Abstract
Wnt signal transduction plays a crucial role in stem cell proliferation and regeneration. When canonical Wnt signaling is low, heads develop, and when it is high, tails are formed. In planarians, Wnt transcription is activated by wounding in a β-catenin–independent way. Hedgehog is one of the signals involved, because it induces regeneration of tails (instead of heads) through the activation of Wnt transcription. Depletion of Smad4 blocks regeneration entirely, which suggests that the bone morphogenetic protein signaling pathway and the Wnt pathway are required for regeneration and body patterning.
Introduction
Animal cells utilize a surprisingly small number of cell-cell signaling pathways to communicate with each other—such as Wnt, Hedgehog, transforming growth factor–β (TGF-β), Notch, receptor tyrosine kinases, and nuclear hormone receptors (1). These conserved signal transduction pathways have been used to generate diverse animal forms during evolution (2). Planarians have long fascinated biologists because an entire animal can regenerate from small fragments of an adult animal (3). Studies on regeneration of the planarian Schmidtea mediterranea have provided important insights into how wound healing is related to the overall position of the wound in the animal (3–5).
Planarian biology
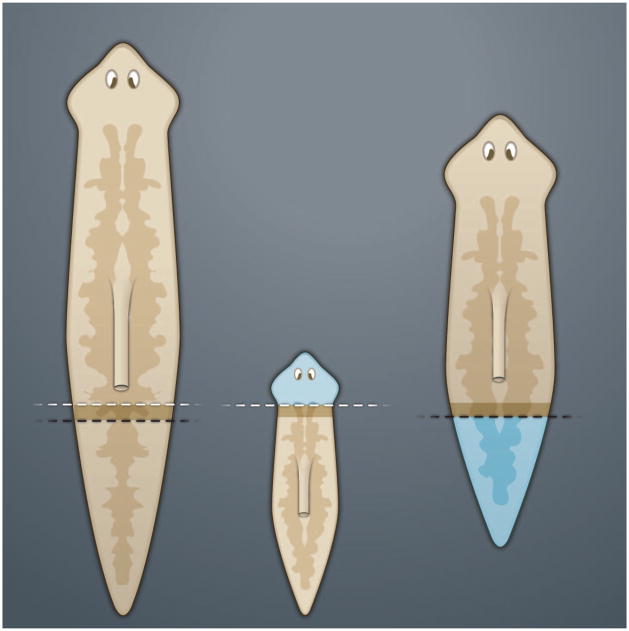
The planarian strain used in modern studies is asexual (because of a chromosomal translocation) and reproduces by stretching its body until it breaks in two, producing two flatworms. The only cells that divide mitotically in these animals are stem cells called neoblasts, which are distributed relatively uniformly throughout the body in a salt-and-pepper fashion (6, 7). During regeneration, neoblasts proliferate and cells accumulate in the wound, forming a regeneration blastema. The body of the adult planarian contains a gradient of positional information, as illustrated by the simple experiment shown in Fig. 1. When a planarian is cut transversely, the caudal fragment will regenerate a head and the anterior piece will regenerate a tail. If two different animals are cut transversely at very similar positions (indicated by the black and white dashed lines in Fig. 1), the resulting blastemas will regenerate entirely different structures (8). Because the cells in these blastemas originate essentially from the same region, the formation of heads or tails depends not on the neoblasts, but rather on the relationship of the wound to an antero-posterior (A-P) gradient of positional information.
Fig. 1.
Planarian head or tail regeneration blastemas sense the A-P positional information in the animal body. In this experiment, though transverse sections (indicated by the white and black dashed lines) were performed at essentially identical levels, the proliferating blastemas (in dark brown) regenerate very different structures (indicated in blue); the posterior fragment also regenerates a pharynx. [Adapted from (22)]
Wnt and A-P patterning
In embryos of the frog Xenopus laevis, A-P polarity is determined by a gradient of canonical Wnt/β-catenin signaling, in which Wnt concentrations are low in the head and high in the tail (9, 10). This key role of Wnt signaling in patterning has now been confirmed during axis formation in most animals (2, 10, 11).
