Abstract
Lesch–Nyhan disease is a neurogenetic disorder caused by mutation of the HPRT1 gene on the X chromosome. There is significant variation in the clinical phenotype, with more than 300 different known mutations. There are few studies that have addressed whether similar mutations result in similar phenotypes across different patients because hypoxanthine–guanine phosphoribosyltransferase (HGprt) deficiency is rare, and most mutations are unique or limited to individual families. However, recent studies have revealed multiple unrelated patients with similar mutations, providing an opportunity to examine genotype–phenotype correlations. We found significant variation among the clinical features of 10 patients from 8 unrelated families all carrying a mutation replacing guanine with adenine at base position 143 (c.143G>A) in the HPRT1 gene. This mutation results in replacement of arginine by histidine at amino acid position 48 (p.arg48his) in the HGprt enzyme. Biochemically, the enzyme exhibits reduced thermal integrity, a mechanism that may explain clinical variation. The literature reveals similar clinical variation among other patients with similar mutations, although the variation is relatively minor across the whole population of patients. Identifiable sources of clinical variation include known limitations of clinical ascertainment and mechanisms that affect residual enzyme activity and stability. These results are helpful for understanding genotype–phenotype correlations and discordance and likely are applicable to other neurogenetic disorders where similar variation occurs.
Introduction
Lesch–Nyhan disease (LND) is a genetic disorder of purine metabolism in which the enzyme, hypoxanthine–guanine phosphoribosyltransferase (HGprt), is defective (Lesch and Nyhan 1964; Seegmiller et al. 1967). The etiology involves a mutation of the HPRT1 gene, which is on the long arm of the X chromosome (Jinnah et al. 2000, 2004). The HGprt protein functions as a dimer or tetramer. It is involved in the purine salvage pathway whereby purine bases are recycled into nucleotides (Jinnah and Friedmann 2001).
Different HPRT1 mutations result in varied levels of residual HGprt enzyme activity as well as a spectrum of disease characteristics. Classic features of LND include hyperuricemia and its sequelae (gout, nephrolithiasis, and tophi), motor disability (dystonia, chorea, and spasticity), intellectual impairment, and self-injurious behavior (Jinnah et al. 2006). In addition to this classic presentation, milder forms of the disease exist in which some clinical features are attenuated or absent (Emmerson and Thompson 1973; Jinnah et al. 2010; Puig et al. 2001). Although there is a continuous spectrum of severity, patients can be clustered into three groups. The most severely affected group has all the classic features of LND. Those in the least severely affected group, HGprt-related hyperuricemia (HRH), exhibit only signs and symptoms related to uric acid overproduction. An intermediate group, HGprt-related neurologic dysfunction (HND), has hyperuricemia and varying neurological abnormalities but no self-injurious behavior (Jinnah et al. 2010).
Although the HPRT1 gene and HGprt protein have been widely studied, few reports have addressed whether similar mutations result in similar phenotypes across different patients (Hladnik et al. 2008; Sarafoglou et al. 2010). The reason for the paucity of such studies is that HGprt deficiency is rare, and most mutations are unique or limited to individual families. In recent studies, multiple unrelated patients with similar mutations were found, providing an opportunity to examine genotype–phenotype correlations (Jinnah et al. 2010). Here we compare the clinical features of ten patients from eight unrelated families with the c.143G>A mutation, which may have arisen independently many times because it is at a CpG motif, where cytosines are frequently methylated and then deaminated to 5-methylcytosine to generate thymine (O'Neill and Finette 1998). We also examine the consequences of the p.arg48his substitution on HGprt enzyme function, and discuss potential mechanisms for genotype–phenotype correlation and discordance.
Materials and methods
Expression vector for HPRT1
The biochemical properties of the c.143G>A mutation leading to p.arg48his were compared with normal human HGprt following expression and purification in vitro. PCR primers for HPRT1 were first designed to add an N-terminal tag containing six histidines for protein purification and cloned into an intermediate vector (pENTRY/nHisHGPRT) using the TOPO™ cloning system (Invitrogen, Carlsbad, CA, USA). The cDNA was then cloned into the pET24d(+) expression vector (Novogen, New Canaan, CT, USA) to create the final vector (pET24d(+)/nHisHGPRT). The c.143G>A mutation was introduced into the parent vector by site-directed mutagenesis with the PCR-based Quick-Change kit from Stratagene (La Jolla, CA, USA), with primers designed via the QuickChange Primer Design Program (http://www.stratagene.com). All coding sequences were confirmed before protein expression.
