR4-dependent. mRNA levels of myeloid-related protein-8 (Mrp8), a putative endogenous activator of TLR4 28, were also increased in ApoE KO mice, but not significantly so. In contrast, TLR4 mRNA levels were unchanged in early lesions of ApoE KO or TLR2 DKO mice vs. those seen in wild-type LCAA segments. These results raise the possibility that TLR4-inducible TLR2 signaling may contribute to lesion formation during early atherogenesis.
Early atherogenesis involves the accumulation of extracellular proteoglycans, which bind lipoproteins and trap them in the subendothelial space where they become modified 1. Two distinct proteoglycans accumulate in early lesions of ApoE KO mice 29, biglycan and perlecan, but it is unknown whether expression of their corresponding mRNAs are increased locally. The levels of both biglycan and perlecan mRNA were similar in the aortic arch of 12 week-old chow-fed WT, ApoE KO and TLR4 DKO mice, and biglycan mRNA was significantly increased rather than reduced in TLR2 DKO mice (Fig. 3). These results thus do not support a role of reduced proteoglycan mRNA expression in the anti-atherogenic effect of TLR deficiency.
Intimal SMC are present in the LCAA at the early lesion stage, and integral to the microarchitecture of nascent lesions
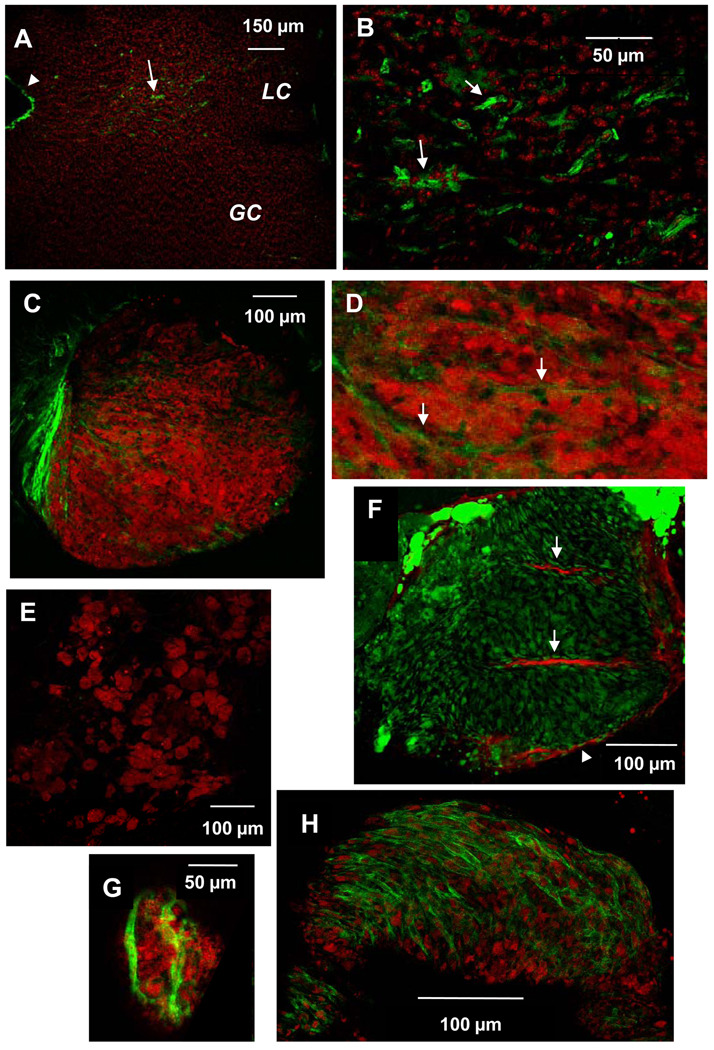
To test whether SMC could contribute to early lesion development in ApoE KO mice in vivo, we determined whether intimal SMC are present in the lesion-prone LCAA of 16 week-old mice. Using en face confocal immunostaining analysis, we found smooth muscle α–actin (SMA)+ cells within the lesion-prone lesser curvature, but these were sparse or absent within the lesion-resistant greater curvature (Fig. 4A, B). In LCAA of older (25 week) ApoE KO mice, most intimal lipid was intracellular, seen as clusters of lipid-laden foam cells (Fig. 4C–F). Notably, SMC were associated with a subset (~50%) of nascent foam cell aggregates (Fig. 4C,F), but absent from others (Fig. 4E). In some cases, spindle-shaped SMC surrounded and permeated spheroidal aggregates of lipid-laden foam cells that comprised a developing lesion (Fig. 4C,D,F; Online Fig. II and III). The spindle-shaped SMC were devoid of lipid, but additional spindle-shaped cells that contained abundant neutral lipid were found directly adjacent to them, raising the possibility that the spindle-shaped foam cells might potentially be derived from SMC. The lesions in TLR4 DKO mice were smaller, as expected, and characterized by small clusters of fewer foam cells that were enveloped by only a few SMC (Fig. 4G), or by sparse foam cells with no associated SMC (not shown). In comparison, a nearly-complete layer of contiguous SMC, reminiscent of a fibrous SMC cap, enveloped large foam cell aggregates within the LCAA of older ApoE KO mice (44 wk; Fig. 4H). The results show that SMC are integral to the microarchitecture of some developing lesions, and are situated to influence foam cell accumulation.
