In the developing organism, cells differentiate, divide and die as part of groups of hundreds or thousands of cells called ‘morphogenetic fields’. Fields have the remarkable property of self-regulation: for example, if the forelimb field is bisected, each half can give rise to a complete limb after transplantation, as discovered by Ross Harrison in 1918. Therefore, cells in the morphogenetic field are capable of long-range communication with each other in order to ascertain their position [1]. This positional information is relayed in the extracellular space in the form of concentration gradients of specific classes of extracellular molecules called ‘morphogens’ that trigger cellular responses by binding and activating cell surface receptors. Here, we focus on a family of morphogens called ‘Bone Morphogenetic Proteins’ (BMPs), which has provided a new paradigm for signaling regulation in the extracellular space.
BMPs were discovered by Marshall Urist in 1965, who found that decalcified bone matrix fragments had bone-inducing activity when transplanted subcutaneously or intramuscularly into rats or rabbits. This activity was then solubilized and purified by Hari Reddi in 1981, and in 1988 BMP2–7 were cloned [2]. BMPs belong to the Transforming Growth Factor β (TGF-β) superfamily, the most numerous group of growth factors in humans. During development, BMPs have been shown to participate in many signaling processes, including organogenesis, tissue type differentiation and dorsal-ventral patterning. In humans, altered BMP signaling is associated with cancer, skeletal and vascular diseases.
Binding of BMPs to cell membrane BMP receptors causes the phosphorylation and activation of transcription factors called ‘Smad1/5/8’ inside the cell. The BMP gradient is controlled by the action of an intricate network of secreted proteins that determine the concentration of active BMP ligands.
In the frog Xenopus laevis, these secreted proteins include BMP-binding molecules that behave as anti- or pro-BMPs and act by sequestering, transporting and solubilizing BMPs. This network of proteins has been conserved throughout the animal kingdom. Many of the Xenopus extracellular BMP regulators are also present in Drosophila, in which they also serve to generate a gradient of dorsal-ventral patterning information. These regulators include Chordin, Crossveinless-2 (CV2), Twisted Gastrulation (Tsg), Tolloid metalloproteinases that cleave and inactivate Chordin, Sizzled (a secreted Frizzled-related protein or sFRP) that is a competitive inhibitor of Tolloid metalloproteinases, the Ont-1 olfactomedin-related protein that promotes Chordin degradation, and Collagen IV, a component of the extracellular matrix that binds BMPs modifying their diffusion [1,3]. Ultimately, these extracellular factors regulate the flow of BMPs within the embryonic morphogenetic field toward regions that require peak BMP activity. The flow of growth factors is essential for the formation of a dorsal-ventral BMP gradient with its highest point in the ventral side of the Xenopus embryo, and for the long-range cell communications required for the self-regulating adjustments of the embryonic morphogenetic field.
The components listed above, however, represent just the tip of the iceberg. Many other secreted BMP signaling antagonists have been discovered, such as Noggin, Follistatin (also an inhibitor of Activin), and members of the Cerberus/Gremlin/Coco/Dan family (which are multivalent BMP inhibitors that also affect other signaling factors such as Nodal and Wnts). Here, we concentrate on those components of the Chordin–BMP pathway that are known to interact genetically with BMPs in Drosophila or zebrafish studies, or that have been shown biochemically to bind to each other in the physiologically relevant, nanomolar range. It is likely that additional molecules will be added to this dorsal-ventral biochemical pathway in due course.
The Chordin–BMP pathway
Over the past decade, considerable progress has been made in understanding the biochemical function of extracellular BMP regulators during Xenopus dorsal-ventral development. A central player is Chordin, a protein abundantly secreted in the dorsal side of the embryo. This region, called Spemann’s organizer, can induce a twinned axis after transplantation. Chordin is essential for this axis-inducing activity, as depletion of Chordin with antisense morpholino oligos results in the loss of all inductive activity of Spemann’s organizer on neighboring cells [1]. BMPs are expressed both on the dorsal side of the embryo, where BMP2 and ADMP are secreted, and on the ventral side, which produces BMP4 and BMP7. Loss of Chordin results in the reduction of dorsal structures and expansion of ventroposterior tissues (ventralization). Depletion of all four BMPs causes the entire ectoderm to become brain tissue, indicating complete dorsalization.