Protein expression and purification
Escherichia coli BL21(DE3) cells were transformed and cultured, and protein expression was induced by isopropyl β-d-1 thiogalactopyranoside (IPTG) for 5 h at 37°C. Bacteria were lysed by sonication and enzymatic digestion. The lysate was then passed through a nickel-based affinity column, which bound the polyhistidine tag. The column was washed with 50 mM Tris–HCl, pH 7.4, 300 mM NaCl, 5% glycerol, 10 mM β-mercaptoethanol and stepwise increasing concentrations of 75 and 100 mM imidazole to eliminate non-specific binding. Purified protein was eluted with 250 mM imidazole, and salts were removed using a PD-10 column (GE Healthcare, Piscataway, NJ, USA). The final protein was concentrated using Amicon ultra centrifugal filter tubes (Millipore, Billerica, MA, USA). Purity was determined by SDS-PAGE followed by staining with Coomassie blue. Protein quantification was carried out using the Bradford method.
Enzyme kinetics
The enzymatic activities of the normal and p.arg48his mutant were tested in vitro. To examine the kinetics towards hypoxanthine or guanine, a total of 200 ng of purified enzyme was tested with 1 mM phosphoribosylpyrophosphate (PRPP) and varying concentrations of hypoxanthine (0–100 μM) or guanine (0–100 μM) at 37°C in a 200 μl reaction volume containing 100 mM Tris–HCl and 12 mM MgCl2 at pH 7.4. To examine kinetics towards PRPP, the reaction contained saturating amounts of hypoxanthine (200 μM) or guanine (200 μM) and varying concentrations of PRPP. Reactions were monitored in quadruplicate in a 96-well UV compatible microplate with a SpectraMax M5e spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Production of IMP from hypoxanthine or GMP from guanine was measured at 245 and 257 nm, respectively. The change in extinction coefficient for hypoxanthine to IMP is 1,770 M−1 cm−1 and for guanine to GMP is 5,146 M−1 cm−1. The Michaelis–Menton Km and Vmax were calculated with SigmaPlot (Systat Software Inc., San Jose, CA, USA) by non-linear regression of initial velocities at each substrate concentration. Enzymes were tested independently three times, and kinetic parameters were averaged and compared via Student's t test with p < 0.05 as the level of significance.
Thermal stability
The thermal stabilities of normal HGprt and the p.arg48his mutant were compared by measuring residual activity after incubation at 37 or 55°C over 32 h. An aliquot of the protein at a concentration of 20 μg/mL was heated to the target temperature and enzyme activity was tested at specific time points in triplicate with of 250 ng of protein, 1 mM PRPP, and 100 μM guanine in 100 mM Tris–HCl with 12 mM MgCl2, pH 7.4.
Results
Clinical features associated with c.143G>A
We identified ten patients from eight different families all with the c.143G>A mutation (Table 1). All exhibited signs of uric acid overproduction. Nine were classified as HND because of evidence for motor or cognitive impairment, while one was classified as HRH. The age at presentation varied from infancy to 28 years. Six presented with motor delay, two presented with renal problems, and one with tophi. Eight had motor impairments, while two did not. Most had low-normal or borderline intelligence. Although none had self-injury, four showed impulsive or oppositional behaviors similar to those of classic LND. Clinical tests for enzyme activity revealed no activity in erythrocyte lysates, but higher levels in intact erythrocytes or cultured fibroblasts.