Figure 4.
SMC are positioned to influence lesion development within the LCAA of ApoE KO mice. Shown are en face confocal images of aortic arch tissue from ApoE KO mice of different ages stained with FITC-labeled anti-smooth muscle α-actin (SMA). A, B: SMC (green) are found within the lesion-prone LCAA (LC) of 16 week-old ApoE KO mice but not in the lesion-resistant greater curvature (GC). Cell nuclei are stained with Topro3 (red); arrowhead indicates SMA staining at cut edge of tissue (SMA antibody penetrates into the media at the cut edges only). B is higher magnification of LC in image A. C–G: Lesions of 25 week-old ApoE KO mice were composed of clusters of lipid-laden foam cells. Neutral lipid was visualized with Oil Red O (red) except in F, in which neutral lipid was visualized with BODIPY (green) and mouse anti-SMA was detected with Cy5-conjugated anti-mouse IgG (red). SMC were found intimately associated with some (C,D,F,G), but not all foam cell aggregates (E). C, D, F: Spindle-shaped SMA+ cells surround (arrowhead) and infiltrate (arrows) a large aggregate of foam cells within ApoE KO LC. F: Spindle-shaped cells adjacent to SMA+ cells contain neutral lipid. G: A few SMC envelop a small cluster of few foam cells within TLR4 DKO LC. H: A contiguous SMC layer covers a later lesion in an older ApoE KO mouse (44 wk). C and F are also presented as online supplemental data, consisting of sequential z-plane images, proceeding from the luminal to medial aspect of the lesion.
TLR4 dependence of SMC cholesteryl ester accumulation and ACAT1 gene expression
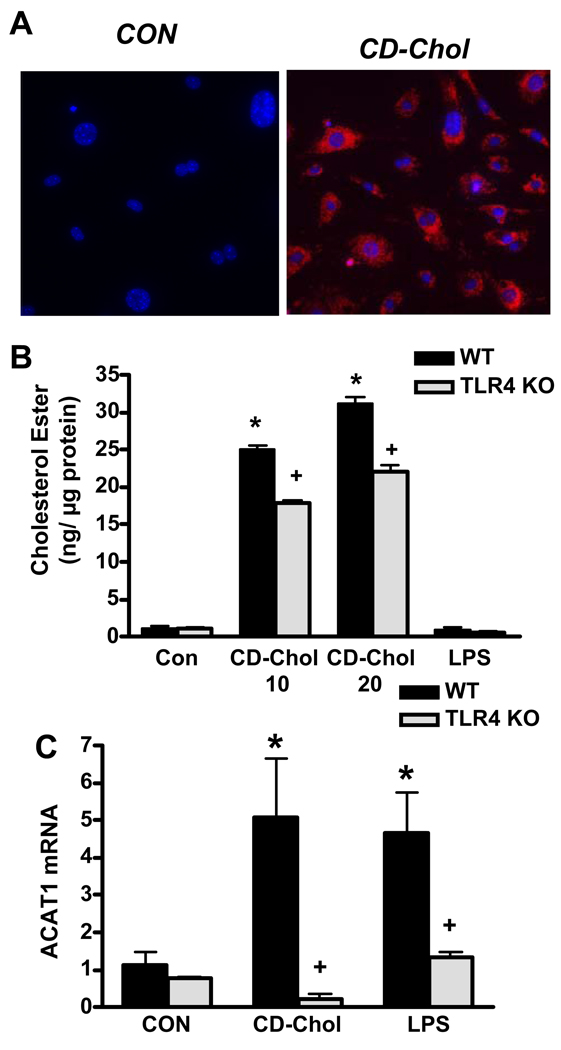
To determine whether TLR4-dependent accumulation of intracellular cholesterol ester in SMC might contribute to intimal lipid accumulation in ApoE KO mice, we compared the cholesterol ester content of wild-type and TLR-deficient mouse aortic SMC incubated in cholesterol-enriched media, an established model of SMC conversion to foam cells 16, 17. Mouse aortic SMC uniformly accumulated abundant cytoplasmic neutral lipid droplets following 68 h incubation with cyclodextrin-cholesterol (Fig. 5A), as previously described. TLR4 deficiency reduced cholesteryl ester accumulation by ~30% vs. that seen in TLR4-expessing SMC (Fig. 5B). ACAT1 is the key cholesterol-esterifying enzyme in most cell types, and its mRNA expression is upregulated during conversion of human SMC to foam