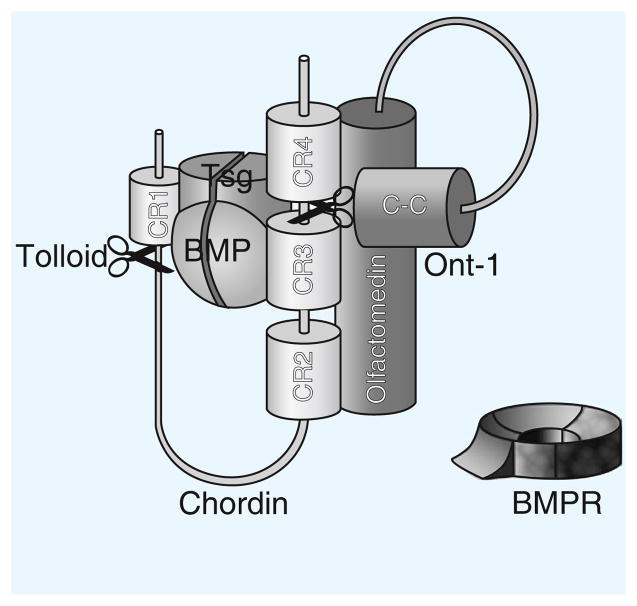
Chordin contains four cysteine-rich domains (related to von Willebrand Factor C, vWF-c, domains) which bind BMPs, inhibiting BMP binding to their receptors (Figure 1). Inhibition is reversible, as active BMPs are released by Tolloid metalloproteinases that cleave Chordin at two specific sites. Tsg binds to both Chordin and BMP, facilitating the formation of Chordin–Tsg–BMP trimolecular complexes that can diffuse in the extracellular space. In this aspect of its function, Tsg is an anti-BMP. However, Tsg also has pro-BMP functions, as it serves to maintain BMPs, which have a strong tendency to bind to extracellular matrix proteins, in a soluble state. In zebrafish, knockdown of Tsg causes dorsalization, revealing that its pro-BMP effect prevails overall [1].
Figure 1.
The Chordin–BMP–Tsg–Tolloid–Ont-1 complex.
Chordin–BMP–Tsg is a trimolecular inhibitory complex that prevents BMP from binding to its receptor (BMPR). Tolloid (scissors) cleaves Chordin at two specific sites, after CR1 and between CR3 and CR4. Ont-1 brings Chordin and Tolloid together, facilitating proteolysis. The olfactomedin domain of Ont-1 interacts with Chordin between CR2 and CR4, while its coiled-coil (C-C) domain binds to Tolloid.
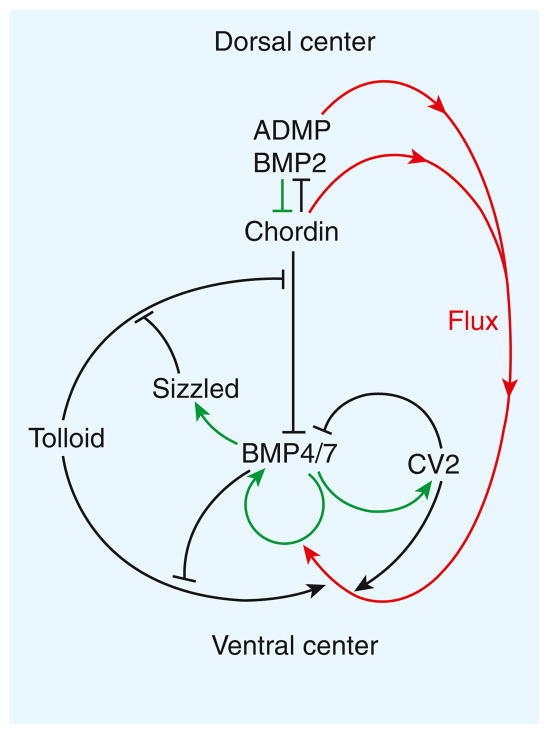
Sizzled, a protein secreted in a ventral region of the gastrulating Xenopus embryo called the ‘ventral center’, provides an additional layer of regulation (Figure 2). Sizzled inhibits the Tolloid protease, competing with its Chordin substrate for the protease’s active site. Sizzled and Chordin are secreted in similar amounts, but unlike Sizzled Chordin is secreted from the dorsal side of the embryo. In zebrafish, chordin or sizzled mutants have very similar loss-of-function phenotypes. Sizzled expression acts as a negative feedback inhibitor of BMP signaling in the ventral center. This is achieved indirectly by inhibition of Tolloid activity, which leads to increased levels of the BMP antagonist Chordin.