Table 1. Clinical phenotype associated with c.143G>A.
| Case ID | Group | Age | Presenting age | Presenting features | Uric acid increased | Motor impairments | Cognition | Behavior | Residual HGprt enzyme activity |
|---|---|---|---|---|---|---|---|---|---|
| 6-6, DoA | HND | 9 | 2 years | Motor delay | Yes | Mild dystonia | Poor attention, IQ = 79 | Impulsive, oppositional | ND in erythrocyte lysates |
| 18-14, TH | HND | 17 | 6 years | Attention deficit disorder | Yes | None | Poor attention, IQ = 89 | Impulsive, oppositional | 39% in cultured fibroblasts |
| 20-16, VC | HND | 18 | <1 year | Motor delay | Yes | Mild dysarthria, hyperreflexia | Significant impairment | Normal | ND in erythrocyte lysates |
| 23-14, GH | HND | 20 | 2.5 years | Clumsy motor skills | Yes | Moderate dystonia | Poor attention, IQ = 83 | Normal | 15% in cultured fibroblasts |
| 28-23, AG | HND | 24 | 13 years | Dystonic gait | Yes | Mild dystonia | Borderline | Normal | 0.3% in erythrocyte lysates; 9.2% in intact erythrocytes |
| VD | HRH | 24 | 15 months | Nephrolithiasis | Yes | None | Normal | Impulsive, oppositional | ND in erythrocyte lysates |
| LA | HND | 26 | <2 years | Motor delay | Yes | Mild dysarthria | NA | Impulsive, oppositional | ND in erythrocyte lysates |
| 30-24, DD | HND | 29 | Early childhood | NA | Yes | Mild dystonia, hyperreflexia, clonus | IQ = 96 | Onychophagia | 20% in cultured fibroblasts |
| 36-16, JB | HND | 37 | 1.5 years | Motor delay | Yes | Mild dystonia, hyperreflexia | Poor school performance | Onychophagia | NA (diagnosis via affected relative) |
| 43-16, CZ | HND | 56 | 28 years | Tophi | Yes | Mild dysarthria, hyperreflexia | NA | Emotional lability | NA (diagnosis via affected relative) |
Clinical features and results of clinical diagnostic testing were collected from prior reports of these same patients (Andres et al. 1987; Jinnah et al. 2010; Larovere et al. 2007; Puig et al. 2001) and/or the medical records. Some information was not available for all cases (NA), and some enzyme results were below detectable limits (ND). Related patients included two American brothers (TH and GH), two French cousins (VD and LA), and three Argentinean cousins (VC, JB, and CZ)
Enzyme kinetics
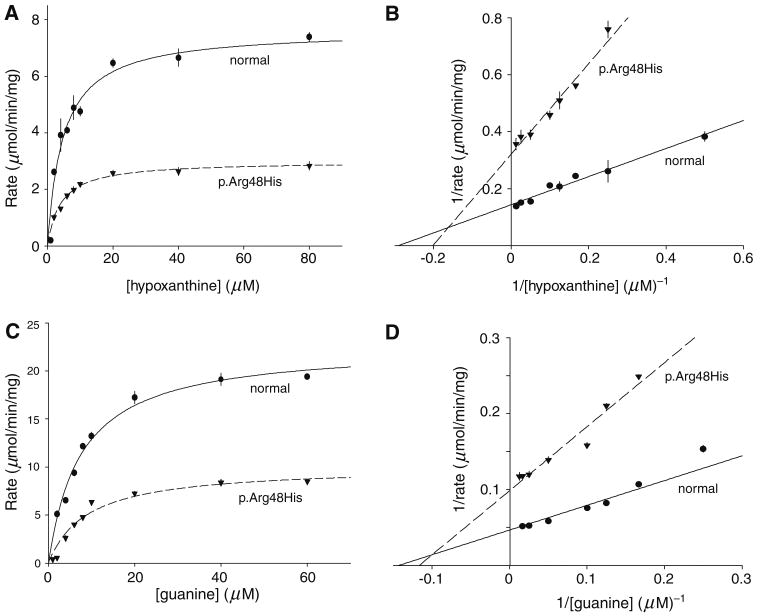
The methods for producing the normal HGprt and p.arg48his mutant reliably generated at least 1 mg/mL of protein with a single major band on SDS-PAGE at the expected molecular weight of 26 KD and approximate purity of 95%. An unpaired t test was conducted to compare the kinetic parameters of freshly prepared mutant enzyme with normal HGprt (Fig. 1; Table 2). There were no significant differences between the normal and the p.arg48his mutant in the Km for either hypoxanthine (p = 0.40) or guanine (p = 0.98). However, the Vmax for p.arg48his was decreased by 33% for hypoxanthine (p < 0.05) and by 37% for guanine (p < 0.002). Kinetic studies varying PRPP concentrations with saturating amounts of hypoxanthine or guanine revealed similar small reductions in Vmax only.
Fig. 1.
Kinetic properties of the human HGprt enzyme. The normal enzyme is shown with solid lines and the p.arg48his mutant with dashed lines. This is one of three representative plots used to generate average values shown in Table 2. a, b Michelis–Menten and Lineweaver–Burke plots for hypoxanthine; c, d similar plots for guanine. Each data point was determined in quadruplicate, and the symbols reflect average ± SD
Table 2. Apparent enzyme kinetics.