cells17, and by NF-κB 30, a key mediator of TLR4-induced gene transcription. Therefore, we tested whether ACAT1 gene expression is increased in a TLR4-dependent manner in SMC incubated with free cholesterol. ACAT1 mRNA levels increased markedly (5-fold) in cholesterol-enriched SMC, and this increase was abolished in TLR4–deficient SMC (Fig. 5C). Similar results were obtained with SMC from ApoE KO mice (data not shown). Exposure to LPS without free cholesterol also induced TLR4-dependent ACAT1 gene expression (Fig. 5C), but not foam cell formation (data not shown), suggesting that both cholesterol-enrichment and TLR4 signaling are required to induce accumulation of cytoplasmic cholesteryl esters in SMC. The results suggest that cholesterol-enriched aortic SMC accumulate cholesteryl esters via a mechanism involving TLR4-dependent ACAT1 gene expression.
Figure 5.
TLR4 deficiency reduces cholesteryl ester accumulation in mouse aortic SMC. A: Intracellular neutral lipid (Oil Red O fluorescence) is abundant in SMC incubated with cyclodextrin-cholesterol (CD-Chol) for 68 h (Blue: Hoescht 33342 nuclear stain). B: TLR4 KO SMC incubated with CD-Chol (10 or 20 µg/ml) show reduced cholesteryl ester content, expressed per µg cellular protein. C: Cholesterol- and LPS-induced ACAT1 gene expression are abolished in TLR4 KO SMC. * P≤ .0002 vs. CON (wild-type SMC without CD-cholesterol or LPS treatment); + P< .0001 vs. SMC treated with CD-cholesterol or LPS.
Cholesterol enrichment stimulates MCP-1, CD68, and Mrp8 mRNA expression: Role of TLR4
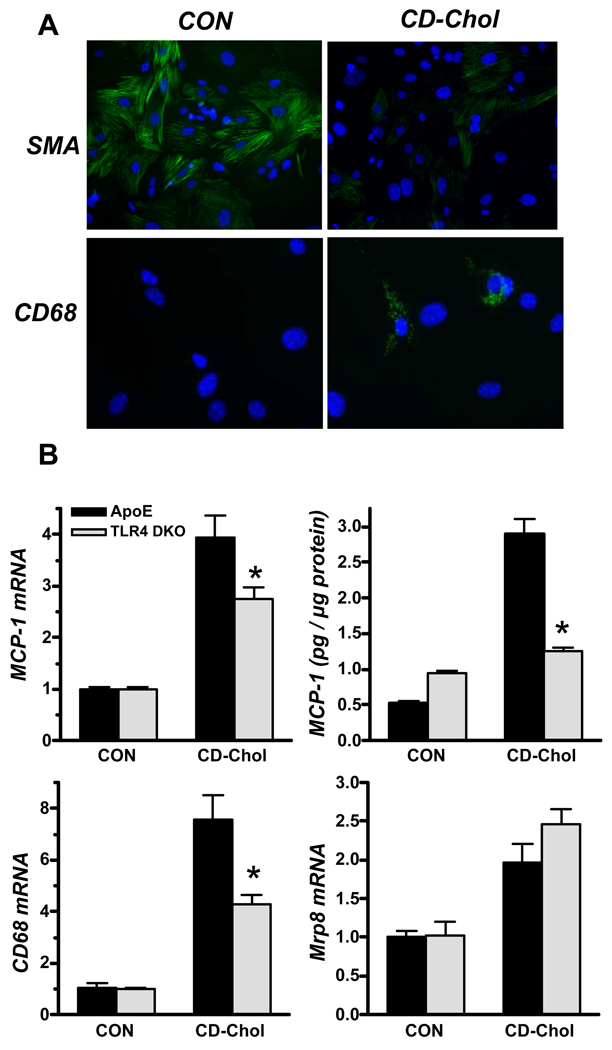
SMC incubated in cholesterol-enriched media lose expression of SMC-specific proteins and gain expression of macrophage-specific proteins 16, 17. In agreement, we found reduced SMA expression in cholesterol-enriched SMC, while a subset of the cells expressed cytoplasmic CD68 (Fig. 6A). Cholesterol enrichment increased CD68 mRNA levels 7.6–fold in ApoE KO SMC, and only 4.3–fold in ApoE/TLR4 DKO SMC. Because TLR4 signaling is a potent inducer of MCP-1 expression in mouse SMC 11, we tested whether cholesterol enrichment increased MCP-1 expression in a TLR4-dependent manner. Cholesterol enrichment increased MCP-1 mRNA levels 4–fold, and MCP-1 release 5-fold in ApoE KO SMC, and both responses were markedly reduced in ApoE/TLR4 DKO SMC (Fig. 6B). Mrp8 mRNA was increased ~2-fold, but its regulation was