Abstract
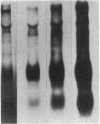
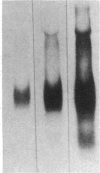
The effects of hypoinsulinemic nonketotic streptozotocin diabetes on hepatic apo B synthesis and secretion was studied in primary cultures of rat hepatocytes. Diabetic rats were characterized by their significantly elevated serum glucose, apo B, and triglyceride levels, while serum insulin levels were less than a third of normal. Serum transminase activities of diabetic rats were significantly elevated when compared with control rats, which was attributed to an increase in liver transaminase activity in diabetic rats. The pattern of enzyme activities of hepatocytes reflected that observed in livers of donor rats and the pattern was retained by primary cultures of hepatocytes over the culture period. Hepatocytes from diabetic rats secreted only one third of the apo B secreted by hepatocytes from control rats, which was determined by monoclonal immunoassay of rat total apo B. Decreases in secretion were confirmed by measurement of secretory [35S]methionine-labeled lipoprotein apo B radioactivity. The decreased apo B content of media of hepatocytes from diabetic rats was not due to increased apo B catabolism since hepatocytes from diabetic rats were shown to degrade less lipoprotein-apo B than hepatocytes from normal rats in control experiments. In addition, the apo B content of detergent-solubilized hepatocytes from diabetic rats was significantly less than that of hepatocytes from control rats. These results suggest that insulin is necessary for normal hepatic apo B synthesis and secretion and that the hyperlipidemia associated with hypoinsulinemia in vivo is primarily of intestinal origin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Chang C. L. The regulation of lipid synthesis in primary cultures of hepatocytes from nonketotic streptozotocin diabetic rats. Metabolism. 1983 Mar;32(3):224–229. doi: 10.1016/0026-0495(83)90186-5. [DOI] [PubMed] [Google Scholar]
- Bar-On H., Roheim P. S., Eder H. A. Serum lipoproteins and apolipoproteins in rats with streptozotocin-induced diabetes. J Clin Invest. 1976 Mar;57(3):714–721. doi: 10.1172/JCI108329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry E. M., Ziv E., Bar-On H. Lipoprotein secretion by isolated perfused livers from streptozotocin-diabetic rats. Diabetologia. 1981 Oct;21(4):402–408. doi: 10.1007/BF00252689. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunzell J. D., Porte D., Jr, Bierman E. L. Abnormal lipoprotein-lipase-mediated plasma triglyceride removal in untreated diabetes mellitus associated with hypertriglyceridemia. Metabolism. 1979 Sep;28(9):901–907. doi: 10.1016/0026-0495(79)90089-1. [DOI] [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- Chait A., Bierman E. L., Albers J. J. Regulatory role of insulin in the degradation of low density lipoprotein by cultured human skin fibroblasts. Biochim Biophys Acta. 1978 May 25;529(2):292–299. doi: 10.1016/0005-2760(78)90072-3. [DOI] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egusa G., Brady D. W., Grundy S. M., Howard B. V. Isopropanol precipitation method for the determination of apolipoprotein B specific activity and plasma concentrations during metabolic studies of very low density lipoprotein and low density lipoprotein apolipoprotein B. J Lipid Res. 1983 Sep;24(9):1261–1267. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Goldberg A. P., Chait A., Brunzell J. D. Postprandial adipose tissue lipoprotein lipase activity in primary hypertriglyceridemia. Metabolism. 1980 Mar;29(3):223–229. doi: 10.1016/0026-0495(80)90063-3. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gotto A. M., Levy R. I., John K., Fredrickson D. S. On the protein defect in abetalipoproteinemia. N Engl J Med. 1971 Apr 15;284(15):813–818. doi: 10.1056/NEJM197104152841503. [DOI] [PubMed] [Google Scholar]