Figure 2.
Self-regulation in Xenopus dorsal-ventral patterning.
The pathway consists of a network of BMP-interacting proteins. The diagram indicates extracellular biochemical protein–protein interactions as black arrows, transcriptional regulation under the control of BMP signaling as green arrows, and the directional flow of Chordin–BMP complexes from the dorsal to the ventral side of the embryo as red arrows.
Tolloid is also under direct negative feedback regulation by BMP (Figure 2). It has been recently found that BMP4 is a non-competitive inhibitor of Tolloid enzymatic activity [2]. In addition to a zinc metalloproteinase region, Tolloids contain five CUB domains and two EGF domains. BMP4 binds to individual CUB domains [2]. This binding provides the molecular explanation for an old mystery: when the first peptide sequences from extracts with bone-inducing activity were obtained, BMP2 to BMP7 turned out to be growth factors, but the first protein identified, designated BMP1, corresponded to a Tolloid enzyme containing three CUB domains, which serve as BMP4/7 binding modules. A total of 53 other extracellular proteins in the human proteome contain CUB domains, raising the interesting possibility that many of these modules may function as BMP/TGF-β regulators.
A recently identified component of the Chordin pathway is Ont1, a member of the olfactomedin family [4]. Olfactomedins contain two distinct domains, a coiled-coil and an olfactomedin domain. It was discovered that the coiled-coil domain of Ont1 binds Tolloid/BMP1, while its olfactomedin domain binds Chordin (Figure 1). Thus, Ont1 functions as an extracellular scaffold protein that brings together Tolloid metalloproteinases and Chordin. Ont1 has a pro-BMP activity because it facilitates the degradation of Chordin. In the future, it will be interesting to ask whether other olfactomedins serve as scaffolds joining extracellular proteases and their substrates.
A role for the extracellular matrix
The extracellular matrix plays a role in formation of the BMP gradient. In Drosophila, type IV Collagen, a basement membrane collagen that is secreted into the perivitelline space of the blastoderm embryo, has been shown to bind the BMP2/4 homologue Dpp [5]. Binding requires a short conserved sequence in the carboxy-terminal region of Drosophila and human Collagen IV. Dpp has been demonstrated to flow towards the dorsal side of the Drosophila embryo [6]. This transport requires both Short gastrulation (Sog), the homologue of Chordin, and Drosophila Tsg. Collagen IV in the extracellular matrix binds both Dpp and Sog, and in the presence of Tsg it greatly facilitates the formation of Sog–Tsg–Dpp complexes, which become soluble and are transported to the dorsal side. In evolutionary terms, the dorsal side of Drosophila is homologous to the ventral (high-BMP) side of vertebrates because an inversion of the dorsal-ventral axis took place during evolution [1]. As Collagen IV facilitates the flow of Dpp, its overall function is to promote peak levels of BMP signaling. This molecular mechanism is likely to be conserved in vertebrates, because binding of BMP4 to human type IV Collagen has been shown [5].