TLR4-independent. MCP-1, CD68, and Mrp8 mRNA upregulation were similar in ApoE KO and ApoE/TLR2 DKO SMC (data not shown). These results suggest that cholesterol enrichment promotes SMC expression of proteins thought to be macrophage-specific, by mechanisms that are at least in part TLR4-dependent, consistent with our hypothesis that TLR4 signaling might play a role in SMC conversion to a macrophage-like phenotype.
Figure 6.
Cholesterol enrichment stimulates expression of MCP-1, CD68 and Mrp 8 in ApoE KO SMC: role of TLR4. A: SMC from ApoE KO and TLR DKO mice were incubated without (control; CON) or with cyclodextrin-cholesterol (CD-Chol) for 24 h, and SMA and CD68 expression were determined by immunofluorescence staining. SMC incubated in media alone express abundant SMA (green; top panel), but no CD68 (green; bottom panel). SMA is diminished in SMC incubated with CD-Chol, while some SMC express intracellular CD68 (Blue: Hoescht 33342 nuclear stain). B: MCP-1, CD68, and Mrp8 mRNA levels were determined by real-time RT-PCR analysis, normalized to β-actin mRNA, and expressed relative to CON levels. MCP-1 release by SMC was determined by ELISA. *P≤ . 05 vs. ApoE KO SMC.
DISCUSSION
Our findings demonstrate an important role of TLR4, and to a lesser extent TLR2, in early intimal foam cell accumulation in hypercholesterolemic ApoE KO mice. We found that TLR4 deficiency markedly reduced aortic lipid accumulation, by ~70–80%, within the LCAA of ApoE KO mice fed chow or short term high cholesterol diet, indicating that such lipid deposition is strongly TLR4-dependent. Our results point to an early, stronger atherogenic influence of TLR4 than suggested by previous studies, which focused on advanced disease in older ApoE KO mice that were fed high-cholesterol diet for many months, and found only a modest or no role of TLR4 9, 10. Such prolonged feeding of ApoE KO mice with high-cholesterol diet produces severe hypercholesterolemia, which may have limited relevance to human atherogenesis, and may have failed to detect the presently-observed critical role of TLR4 in early lesions due to later activation of additional receptors. The TLR4- and TLR2-induced effects reported here occurred in the absence of altered serum cholesterol or triglyceride levels, indicating that signaling via these receptors increases the susceptibility of the artery wall to inflammation and lipid deposition.