| Km (μM) | Vmax (μM/min/mg protein) | |
|---|---|---|
| Hypoxanthine | ||
| Normal HGprt | 5.6 ± 1.9 | 8.0 ± 2.1 |
| p.arg48his | 6.8 ± 2.4 | 5.3 ± 1.9* |
| Guanine | ||
| Normal HGprt | 7.9 ± 0.5 | 18.0 ± 3.1 |
| p.arg48his | 7.8 ± 2.0 | 11.3 ± 2.3* |
Kinetic properties of the normal and p.arg48his mutant HGprt enzyme were determined from three independent assays and combined to give average values ± SD. Asterisks denote significant differences after unpaired t tests (p < 0.05)
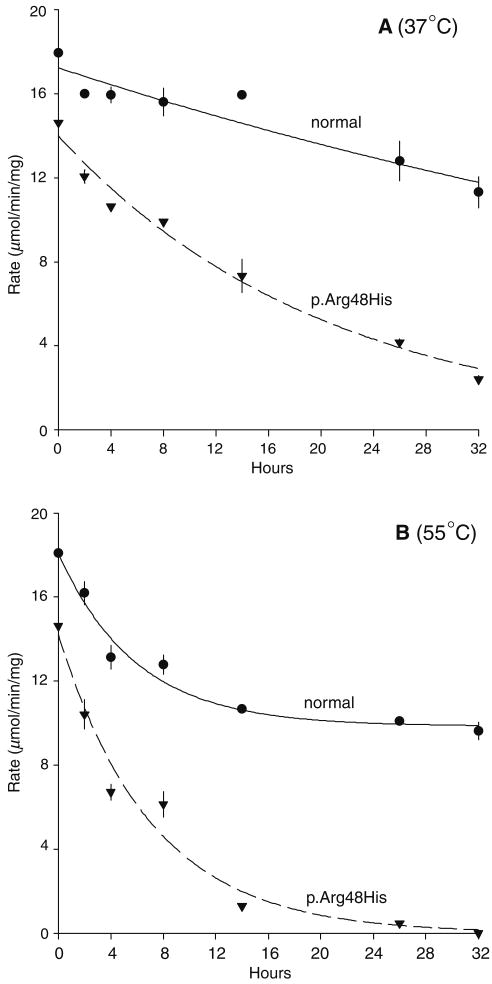
The small decrease in activity of freshly prepared mutant p.arg48his enzyme contrasts with the much larger decreases in activity obtained from cultured fibroblasts or erythrocytes reported from clinical diagnostic testing of the patients (Table 1). One explanation for this discrepancy is an unstable protein that loses activity more rapidly than the normal enzyme over time. The thermal integrity of freshly prepared enzyme therefore was tested. Compared to the normal enzyme, the p.arg48his mutant demonstrated more rapid loss of activity over time after incubation at 37 or 55°C. After 32 h, the p.arg48his mutant displayed only 16% of its starting activity at 37°C and undetectable activity at 55°C whereas the normal HGprt enzyme retained 63% of its starting activity at 37°C and 53% of its activity at 55°C (Fig. 2). These results demonstrate poor thermal stability of the p.arg48his mutant.
Fig. 2.
Thermal integrity of the human HGprt enzyme and the p.arg48his mutant. The normal enzyme is shown with solid lines and the mutant with dashed lines after various time points of incubation at 37 or 55°C. Each data point was determined in triplicate, and symbols depict average ± SD
Discussion
Understanding how specific genetic changes correlate with clinical phenotypes is a fundamental goal in human genetics. Genotype–phenotype correlations are important for their prognostic value, as well as for understanding basic mechanisms of pathogenesis. Historically, HPRT1 mutations provided one of the first models for studying genotype–phenotype correlations (Stein and Morrison 1985; Stout and Caskey 1985). Although early studies suggested that mutations encoding the active site of the enzyme would be associated most closely with disease, subsequent studies demonstrated that mutations were spread throughout the gene and upstream regulatory sequences (Jinnah et al. 2000, Jinnah et al. 2004). Presumably, mutations distant from the active site affect enzyme activity by interfering with protein expression, stability, dimerization or by causing other deleterious conformational changes (Duan et al. 2004). While there is no obvious correlation between the location of gene mutations and the clinical phenotype, the predicted consequence of the mutation does correlate with clinical phenotype (de Gemmis et al. 2010; Jinnah et al. 2000, 2004; Jurecka et al. 2008). Specifically, mutations that predict null enzyme are most often are associated with the most severe phenotype (LND), while mutations that may permit residual enzyme activity typically are associated with attenuated clinical phenotypes (HND or HRH). In view of these observations, efforts to advance understanding of genotype–phenotype correlations for HPRT1 have moved beyond the gene to the biochemical consequences of mutations.
Beyond genotype–phenotype correlations
In general, clinical severity appears inversely correlated with residual enzyme activity in studies of living cell preparations that most closely mimic the natural state in vivo (Fairbanks et al. 1987; Page et al. 1981; Puig et al. 2001). Less than 1.5% of normal enzyme activity typically is found among patients with the most severe phenotype (LND), while >8% activity more typically is associated with the mildest phenotype (HRH). Intermediate activity is seen in the intermediate phenotypes (HND). These studies have provided good evidence that HPRT1 mutations cause disease primarily through their influence on HGprt enzyme activity, with only minor influence from other factors (Jinnah and Friedmann 2001).
On the other hand, there are individuals in which the clinical phenotype does not correlate with apparent HGprt enzyme activity. Some individuals with the classic LND phenotype and greater than expected residual HGprt activity have been reported (Holland et al. 1976; Rijksen et al. 1981) as have other individuals with attenuated phenotypes and no detectable HGprt activity (Cossu et al. 2002; Hersh et al. 1986). These discrepancies typically arise when HGprt is measured via assays that do not replicate the natural state of the enzyme in vivo (Jinnah et al. 2004). For example, mutations resulting in enzymes that are structurally unstable outside of the normal cellular environment may yield unnaturally low activity when measured via assays employing cell lysates (Bakay et al. 1979; Dancis et al. 1973; Fairbanks et al. 1987). Similarly, mutations that reduce the affinity of HGprt towards its substrates may result in artificially high activity when measured via assays employing substrate concentrations that exceed physiological levels (McDonald and Kelley 1972; Zoref-Shani et al. 2000). When cases evaluated with HGprt assays that are susceptible to artifactual results are excluded and more naturalistic assays are considered, there is a good correlation between residual enzyme function and clinical severity (Fairbanks et al. 1987; Page et al. 1981; Puig et al. 2001).