Crossveinless-2 provides a sink for Chordin–Tsg–BMP flow
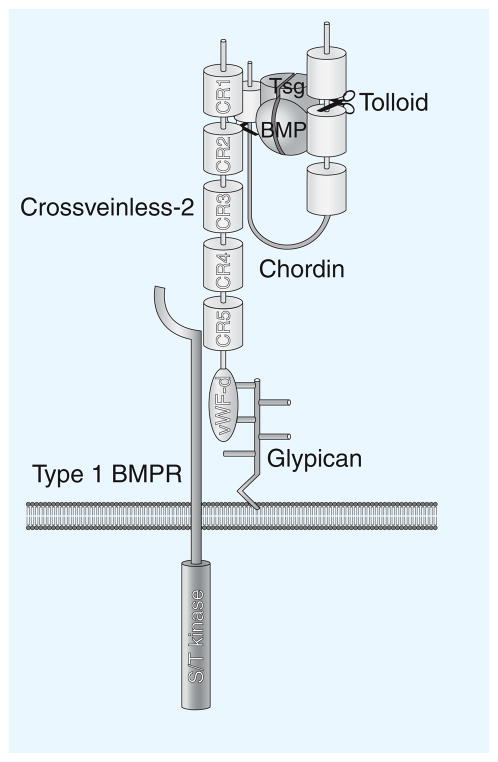
A molecule that in recent years has captivated the attention of scientists in the BMP field is CV2. It was first identified in Drosophila as a mutation eliminating the formation of wing crossveins, structures that require high-BMP signaling. This suggested that CV2 would function as a pro-BMP. However, once the gene was cloned, initially by Seth Blair, both pro- and anti-BMP functions became evident. CV2 is a secreted protein containing five CR Chordin-like domains (Figure 3). The carboxyl terminus of CV2 has a vWF-d domain, which binds to heparan sulfate proteoglycans, such as glypicans attached to the plasma membrane via a Glycosyl-Phosphatidyl-Inositol (GPI) hydrophobic anchor. This limits the ability of CV2 to diffuse in the extracellular space, so that it remains tethered to the cells in which it was produced.
Figure 3.
CV2–Chordin interaction.
Membrane-tethered CV2 serves as a sink for Chordin–Tsg–BMP on the ventral side of frog embryos. CV2 also interacts with the type I BMP transmembrane receptor (a serine/threonine protein kinase that phosphorylates Smad1/5/8). This concentrates BMPs in the vicinity of their receptors once they have been released by Chordin after Tolloid cleavage (scissors). CV2 is also involved in the endocytosis of BMPs and possibly of Chordin and proteolytic Chordin fragments, removing them from the extracellular space.
CV2 transcription is activated by high BMP signaling. In the Xenopus gastrula, CV2 is expressed in the ventral center. Like Chordin, it inhibits BMP signaling by binding to BMPs. In addition, CV2 triggers the clearing and inactivation of BMPs from the extracellular space through an endocytic trap mechanism. Therefore, in this aspect of its function, CV2 serves as a BMP-feedback inhibitor (Figure 2).
How does the pro-BMP function of CV2 arise? Biochemical studies in Xenopus provided the molecular explanation: CV2 binds with high affinity to Chordin [7]. CV2 binds with even higher affinity to Chordin–BMP complexes, as well as to Chordin fragments generated by digestion with Tolloid protease. Thus, CV2 tethered to the cell membrane of ventral cells serves as a molecular sink (Figure 3), concentrating Chordin–Tsg–BMP complexes made in more dorsal regions of the frog embryo. Once in the ventral side, BMPs are released from Chordin complexes by Tolloid digestion, allowing peak BMP signaling to take place. Therefore, CV2 has a pro-BMP function because it promotes the flux of BMP morphogens to the region in which CV2 is expressed. Drosophila CV2 binds to the type I BMP receptor Thickveins as well as to Sog. The proximity between a cell surface BMP-binding protein and its BMP receptor may also help promote signaling at low CV2 levels [3].
As most of the dissociation or enzymatic constants of this network of interacting extracellular proteins have been determined experimentally, the Chordin–Tsg–BMP–Tolloid–CV2 pathway is ideally suited for mathematical modeling by reaction-diffusion equations [2]. Using this type of approach, it has been found that the flux of growth factors is essential to ensure robustness in the size adjustments embryonic morphogenetic fields must undergo after experimental manipulations [8]. In Drosophila, the crucial role played by the flux of Sog–Tsg–Dpp towards the site of peak BMP signaling has been documented in many modeling and experimental studies [3,6].