Both TLR4 and TLR2 contributed strongly to the upregulation of the IL-1α and IL-1RA genes seen in lesional tissue of ApoE-deficient mice, and TLR4 deficiency lowered serum IL-1α, providing further evidence for a role of IL-1 α in the proatherogenic effects of TLR4. IL-1 receptor signaling plays a pro-atherogenic role in ApoE KO mice 20 and IL-1α promotes multiple cellular responses relevant to early atherogenesis, including MCP-1 expression and SMC proliferation 31, 32. IL-1 signaling also disrupts LDL receptor regulation and induces foam cell formation in human coronary artery SMC 33, suggesting a potential mechanism for increased cellular lipid accumulation. Together, these results raise the possibility that TLR-dependent IL-1α gene expression may contribute to intimal lipid accumulation.
TLR2 gene expression was also upregulated within lesional aortic tissue of ApoE KO mice, but not that of TLR4-deficient mice. Local upregulation of TLR2 might contribute to intimal lipid accumulation in ApoE KO mice, as TLR2 deficiency reduced it, albeit somewhat less so than TLR4. TLR4 signaling upregulates TLR2 gene expression in endothelial cells, SMC, and macrophages in vitro 12, 34, 35, suggesting that multiple cell types might account for the observed TLR4-dependent, local TLR2 gene expression in ApoE-deficient mice. In LDL receptor-deficient mice, TLR2 expression was regionally upregulated in the LCAA, specifically within endothelial cells 7. Thus, endothelial cell-specific expression might similarly account for TLR2 upregulation seen here in the LCAA of ApoE KO mice.
Our main findings point to a proatherogenic role of TLR4, raising the important question of how TLR4 becomes activated in ApoE KO mice. One possibility is that circulating LPS provides a tonic stimulus to TLR4 signaling, as LPS can be found in the serum of apparently healthy men and laboratory mice, particularly those consuming a high fat diet 36, 37. Although the observed levels of serum LPS in ApoE KO mice were seemingly low (~100 pg/ml), and similar to those in wild-type mice, ApoE-deficient mice are reportedly more sensitive to LPS than wild-type mice, exhibiting markedly enhanced cytokine production and mortality after systemic LPS administration in vivo 38–40. Thus, low levels of LPS may be sufficient to prime the inflammatory response in athero-prone regions of the vasculature. LPS-driven TLR4 signaling has also been postulated to promote human arterial disease 41. For example, epidemiological evidence indicates that low-grade endotoxemia (>50 pg/ml) is a strong risk factor for progression of early carotid artery atherosclerosis in humans 42. Also, human macrophages, endothelial cells and SMC are exquisitely sensitive to LPS, as concentrations as low as 30–100 pg/ml stimulate VCAM-1 expression and MCP-1 release 43–45, suggesting that circulating LPS levels in healthy people may be capable of stimulating atherogenic inflammation.
An alternative hypothesis for how atherogenic TLR4 activation occurs in ApoE-deficient mice is that non-microbial, endogenous TLR4 agonist ligands contribute to a “sterile inflammatory response”. This idea is supported by findings that advanced lesions in older, Western diet-fed ApoE KO mice are similar whether the mice are gnotobiotic and raised in a “sterile” environment free of bacteria and known pathogens, or raised in the presence of ambient pathogens 10. One class of candidate hypercholesterolemia-associated TLR4 ligands includes molecules associated with minimally or moderately oxidized forms of LDL, which stimulate TLR4-dependent fluid phase LDL uptake, a potential mechanism of foam cell formation 46, and expression of multiple chemokines 47. Additional putative endogenous TLR4 ligands include intracellular proteins released by stressed or dying cells, such as Mrp8 28. We found that Mrp8 mRNA was expressed in the LCAA of ApoE KO mice, suggesting that Mrp8 may contribute to TLR4 activation in LCAA lesions. Mrp8 mRNA levels were also increased in SMC incubated in cholesterol-enriched media, suggesting the possibility that TLR4-dependent expression of pro-atherogenic genes in cholesterol-enriched SMC, and/or macrophages 48 may potentially involve enhanced expression of Mrp8, which promotes TLR4 signaling.