- HENRY R. J., CHIAMORI N., GOLUB O. J., BERKMAN S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am J Clin Pathol. 1960 Oct;34:381–398. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Levy E., Shafrir E., Ziv E., Bar-On H. Composition, removal and metabolic fate of chylomicrons derived from diabetic rats. Biochim Biophys Acta. 1985 May 17;834(3):376–385. doi: 10.1016/0005-2760(85)90011-6. [DOI] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P. Hypolipidemia. Med Clin North Am. 1982 Mar;66(2):469–484. doi: 10.1016/s0025-7125(16)31431-6. [DOI] [PubMed] [Google Scholar]
- Marsh J. B., Sparks C. E. Hepatic secretion of lipoproteins in the rat and the effect of experimental nephrosis. J Clin Invest. 1979 Nov;64(5):1229–1237. doi: 10.1172/JCI109577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä E. A., Huttunen J. K., Ehnholm C. Postheparin plasma lipoprotein lipase and hepatic lipase in diabetes mellitus. Relationship to plasma triglyceride metabolism. Diabetes. 1977 Jan;26(1):11–21. doi: 10.2337/diab.26.1.11. [DOI] [PubMed] [Google Scholar]
- O'Looney P., Irwin D., Briscoe P., Vahouny G. V. Lipoprotein composition as a component in the lipoprotein clearance defect in experimental diabetes. J Biol Chem. 1985 Jan 10;260(1):428–432. [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Gotto A. M., Jr, Patsch J. R. Effects of insulin on lipoprotein secretion in rat hepatocyte cultures. The role of the insulin receptor. J Biol Chem. 1986 Jul 25;261(21):9603–9606. [PubMed] [Google Scholar]
- Reaven E. P., Reaven G. M. Mechanisms for development of diabetic hypertriglyceridemia in streptozotocin-treated rats. Effect of diet and duration of insulin deficiency. J Clin Invest. 1974 Nov;54(5):1167–1178. doi: 10.1172/JCI107860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckless J. P., Betteridge D. J., Wu P., Payne B., Galton D. J. High-density and low-density lipoproteins and prevalence of vascular disease in diabetes mellitus. Br Med J. 1978 Apr 8;1(6117):883–886. doi: 10.1136/bmj.1.6117.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röschlau P., Bernt E., Gruber W. Enzymatische Bestimmung des Gesamt-Cholesterins im Serum. Z Klin Chem Klin Biochem. 1974 Sep;12(9):403–407. [PubMed] [Google Scholar]
- Salhanick A. I., Konowitz P., Amatruda J. M. Potentiation of insulin action by a sulfonylurea in primary cultures of hepatocytes from normal and diabetic rats. Diabetes. 1983 Mar;32(3):206–212. doi: 10.2337/diab.32.3.206. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Birge C., Miller J. P., Kessler G., Santiago J. Apolipoprotein B levels and altered lipoprotein composition in diabetes. Diabetes. 1974 Oct;23(10):827–834. doi: 10.2337/diab.23.10.827. [DOI] [PubMed] [Google Scholar]
- Sparks C. E., Dehoff J. L., Capuzzi D. M., Pietra G., Marsh J. B. Proteolysis of very low density lipoprotein in perfused lung. Biochim Biophys Acta. 1978 Apr 28;529(1):123–130. doi: 10.1016/0005-2760(78)90110-8. [DOI] [PubMed] [Google Scholar]
- Sparks C. E., Marsh J. B. Metabolic heterogeneity of apolipoprotein B in the rat. J Lipid Res. 1981 Mar;22(3):519–527. [PubMed] [Google Scholar]
- Sparks C. E., Sparks J. D., Bolognino M., Salhanick A., Strumph P. S., Amatruda J. M. Insulin effects on apolipoprotein B lipoprotein synthesis and secretion by primary cultures of rat hepatocytes. Metabolism. 1986 Dec;35(12):1128–1136. doi: 10.1016/0026-0495(86)90026-0. [DOI] [PubMed] [Google Scholar]
- Sparks J. D., Bolognino M., Trax P. A., Sparks C. E. The production and utility of monoclonal antibodies to rat apolipoprotein B lipoproteins. Atherosclerosis. 1986 Sep;61(3):205–211. doi: 10.1016/0021-9150(86)90139-5. [DOI] [PubMed] [Google Scholar]
- Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969 Mar;22(2):158–161. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelberg K. H., Gries F. A., Moschinski D. Hepatic production of VLDL-triglycerides. Dependence of portal substrate and insulin concentration. Horm Metab Res. 1980 Dec;12(12):688–694. doi: 10.1055/s-2007-999233. [DOI] [PubMed] [Google Scholar]