Abstract
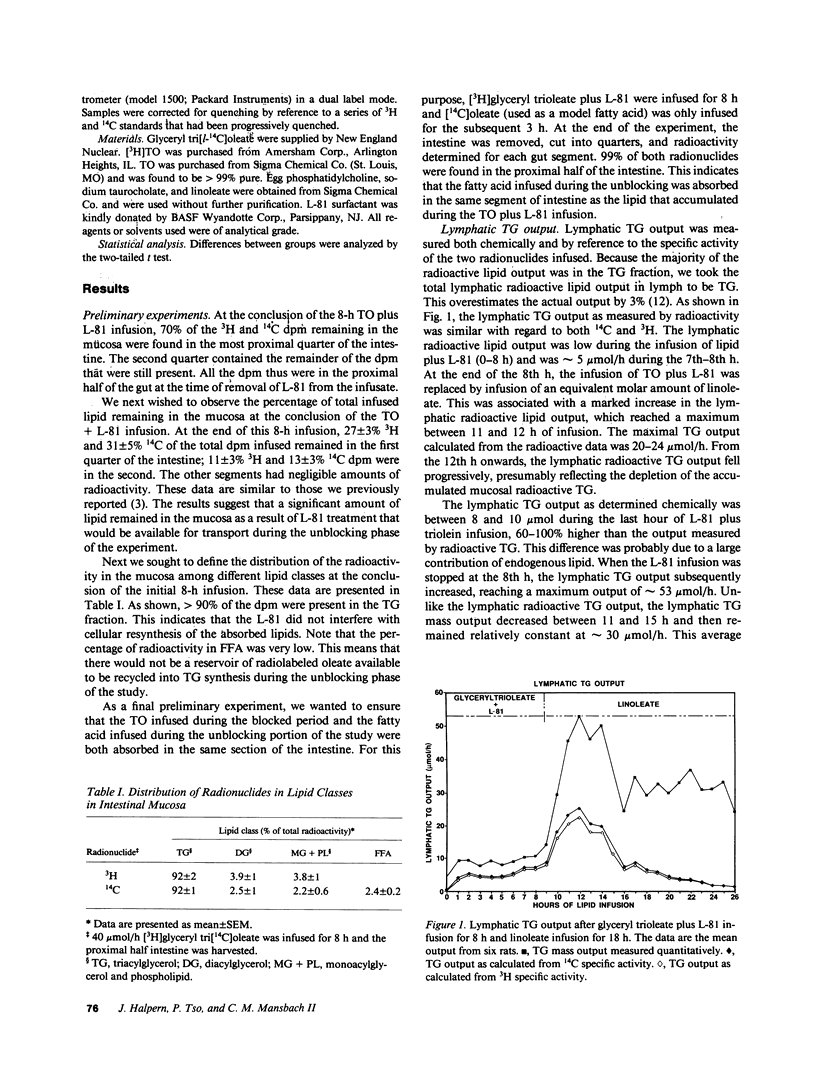
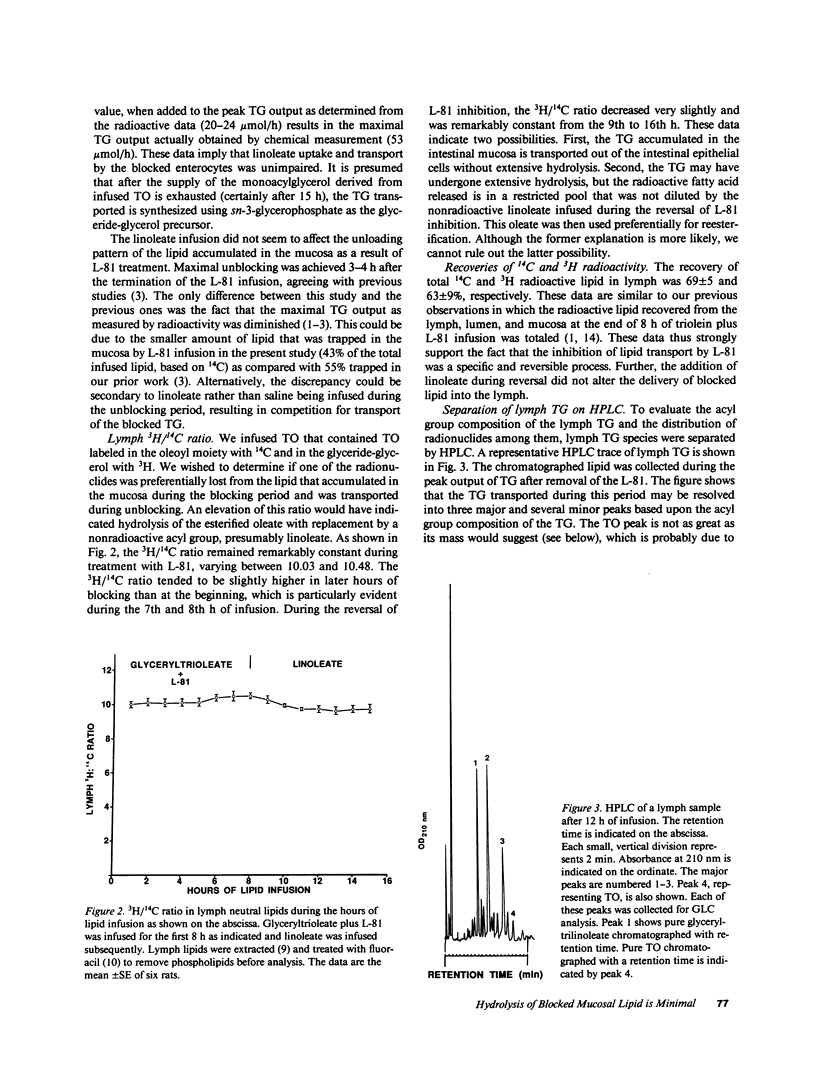
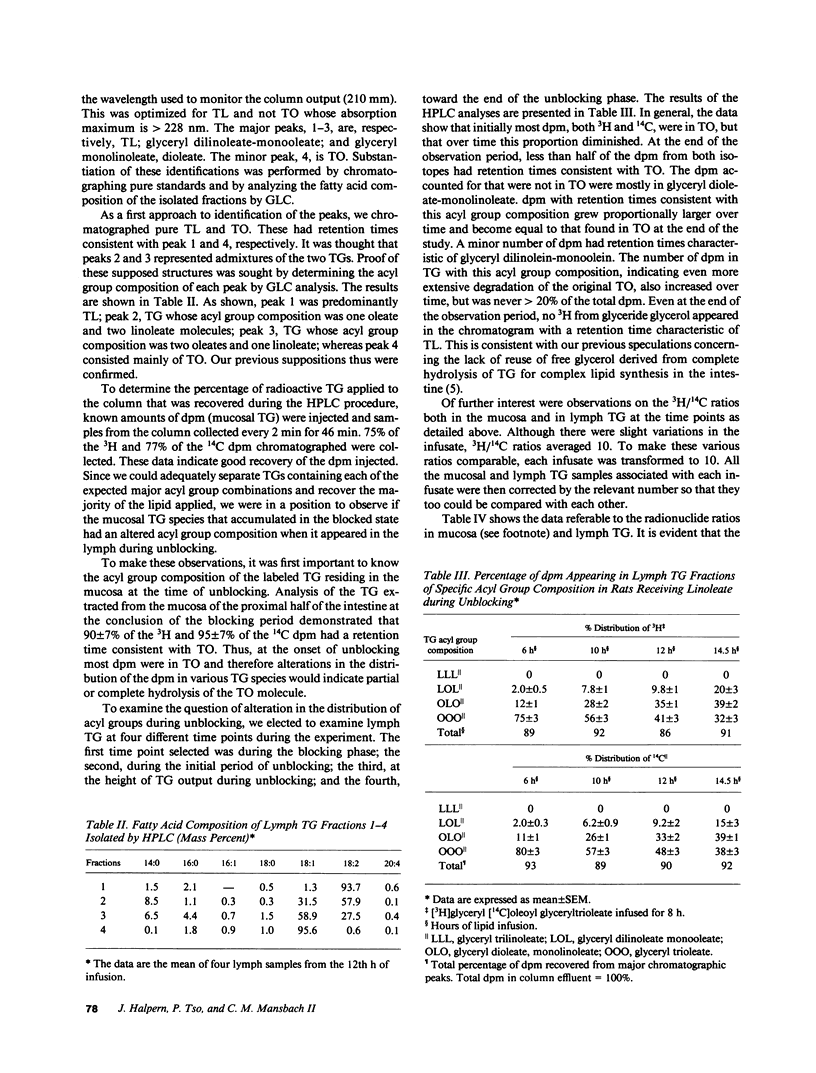
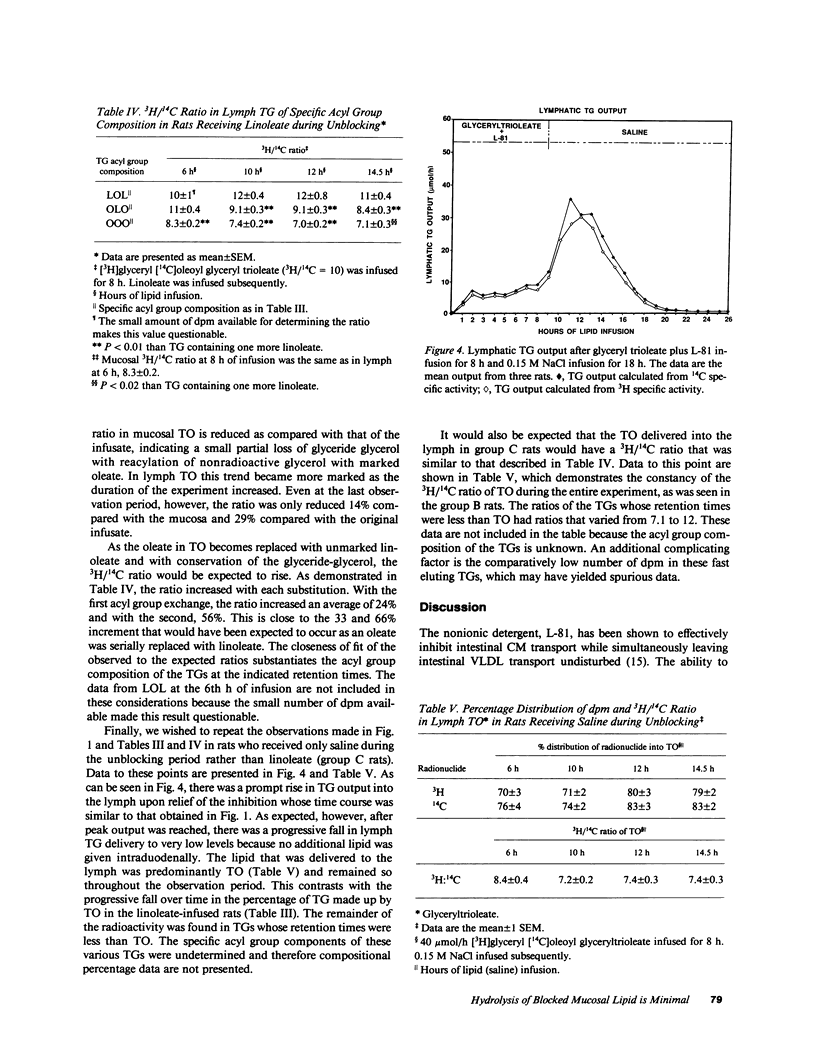
The nonionic detergent, Pluronic L-81 (L-81) has been shown to block the transport of intestinal mucosal triacylglycerol (TG) in chylomicrons. This results in large lipid masses within the enterocyte that are greater in diameter than chylomicrons. On removal of L-81, mucosal TG is rapidly mobilized and appears in the lymph. We questioned whether the blocked TG requires partial or complete hydrolysis before its transport. Rats were infused intraduodenally with [3H]glyceryl, [14C]oleoyl trioleate (TO) and 0.5 mg L-81/h for 8 h, followed by 120 mumol/h linoleate for 18 h. Mesenteric lymph was collected and analyzed for TG content and radioactivity. An HPLC method was developed to separate TG on the basis of its acyl group species. The assumed acyl group composition was confirmed by gas liquid chromatography analysis. TG lymphatic output was low for the first 8 h but increased to 52 mumol/h at the 11th h of infusion (3 h after stopping L-81). 38% of the infused TO was retained in the mucosa after the 8-h infusion. 