Self-regulation and transcriptional control
How does a Xenopus embryo re-form the entire body axis after being cut in half? The key to understanding this lies in the opposite transcriptional control of the dorsal and ventral centers. The transcription of ventral center genes is activated by BMP signaling (via Smad1/5/8 transcription factors), while the transcription of dorsal genes is repressed by BMP (Figure 2).
A molecular see-saw is established, such that, for example, if BMP levels are lowered, BMP2 and ADMP levels increase in the dorsal center, restoring the gradient. If BMP levels increase, Sizzled levels rise, inhibiting BMP signaling indirectly through the inhibition of Tolloid enzyme activity and the consequent increase in the levels of the Chordin BMP antagonist (Figure 2). The Chordin–BMP biochemical pathway is self-adjusting due to a combination of intracellular transcriptional regulation and extracellular biochemical events that drive the flow of BMPs within the field [1].
Morphogenetic fields
Why is self-regulation of the BMP gradient so precisely regulated? In the case of Xenopus embryos, the egg is laid in a pond where temperature will undoubtedly fluctuate. Mesodermal cells envelop the yolky endoderm, with the blastopore becoming ever smaller yet maintaining a constant gradient of BMP signaling. The ability of cells at opposite poles of the embryo to communicate over long distances — the frog gastrula contains about 10,000 cells — and to introduce self-adjustments, is an essential biological property. This is because the BMP morphogen gradient is responsible for histotypic differentiation in the embryo. For example, in the mesodermal germ layer, at the low end of the BMP gradient, prechordal plate and notochord differentiate. As BMP levels increase towards the ventral side, somite (muscle), kidney, lateral plate (body wall) and finally blood differentiation is elicited. The embryo gets only one chance to get allocation of its tissues right, and this has to be perfectly achieved time after time.
A very important outstanding question is whether the molecular mechanisms discovered in the morphogenetic field of early embryos also operate at later stages of development. Many self-regulating fields have been described by experimental embryologists — such as limb, heart, eye, lens, olfactory, and other organ-fields — but we do not know how any of these achieve self-regulation. One idea is that cells sense the positional information of their immediate neighbors, generating a system of polar coordinates that can explain many experimental observations in limb regeneration. Models in which physical morphogen gradients are maintained by the physicochemical flow of proteins within the organ-field would represent a significant departure from current wisdom. Yet, useful developmental circuits have a tendency to be used over and over in animals, not just in the embryo but also in the adult.
One hint that protein flow might take place at later developmental stages comes from vertebral development in the mouse embryo. Knockout of CV2 in mice decreased BMP signaling in the intervertebral disc, even though CV2 protein is secreted and retained by prospective vertebral body cartilage [9]. Therefore, loss of CV2 has long-range effects in the vertebral morphogenetic field (about 85 microns at embryonic day 12.5). It is possible that CV2 in cartilage may act as a sink for Chordin–Tsg–BMP4 transported from the intervertebral disc. Chordin protein is secreted by intervertebral disc cells but accumulates in the vertebral body, and this flow of protein has a complete requirement for CV2. The long-range inhibition of Smad1/5/8 phosphorylation [9] may be due to BMP inhibition caused locally by Chordin that is unable to flow towards its CV2 sink. Although a function for protein flow in later cell fields remains speculative, it is now clear that the Chordin–Tsg–BMP–Tolloid–CV2 pathway, involving reciprocal transcriptional regulation at opposite poles and protein flow, constitutes the ancestral mechanism of dorsal-ventral patterning. It determines dorsal-ventral positional information in the early morphogenetic field of embryos as diverse as those of Xenopus, zebrafish, amphioxus, hemichordates, spiders, beetles and Drosophila and presumably in most bilateral animals [1].
Further reading
- 1.De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 2009;126:925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HX, Mendes FA, Plouhinec JL, De Robertis EM. Enzymatic regulation of pattern. BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev. 2009;23:2551–2562. doi: 10.1101/gad.1839309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inomata H, Haraguchi T, Sasai Y. Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell. 2008;134:854–865. doi: 10.1016/j.cell.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 6.Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell. 2008;15:248–260. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 9.Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM. Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted Gastrulation. Dev Biol. 2008;323:6–18. doi: 10.1016/j.ydbio.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]