Genotype–phenotype discordance and clinical ascertainment
If residual HGprt enzyme activity is the primary determinant of the clinical phenotype, then it seems reasonable to expect that identical HPRT1 mutations will cause the same clinical phenotype. Indeed, the majority of recurrent mutations produce a similar phenotype even in unrelated patients, with little variability. However, there are instances of the same mutation producing discordant phenotypes (Hladnik et al. 2008; Jinnah et al. 2010; Sarafoglou et al. 2010). These exceptions might indicate that factors other than HGprt activity can influence the phenotype.
Any potential explanation for discordant phenotypes first must acknowledge several limitations of clinical ascertainments. The first involves the rigor of clinical evaluations. Subtle neurological defects frequently are missed without a formal neurological evaluation, and cognitive impairments often elude clinical detection without neuropsychological testing. Some of our cases would readily have been misclassified as HRH without formal neurological and cognitive evaluation that revealed significant impairments (Table 1).
A second limitation of clinical ascertainment involves age at evaluation. Mild deficits in motor or cognitive skills are difficult to measure in very young children, so very young patients are prone to misclassification. These skills also change with age. A recent example is three patients from the same family with a c.500G>T mutation. Apparent variations in their motor skills might be attributable to comparing toddlers with adults (Sarafoglou et al. 2010). The discordant phenotype in 1 of 13 cases with the c.508C>T stop mutation provides another example (Jinnah et al. 2000). The only boy who did not engage in self-injurious behavior was 11 years old at the time of the report. Since self-injury can emerge later, the original phenotypic classification might change as the patient ages.
Another problem of clinical ascertainment involves the classification of patients. Although they are clustered into three distinct groups for heuristic reasons, the reality is a more continuous and graded spectrum (Jinnah et al. 2010). This spectrum means that some patients may fall between rather than within groups. For example, there are patients with late-onset self-injury, or whose self-injury is so mild that it is interpreted as accidental (Jinnah et al. 2010). These patients may be misclassified as HND rather than LND. A recent example involves the discordant phenotypes observed among five members of one family carrying a c.485+2T>C splicing mutation (Hladnik et al. 2008). All had severe gait disability, although four could walk with some difficulty. Only one had overt self-injury, though it was unusually late in onset (age 20). Two suffered repeated injuries to their chins from falls. The observation that injuries were limited to the chin raises questions regarding whether these injuries were entirely accidental, since fall-related injuries should occur at random sites. Similar suspicions regarding accidental versus self-injurious tendencies have been reported for patients with multiple recurrent injuries from “bad wheelchair driving” since such injuries are not common among other developmentally disabled people (Jinnah et al. 2010). Thus, even the definition of self-injurious behavior sometimes falls into question. These examples demonstrate the challenges of classifying patients with a continuously graded spectrum of severity into specific subgroups. Thus, “discordant” phenotypes must be judged only after careful consideration of the methods used for clinical ascertainment and classification.
Biological mechanisms for genotype–phenotype discordance
Biological explanations for discordant phenotypes involve both genetic and non-genetic modifiers in addition to the original HPRT1 mutation. These modifiers may act independent from HGprt, but some may act by modifying HGprt activity itself. In fact, this mechanism has been established for HPRT1 splicing mutations, where a major mRNA species corresponding to HPRT1 is either absent or incorrect in size (De Gregorio et al. 2005; Hunter et al. 1996; O'Neill et al. 1998; Torres et al. 2010). However, a small proportion of correctly spliced transcripts may occur due to variation in the fidelity of splicing machinery. Since splicing machinery is inherited independently from HPRT1, the proportion of normal transcripts for HGprt and subsequent residual enzyme activity may vary among individuals carrying the same splicing mutation. This mechanism may explain why clinical variation is more common with splicing mutations (Hladnik et al. 2008; Jinnah et al. 2000).