Our in vivo immunoconfocal findings revealed an intimate relationship between SMC and developing lesions, supporting a possible role of SMC in modulating foam cell accumulation. First, we found that SMC are prevalent in the intima of the lesion-susceptible lesser curvature, but not the lesion-resistant greater curvature of the aortic arch at the early lesion stage. MCP-1 released by such intimal SMC may promote monocyte recruitment, a possibility supported by our findings that cholesterol-enriched SMC express MCP-1, and that the lesion-prone LCAA expresses MCP-1 mRNA in a TLR4-dependent manner. Second, we discovered that bands of SMC surround and permeate developing clusters of lipid-laden foam cells, suggesting that paracrine actions of SMC-derived cytokines or chemokines may also influence foam cell accumulation in nascent lesions. Furthermore, the spindle-shaped SMC that invested developing lesions were intimately associated with SMA-negative spindle-shaped cells that contained abundant neutral lipid, although they themselves appeared to contain none. The origin of these novel spindle-shaped lipid-laden cells remains to be determined, but their close anatomic association with SMC suggests that they might be SMC-derived. This hypothesis is supported by the findings that ACAT1 gene expression and intracellular cholesterol ester accumulation were increased in a TLR4-dependent manner in SMC incubated in cholesterol-enriched media, while SMA expression is simultaneously diminished. Also supporting this idea, some of the lipid-laden cells present in atherosclerotic lesions of human and primates appear to be of SMC origin 49–51.
These findings suggest that TLR4 promotes early foam cell accumulation and expression of IL-1α and other pro-inflammatory mediators at lesion-prone aortic sites in ApoE KO mice. We found that intimal SMC are situated within nascent lesions in a manner suggesting that they could influence foam cell accumulation. SMC that are intimately associated with nascent lesions may release pro-inflammatory chemokines in a cholesterol-enriched environment, accumulate intracellular cholesterol ester, or convert to a macrophage-like phenotype via mechanisms involving TLR4 signaling.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Alenka Lovy-Wheeler and Rob Jackson, Tufts Center for Neuroscience Research, for assistance with confocal microscopy. We also thank Dr. Richard Karas for review of the manuscript and Dr. Douglas Golenbock, University of Massachusetts Medical School, for kindly providing TLR4 KO mice.
SOURCES OF FUNDING
This work was supported by grants HL47569 (DB), AG020255 (KJM), HL066467 (JBG), and P30 NS047243 (RJ) from the National Institutes of Health, and A2008-130 from the American Health Assistance Foundation (KJM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atheroclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 4.Edfeldt K, Swedenborg J, Hansson GK, Yan Z. Expression of toll-like receptor in human atherosclerotic lesions. A possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 5.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westerterp M, Berbee JF, Pires NM, van Mierlo GJ, Kleemann R, Romijn JA, Havekes LM, Rensen PC. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116:2173–2181. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
- 7.Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, 3rd, Genco CA. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright SD, Burton C, Hernandez M, Hassing H, Montenegro J, Mundt S, Patel S, Card DJ, Hermanowski-Vosatka A, Bergstrom JD, Sparrow CP, Detmers PA, Chao YS. Infectious agents are not necessary for murine atherogenesis. J Exp Med. 2000;191:1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: Role of efficient Toll-like receptor 4 signaling. Am J Physiol (Heart and Circ Physiol) 2005;289:H1069–H1076. doi: 10.1152/ajpheart.00143.2005. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Coriolan D, Schultz K, Golenbock DT, Beasley D. Toll-like receptor 2 mediates persistent chemokine release by Chlamydia pneumoniae-infected vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:2308–2314. doi: 10.1161/01.ATV.0000187468.00675.a3. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol. 2007;27:1159–1165. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 14.Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 15.Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- 16.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A. 2003;100:13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rong JX, Kusunoki J, Oelkers P, Sturley SL, Fisher EA. Acyl-coenzymeA (CoA):cholesterol acyltransferase inhibition in rat and human aortic smooth muscle cells is nontoxic and retards foam cell formation. Arterioscler Thromb Vasc Biol. 2005;25:122–127. doi: 10.1161/01.ATV.0000148202.49842.3b. [DOI] [PubMed] [Google Scholar]