The laboratory of Sánchez Alvarado discovered that planarians are exquisitely sensitive to RNA interference (RNAi) of gene expression triggered by feeding Escherichia coli expressing double-stranded RNA (dsRNA) or by injecting dsRNA (12). High-throughput screens led to the identification of genes required for regeneration and stem cell maintenance (13). Among these, some of the most informative were those encoding components of the Wnt/β-catenin pathway. Depletion of β-catenin or Disheveled (a Wnt transducer) resulted in the regeneration of heads instead of tails (14, 15). Conversely, RNAi for adenomatous polyposis coli (APC), which encodes a protein that normally facilitates the degradation of β-catenin, caused the regeneration of tails instead of heads (14). Depletion of wntless, a gene required for Wnt secretion, or of wntP-1, a gene encoding the posterior-most planarian Wnt growth factor, promoted head formation (16). The observation that WntP-1 was required for tail formation provided the starting point for the new investigations reviewed here.
Wnt and regeneration
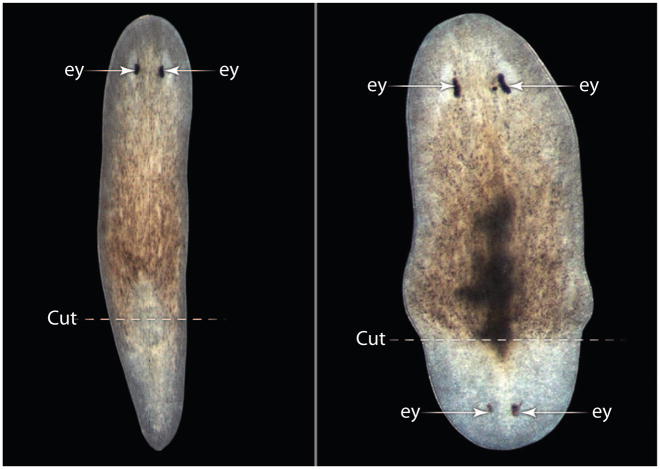
Planarians depleted of WntP-1 regenerate heads in place of tails (16, 17) in about 25% of animals (Fig. 2). wntP-1 transcripts were unexpectedly detected in both anterior and posterior wounds during the first day after the operation, even though the gene is normally expressed only posteriorly. wntP-1 was present in any type of wound (such as diagonal, longitudinal, or puncture), which indicated a coupling between its early expression and regeneration. However, after 3 days, wntP-1 expression was maintained only in its normal posterior domain (17).
Fig. 2.
Wnt is required for the regeneration of tails in planarians. Anterior fragments treated with control RNAi regenerate normal tails posterior to the cut (right), whereas wntP-1 RNAi causes the formation of posterior heads (left). Note that the posterior head develops eye (ey) photoreceptors and that tissues resulting from proliferation of regeneration blastema are less pigmented than preexisting tissue. [Image: Christian P. Petersen and Peter W. Reddien, Whitehead Institute for Biomedical Research and Massachusetts Institute of Technology. Adapted from (3)]
Nine planarian Wnt genes have been identified to date. wntP-2 is expressed in the posterior half of the animal, and double knock-down of WntP-2 and WntP-1 increased the frequency of posterior heads (17). During regeneration, wntP-2 is expressed in posterior wounds after wntP-1, and this expression requires β-catenin and wntP-1. Furthermore, the early expression of wntP-1 in all wounds is independent of Wnt signaling, which indicates that the early regenerative response to wounding is activated by a different signal transduction pathway acting upstream of Wnt.
A component of this upstream signal is Hedgehog (Hh) signaling. Most of the Hh pathway components have been cloned in planarians and show relatively uniform distribution patterns (3). Reducing Hh signaling with hh RNAi inhibited tail regeneration. Activating the Hh pathway with patched RNAi (Patched normally inhibits Hh signaling) caused overexpression of wntP-1 and regeneration of tails instead of heads at the anterior end (3). Double knock-down of WntP-1 and Patched reversed the high Hh signaling phenotype and caused the regeneration of heads at both ends. Thus, the effects of Hh are mediated by the transcriptional activation of WntP-1 at wound sites (3).