Our studies of the p.arg48his mutant provide evidence for another mechanism whereby genetic and non-genetic factors may influence the clinical phenotype through modifications of HGprt enzyme activity. The mechanisms responsible for thermal instability of the p.arg48his mutant are not known, but it is in an alpha-2 helix at the interface between dimerization of the protein, where it may destabilize subunit aggregation required for enzymatic activity. An increase in monomers that may be more prone to thermal denaturation may affect overall enzyme stability. For a thermally unstable protein, enzyme activity will depend on mechanisms responsible for synthesis of new protein, as well as turnover or repair of damaged protein. Since these mechanisms are inherited independently from HPRT1, different individuals may exhibit different levels of HGprt activity even with the same mutation. It is interesting to speculate on potential environmental influences mediated through modification of HGprt activity for a thermally unstable protein. Specifically, a patient with many severe or prolonged febrile episodes may suffer more rapid loss of a thermally unstable protein, and thereby have lower HGprt enzyme activity during a critical period of early development. In contrast, another patient with the same mutation but without severe or frequent febrile episodes may retain relatively higher HGprt activity and therefore be spared the pathophysiological consequences of low HGprt activity during early development. Thus, the physiological consequences of the same mutation may be influenced by childhood infections. This mechanism is not likely to be unique to the p.arg48his mutant, as others also have described thermally unstable HGprt mutants (Snyder et al. 1984; Uitendaal et al. 1978).
Conclusions
In general, similar clinical phenotypes result from similar mutations in the HPRT1 gene, with relatively minor variations. This relationship between genotype and phenotype most likely is determined by the effect the gene mutation has on residual HGprt enzyme function. Discordant phenotypes from the same mutation may be related to limitations of clinical ascertainment and classification, and from the fact that some mutations interact with other biological processes to influence final HGprt enzyme activity.
These observations are important for guiding counseling regarding prognosis. A new patient who is found to have a previously described mutation is likely to have a phenotype resembling that associated with the previously found mutation. The prognosis for novel mutations not previously described is more challenging, unless the mutation has a predictable effect on enzyme function, such as an early nonsense mutation or one that alters the reading frame to produce a null protein. Testing residual enzyme activity may therefore be needed in some cases, provided that the method avoids artifacts associated with unnatural conditions.
Acknowledgments
These studies were supported by grants from the NIH (HD 053312 and DK 082840), Fondo de Investigaciones Sanitarias FIS 06/0019 and FIS 08/0009 and CIBERER (Centro de Investigaciones Biomedicas en Red para el Estudio de las Enfermedades Raras), Lesch–Nyhan Action, and Fondation Jerome Lejeune and Association Malaury.
Contributor Information
Radhika Sampat, Departments of Neurology, Human Genetics and Pediatrics, Emory University, Suite 6300 Woodruff Memorial Building, 101 Woodruff Circle, Atlanta, GA 30322, USA.
Rong Fu, Departments of Neurology, Human Genetics and Pediatrics, Emory University, Suite 6300 Woodruff Memorial Building, 101 Woodruff Circle, Atlanta, GA 30322, USA.
Laura E. Larovere, Centro de Estudio de las Metabolopatias Congenitas, Universidad Nacional de Cordoba, Cordoba, Argentina
Rosa J. Torres, Division of Clinical Biochemistry and Genetic Institute, IdiPaz Hospital Universitario La Paz, Universidad Autonoma de Madrid, Madrid, Spain
Irene Ceballos-Picot, Metabolic Biochemistry Laboratory, Necker-Enfants Malades Hospital, APHP and Paris Descartes University, Paris, France.
Michel Fischbach, Pediatrics and Nephrology, University Hospital Hautepierre, Strasbourg, France.
Raquel de Kremer, Centro de Estudio de las Metabolopatias Congenitas, Universidad Nacional de Cordoba, Cordoba, Argentina.
David J. Schretlen, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, Baltimore, MD, USA; Department of Radiology, Johns Hopkins University, Baltimore, MD, USA
Juan Garcia Puig, Division of Internal Medicine, IdiPaz Hospital Universitario La Paz, Universidad Autonoma de Madrid, Madrid, Spain.
H. A. Jinnah, Email: hjinnah@emory.edu, Departments of Neurology, Human Genetics and Pediatrics, Emory University, Suite 6300 Woodruff Memorial Building, 101 Woodruff Circle, Atlanta, GA 30322, USA.