- 18.Meiner VL, Cases S, Myers HM, Sande ER, Bellosta S, Schambelan M, Pitas RE, McGuire J, Herz J, Farese RV., Jr Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci U S A. 1996;93:14041–14046. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 20.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 21.Seckinger P, Klein-nulend J, Alander C, Thompson R, Dayer J, Raisz L. Natural and recombinant human IL-1 receptor antagonists block the effects of IL-1 on bone resorption and prostaglandin production. 1990;145:4181–4184. [PubMed] [Google Scholar]
- 22.McIntyre KW, Stepan GJ, Kolinsky KD, Benjamin WR, Plocinski JM, Kaffka KL, Campen CA, Chizzonite RA, Kilian PL. Inhibition of interleukin 1 (IL-1) binding and bioactivity in vitro and modulation of acute inflammation in vivo by IL-1 receptor antagonist and anti-IL-1 receptor monoclonal antibody. Journal of Experimental Medicine. 1991;173:931–939. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 24.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 25.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 26.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 27.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 28.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 29.Kunjathoor VV, Chiu DS, O'Brien KD, LeBoeuf RC. Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:462–468. doi: 10.1161/hq0302.105378. [DOI] [PubMed] [Google Scholar]
- 30.Lei L, Xiong Y, Chen J, Yang JB, Wang Y, Yang XY, Chang CC, Song BL, Chang TY, Li BL. TNF-alpha stimulates the ACAT1 expression in differentiating monocytes to promote the CE-laden cell formation. J Lipid Res. 2009;50:1057–1067. doi: 10.1194/jlr.M800484-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beasley D, Cooper A. Constitutive expression of interleukin-1α precursor promotes human vascular smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol. 1999;276:H901–H912. doi: 10.1152/ajpheart.1999.276.3.H901. [DOI] [PubMed] [Google Scholar]
- 32.Schultz K, Murthy V, Tatro JB, Beasley D. Endogenous interleukin-1 alpha promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2927–H2934. doi: 10.1152/ajpheart.00700.2006. [DOI] [PubMed] [Google Scholar]
- 33.Ruan XZ, Moorhead JF, Tao JL, Ma KL, Wheeler DC, Powis SH, Varghese Z. Mechanisms of dysregulation of low-density lipoprotein receptor expression in vascular smooth muscle cells by inflammatory cytokines. Arterioscler Thromb Vasc Biol. 2006;26:1150–1155. doi: 10.1161/01.ATV.0000217957.93135.c2. [DOI] [PubMed] [Google Scholar]
- 34.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166:2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 35.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 36.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 37.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 38.de Bont N, Netea MG, Demacker PN, Verschueren I, Kullberg BJ, van Dijk KW, van der Meer JW, Stalenhoef AF. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J Lipid Res. 1999;40:680–685. [PubMed] [Google Scholar]
- 39.Van Oosten M, Rensen PC, Van Amersfoort ES, Van Eck M, Van Dam AM, Breve JJ, Vogel T, Panet A, Van Berkel TJ, Kuiper J. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J Biol Chem. 2001;276:8820–8824. doi: 10.1074/jbc.M009915200. [DOI] [PubMed] [Google Scholar]
- 40.Ali K, Middleton M, Pure E, Rader DJ. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ Res. 2005;97:922–927. doi: 10.1161/01.RES.0000187467.67684.43. [DOI] [PubMed] [Google Scholar]
- 41.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 42.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease. J Amer Coll Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 43.Pugin J, Schurer-Maly C, Leturcq D, Moriarty A, Ulevitch R, Tobias P. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice JB, Stoll LL, Li WG, Dennin GM, Weydert J, Charipar E, Richenbacher WE, Miller FJ, Jr, Weintraub NL. Low-level endotoxin induces potent inflammatory activation of human blood vessels. Inhibition by statins. Arterioscler Thromb Vasc Biol. 2003;23:1576–1582. doi: 10.1161/01.ATV.0000081741.38087.F9. [DOI] [PubMed] [Google Scholar]
- 45.Stoll LL, Denning GM, Li WG, Rice JB, Harrelson AL, Romig SA, Gunnlaugsson ST, Miller FJJ, Weintraub NL. Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. J. Immunol. 2004;173:1336–1343. doi: 10.4049/jimmunol.173.2.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009;104:455–465. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katsuda S, Boyd HC, Fligner C, Ross R, Gown AM. Human atherosclerosis. III. Immunocytochemical analysis of the cell composition of lesions of young adults. Am J Pathol. 1992;140:907–914. [PMC free article] [PubMed] [Google Scholar]
- 50.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 51.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984;4:323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.