The intimate relation between Wnt and regeneration is becoming increasingly apparent in other organisms. In Hydra, a cnidarian that also has unlimited regenerative plasticity, polyp development is mediated by the Wnt/β-catenin pathway (18). The expansion of mammalian stem cells requires Wnt signaling, usually in conjunction with growth factors that activate a receptor tyrosine kinase (19). For example, villus regeneration in the intestine can be blocked by the Wnt inhibitor Dickkopf-1 (20). The importance of these findings for regenerative medicine is illustrated by the discovery that injection of liposomal vesicles containing Wnt3a into mouse fractures accelerates bone regeneration (21). In the future, it will be informative to identify wound-responsive enhancer elements in genes such as wntP-1 or its mammalian orthologs. Reporter genes activated by wounding may help to establish whether Hh is generally required for wound repair. Another candidate pathway is fibroblast growth factor (FGF)–mitogen-activated protein kinase (MAPK), which is readily activated by wounding in vertebrate embryos (22).
Regeneration and morphogenetic fields
Planarian regeneration leads to the most perfect pattern possible. Multiple deep cuts in the head region can lead to animals with many little heads in which A-P and dorsal-ventral (D-V) patterns are seamlessly integrated (Fig. 3). How is this regulatory feat achieved? Self-regulating morphogenetic fields are of interest to developmental biologists, because they explain, for example, the pattern regeneration observed when an early embryo is cut in half, which leads to the formation of identical twins in sea urchin, Xenopus, or chick embryos (23, 24).
Fig. 3.
Self-regulation of pattern formation in the head field of planaria after a series of anterior cuts. [Adapted from (8)]
In Xenopus, D-V patterning is established by a gradient of bone morphogenetic protein (BMP) signaling. BMPs are members of the TGF-β family, which also has a second branch represented by the Nodal and Activin subfamily of growth factors. A self-regulating D-V patterning field is established by a network of secreted proteins involving dorsal and ventrally expressed BMPs, the BMP-binding protein Chordin, and Tolloid—a metalloproteinase that degrades Chordin, which releases active BMPs (24). Binding of BMPs to the BMP receptor triggers the phosphorylation and activation of the Smad1 transcription factor, which, together with its cofactor Smad4, transduces the BMP signal. In addition, Smad1 is phosphorylated by glycogen synthase kinase–3 (GSK3), which promotes its degradation. Phosphorylation of Smad1 by GSK3 is inhibited by Wnt/β-catenin signaling (25), and this provides a mechanism for integration of the A-P (Wnt) and D-V (BMP) patterning systems that has been conserved between vertebrates and Drosophila (26).
In invertebrates such as planarians, the D-V axis is inverted compared with vertebrates during evolution (2), so that BMP4 is expressed dorsally (4). The central nervous system (CNS) is located ventrally, but when BMP4 was depleted, a duplicated CNS (including an extra pair of eyes) was formed ectopically on the dorsal side (27). RNAi against tolloid, BMP4, or Smad4 caused animals to form two ventral sides, some of which even glided upside down on their former dorsal surface. These genes encoding BMP pathway components are also required for regeneration, particularly in the midline region (4). The most spectacular phenotype is that induced by Smad4 RNAi, which blocks the formation of all regeneration blastemas (4).
Smad4 is essential for regeneration and required for the transduction of both BMP and Activin-Nodal signals. Wnt can prolong the signal of the Smad4 binding partner, Smad1, by inhibiting its degradation through GSK3 (25). Planarian Smad1 contains putative GSK3 phosphorylation sites (28) and could therefore be potentially regulated by Wnt. The mammalian Activin-Nodal transcription factor Smad3 might also be regulated by GSK3 and Wnt (29). Thus, Smads provide a good entry point to investigate how a harmonious body plan, in which A-P and D-V patterns are integrated, is formed and regenerated. There is still room for a role for TGF-β signaling in A-P patterning: In zebrafish and Xenopus, coexpression of Wnt, Nodal, and BMP facilitates tail formation, whereas inhibition of these growth factors promotes head formation (30, 31).