References
- Andres A, Praga M, Ruilope LM, Martinez JM, Millet VG, Bellow I, Rocicio JL. Partial deficit of hypoxanthine guanine phosphoribosyl transferase presenting as acute renal failure. Nephron. 1987;46:179–181. doi: 10.1159/000184337. [DOI] [PubMed] [Google Scholar]
- Bakay B, Nissinen E, Sweetman L, Francke U, Nyhan WL. Utilization of purines by an HPRT variant in an intelligent, nonmutilative patient with features of the Lesch–Nyhan syndrome. Pediatr Res. 1979;13:1365–1370. doi: 10.1203/00006450-197912000-00013. [DOI] [PubMed] [Google Scholar]
- Cossu A, Micheli V, Jacomelli G, Carcassi A. Kelley–Seegmiller syndrome in a patient with complete hypoxanthine-guanine phosphoribosyltransferase deficiency. Clin Exp Rhematol. 2002;19:851–853. [PubMed] [Google Scholar]
- Dancis J, Yip LC, Cox RP, Piomelli S, Balis ME. Disparate enzyme activity in erythrocytes and leukocytes: a variant of hypoxanthine phosphoribosyl-transferase deficiency with an unstable enzyme. J Clin Invest. 1973;52:2068–2074. doi: 10.1172/JCI107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gemmis P, Anesi L, Lorenzetto E, Gioachini I, Fortunati E, Zandona G, Fanin E, Fairbanks L, Andrighetto G, Parmigiani P, et al. Analysis of the HPRT1 gene in 35 Italian Lesch–Nyhan families: 45 patients and 77 potential female carriers. Mutat Res. 2010;692:1–5. doi: 10.1016/j.mrfmmm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- De Gregorio L, Jinnah HA, Nyhan WL, Trombley L, O'Neill JP. Lesch–Nyhan disease in one member of a female monozygotic twin pair heterozygous for a mutation in HPRT. Mol Genet Metab. 2005;85:70–77. doi: 10.1016/j.ymgme.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Duan J, Nilsson L, Lambert B. Structural and functional analysis of mutations at the human hypoxanthine phosporibosyl transferase (HPRT1) locus. Hum Mutat. 2004;23:599–611. doi: 10.1002/humu.20047. [DOI] [PubMed] [Google Scholar]
- Emmerson BT, Thompson L. The spectrum of hypoxanthine-guanine phosphoribosyltranferase deficiency. Quart J Med. 1973;166:423–440. [PubMed] [Google Scholar]
- Fairbanks LD, Simmonds HA, Webster DR. Use of intact erythrocytes in the diagnosis of inherited purine and pyrimidine disorders. J Inherit Metab Dis. 1987;10:174–186. doi: 10.1007/BF01800045. [DOI] [PubMed] [Google Scholar]
- Hersh JH, Page T, Hand ME, Seegmiller JE, Nyhan WL, Weisskopf B. Clinical correlations in partial hypoxanthine guanine phosphoribosyltransferase deficiency. Pediatr Neurol. 1986;2:302–304. doi: 10.1016/0887-8994(86)90025-1. [DOI] [PubMed] [Google Scholar]
- Hladnik U, Nyhan WL, Bertelli M. Variable expression of HPRT deficiency in 5 members of a family with the same mutation. Arch Neurol. 2008;65:1240–1243. doi: 10.1001/archneur.65.9.1240. [DOI] [PubMed] [Google Scholar]
- Holland MJ, DiLorenzo AM, Dancis J, Balis ME, Yu TF, Cox RP. Hypoxanthine phosphoribosyltransferase activity in intact fibroblasts from patients with X-linked hyperuricemia. J Clin Invest. 1976;57:1600–1605. doi: 10.1172/JCI108430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter TC, Melancon SB, Dallaire L, Taft S, Skopek TR, Albertini RJ, O'Neill JP. Germinal HPRT splice donor site mutation results in multiple RNA splicing products in T-lymphocyte cultures. Somat Cell Mol Genet. 1996;22:145–150. doi: 10.1007/BF02369904. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Friedmann T. Lesch–Nyhan disease and its variants. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th. McGraw-Hill; New York: 2001. pp. 2537–2570. [Google Scholar]
- Jinnah HA, DeGregorio L, Harris JC, Nyhan WL, O'Neill JP. The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat Res. 2000;463:309–326. doi: 10.1016/s1383-5742(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Harris JC, Nyhan WL, O'Neill JP. The spectrum of mutations causing HPRT deficiency: an update. Nucleosides Nucleotides Nucleic Acids. 2004;23:1153–1160. doi: 10.1081/NCN-200027400. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Ceballos-Picot I, Torres RJ, Visser JE, Schretlen D, Verdu A, Larovere LE, Chen CJ, Cossu A, Wu CH, Sampat R, Change SJ, de Kremer RD, Nyhan WL, Harris JC, Reich SG, Puig JG. Attenuated variants of Lesch–Nyhan disease. Brain. 2010;133:671–689. doi: 10.1093/brain/awq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurecka A, Popowska E, Tylki-Szymanska A, Kubalska J, Ciara E, Krumina Z, Sykut-Cegielska J, Pronicka E. Hypoxanthine-guanine phosphoribosylotransferase deficiency: the spectrum of Polish mutations. J Inherit Metab Dis. 2008:136–140. doi: 10.1007/s10545-008-1013-8. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Visser JE, Harris JC, Verdu A, Larovere L, Ceballos-Picot I, Neychev V, Torres RJ, Dulac O, Desguerre I, Schretlen DJ, Robey KL, Barabas G, Bloem BR, Nyhan WL, Kremer R, Eddey GE, Puig JG, Reich SG. Delineation of the motor disorder of Lesch–Nyhan disease. Brain. 2006;129:1201–1217. doi: 10.1093/brain/awl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larovere LE, O'Neill JP, Randall M, Fairbanks LD, Guelbert N, Czornyi L, de Kremer RD. Hypoxanthine-guanine phosphoribosyltransferase deficiency: biochemical and molecular findings in six Argentine patients. Nucleosides Nucleotides Nucleic Acids. 2007;26:255–258. doi: 10.1080/15257770701257269. [DOI] [PubMed] [Google Scholar]
- Lesch M, Nyhan WL. A familial disorder of uric acid metabolism and central nervous system function. Am J Med. 1964;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Kelley WN. Lesch–Nyhan syndrome: absence of the mutant enzyme in erythrocytes of a heterozygote for both normal and mutant hypoxanthine-guanine phosphoribosyl transferase. Biochem Genet. 1972;6:21–26. doi: 10.1007/BF00485961. [DOI] [PubMed] [Google Scholar]
- O'Neill JP, Finette BA. Transition mutations at CpG dinucleotides are the most frequent in vivo spontaneous single-base substitution mutation in the human HPRT gene. Environ Mol Mutagen. 1998;32:188–191. doi: 10.1002/(sici)1098-2280(1998)32:2<188::aid-em16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- O'Neill JP, Rogan PK, Cariello N, Nicklas JA. Mutations that alter RNA splicing of the human HPRT gene: a review of the spectrum. Mutat Res. 1998;411:179–214. doi: 10.1016/s1383-5742(98)00013-1. [DOI] [PubMed] [Google Scholar]
- Page T, Bakay B, Nissinen E, Nyhan WL. Hypoxanthine-guanine phosphoribosyltranferase variants: correlation of clinical phenotype with enzyme activity. J Inherit Metab Dis. 1981;4:203–206. doi: 10.1007/BF02263652. [DOI] [PubMed] [Google Scholar]
- Puig JG, Torres RJ, Mateos FA, Ramos TH, Arcas JM, Buno AS, O'Neill P. The spectrum of hypoxanthine-guanine phosphoribosyltransferase deficiency: clinical experience based on 22 patients from 18 Spanish families. Medicine. 2001;80:102–112. doi: 10.1097/00005792-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Rijksen G, Staal GEJ, van der Vlist MJM, Beemer FA, Troost J, Gutensohn W, van Laarhoven JPRM, De Bruyn CHMM. Partial hypoxanthine-guanine phosphoribosyl transferase deficiency with full expression of the Lesch–Nyhan syndrome. Hum Genet. 1981;57:39–47. doi: 10.1007/BF00271165. [DOI] [PubMed] [Google Scholar]
- Sarafoglou K, Grosse-Redlinger K, Boys CJ, Charnas L, Otten N, Broock R, Nyhan WL. Lesch–Nyhan variant syndrome: variable presentation in 3 affected family members. Arch Neurol. 2010;67:761–764. doi: 10.1001/archneurol.2010.116. [DOI] [PubMed] [Google Scholar]
- Seegmiller JE, Rosenbloom FM, Kelley WN. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967;155:1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Snyder FF, Chudley AE, MacLeod PM, Carter RJ, Fung E, Lowe JK. Partial deficiency of hypoxanthine-guanine phosphoribosyltransferase with reduced affinity for PP-ribose-P in four related males with gout. Hum Genet. 1984;67:18–22. doi: 10.1007/BF00270552. [DOI] [PubMed] [Google Scholar]
- Stein SA, Morrison MR. The molecular biology of Lesch–Nyhan syndrome. Trends Neurosci. 1985;8:148–150. [Google Scholar]
- Stout JT, Caskey CT. HPRT: gene structure, expression, and mutation. Ann Rev Genet. 1985;19:127–148. doi: 10.1146/annurev.ge.19.120185.001015. [DOI] [PubMed] [Google Scholar]
- Torres RJ, Garcia MG, Puig JG. Partial HPRT deficiency phenotype and incomplete splicing mutation. Nucleosides Nucleotides Nucleic Acids. 2010;29:295–300. doi: 10.1080/15257771003730250. [DOI] [PubMed] [Google Scholar]
- Uitendaal MP, de Bruyn CH, Oei TL, Hosli P. Molecular and tissue-specific heterogeneity in HPRT deficiency. Biochem Genet. 1978;16:1187–1202. doi: 10.1007/BF00484539. [DOI] [PubMed] [Google Scholar]
- Zoref-Shani E, Feinstein S, Frishberg Y, Sperling O. Kelley–Seegmiller syndrome due to a unique variant of hypoxanthine-guanine phosphoribosyltransferase: reduced affinity for 5-phosphoribosy1-pyrophosphate manifested only at low, physiological substrate concentrations. Biochim Biophys Acta. 2000;1500:197–203. doi: 10.1016/s0925-4439(99)00103-9. [DOI] [PubMed] [Google Scholar]