A final issue concerns the role of neoblasts in the planarian body plan. These stem cells can be eliminated by x-ray irradiation. Neoblast-depleted planarians can still activate transcription of wntP-1, wntP-2, and BMP4 after wounding (4, 17). Therefore, the key to the gradient of positional information in planarians resides in the nondividing differentiated cells. The stem cells seem to proliferate and differentiate in response to instructions present as a blueprint in somatic nondividing cells.
Studies in the planarian model system have provided powerful new insights into the role of Wnt in the wounding response, regeneration, and stem cell proliferation. There is hope that future studies may illuminate how gradients of positional information are maintained along the body axes of an adult animal.
Acknowledgments
Funding: Work in our laboratory is supported by NIH grant HD21502-23. E.M.D.R. is a Howard Hughes Medical Institute investigator.
References and Notes
- 1.Wineberg RA. Biology of Cancer. Garland Science; Hamden, CT: 2006. pp. 119–158. [Google Scholar]
- 2.De Robertis EM. Evo-devo: Variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen CP, Reddien PW. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci USA. 2009;106:17061–17066. doi: 10.1073/pnas.0906823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rink JC, Gurley KA, Elliott SA, Sánchez Alvarado A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddien PW, Bermange AL, Kicza AM, Sánchez Alvarado A. BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development. 2007;134:4043–4051. doi: 10.1242/dev.007138. [DOI] [PubMed] [Google Scholar]
- 6.Baguña J, Saló E, Auladell C. Regeneration and pattern formation in planarians III. Evidence that neoblasts are totipotent stem cells and the source of blastema cells. Development. 1989;107:77–86. [Google Scholar]
- 7.Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- 8.Huxley JS, de Beer GR. The Elements of Experimental Embryology. Cambridge Univ. Press; Cambridge: 1934. [Google Scholar]
- 9.Kiecker C, Niehrs C. A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 10.Niehrs C. On growth and form: A Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 11.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Newmark PA, Reddien PW, Cebrià F, Alvarado A Sánchez. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc Natl Acad Sci USA. 2003;100(suppl 1):11861–11865. doi: 10.1073/pnas.1834205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sánchez Alvarado A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell. 2005;8:635–649. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurley KA, Rink JC, Sánchez Alvarado A. β-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen CP, Reddien PW. Smed-β-catenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- 16.Adell T, Saló E, Boutros M, Bartscherer K. Smed-Evi/Wntless is required for β-catenin-dependent and -independent processes during planarian regeneration. Development. 2009;136:905–910. doi: 10.1242/dev.033761. [DOI] [PubMed] [Google Scholar]
- 17.Morgan TH. Experimental studies on the regeration of Planaria maculata. Dev Genes Evol. 1898;7:364–397. [Google Scholar]
- 18.Lengfeld T, Watanabe H, Simakov O, Lindgens D, Gee L, Law L, Schmidt HA, Ozbek S, Bode H, Holstein TW. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev Biol. 2009;330:186–199. doi: 10.1016/j.ydbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Nusse R, Fuerer C, Ching W, Harnish K, Logan C, Zeng A, ten Berge D, Kalani Y. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol. 2008;73:59–66. doi: 10.1101/sqb.2008.73.035. [DOI] [PubMed] [Google Scholar]
- 20.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2:29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 22.Christen B, Slack JM. Spatial response to fibroblast growth factor signalling in Xenopus embryos. Development. 1999;126:119–125. doi: 10.1242/dev.126.1.119. [DOI] [PubMed] [Google Scholar]
- 23.De Robertis EM, Morita EA, Cho KWY. Gradient fields and homeobox genes. Development. 1991;112:669–678. doi: 10.1242/dev.112.3.669. [DOI] [PubMed] [Google Scholar]
- 24.De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 2009;126:925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eivers E, Demagny H, De Robertis EM. Integration of BMP and Wnt signaling via vertebrate Smad1/5/8 and Drosophila Mad. Cytokine Growth Factor Rev. 2009;20:357–365. doi: 10.1016/j.cytogfr.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina MD, Saló E, Cebrià F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol. 2007;311:79–94. doi: 10.1016/j.ydbio.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Demagny H, De Robertis EM. unpublished observations. [Google Scholar]
- 29.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3-β control Smad3 protein stability and modulate TGF-β signaling. Genes Dev. 2008;22:106–120. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- 31.Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]