95% of mucosal TG was TO, 92% of the radioactivity was in TG, and 2.4% of the 14C disintegrations per minute was in fatty acid. HPLC analysis of lymph at 6, 10, 12, and 14.5 h of infusion showed a progressive rise in TG composed of one linoleate and two oleates, to 39%; and in TG composed of two linoleates and one oleate to 20% at 14.5 h of infusion. On a mass basis, however, 80% of the TG acyl groups were oleate. 3H/14C ratios in the various TG acyl group species reflected the decrease in oleate. We conclude that first, unlike liver, most mucosal TG is not hydrolyzed before transport. The mechanism of how the large lipid masses present in mucosal cells after L-81 infusion are converted to the much smaller chylomicrons is unknown. Second, the concomitant infusion of linoleate did not impair lymph TG delivery after L-81 blockade.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLANKENHORN D. H., AHRENS E. H., Jr Extraction, isolation, and identification of hydrolytic products of triglyceride digestion in man. J Biol Chem. 1955 Jan;212(1):69–81. [PubMed] [Google Scholar]
- BORGSTROM B. Investigation on lipid separation methods. Separation of phospholipids from neutral fat and fatty acids. Acta Physiol Scand. 1952 Jun 6;25(2-3):101–110. doi: 10.1111/j.1748-1716.1952.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Burrier R. E., Brecher P. Hydrolysis of triolein in phospholipid vesicles and microemulsions by a purified rat liver acid lipase. J Biol Chem. 1983 Oct 10;258(19):12043–12050. [PubMed] [Google Scholar]
- Coates P. M., Brown S. A., Jumawan J., Koldovský O. Characteristics and postnatal development of the acid lipase activity of the rat small intestine. Biochem J. 1977 Sep 15;166(3):331–338. doi: 10.1042/bj1660331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hyun S. A., Vahouny V., Treadwell C. R. Portal absorption of fatty acids in lymph- and portal vein-cannulated rats. Biochim Biophys Acta. 1967 Apr 4;137(2):296–305. doi: 10.1016/0005-2760(67)90105-1. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Mansbach C. M., 2nd, Arnold A., Cox M. A. Factors influencing triacylglycerol delivery into mesenteric lymph. Am J Physiol. 1985 Nov;249(5 Pt 1):G642–G648. doi: 10.1152/ajpgi.1985.249.5.G642. [DOI] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd, Arnold A., Garrett M. Effect of chloroquine on intestinal lipid metabolism. Am J Physiol. 1987 Nov;253(5 Pt 1):G673–G678. doi: 10.1152/ajpgi.1987.253.5.G673. [DOI] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd, Arnold A. Steady-state kinetic analysis of triacylglycerol delivery into mesenteric lymph. Am J Physiol. 1986 Aug;251(2 Pt 1):G263–G269. doi: 10.1152/ajpgi.1986.251.2.G263. [DOI] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd Complex lipid synthesis in hamster intestine. Biochim Biophys Acta. 1973 Feb 14;296(2):386–402. doi: 10.1016/0005-2760(73)90097-0. [DOI] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd, Parthasarathy S. A re-examination of the fate of glyceride-glycerol in neutral lipid absorption and transport. J Lipid Res. 1982 Sep;23(7):1009–1019. [PubMed] [Google Scholar]
- McDonald G. B., Saunders D. R., Weidman M., Fisher L. Portal venous transport of long-chain fatty acids absorbed from rat intestine. Am J Physiol. 1980 Sep;239(3):G141–G150. doi: 10.1152/ajpgi.1980.239.3.G141. [DOI] [PubMed] [Google Scholar]
- Mooney R. A., Lane M. D. Formation and turnover of triglyceride-rich vesicles in the chick liver cell. Effects of cAMP and carnitine on triglyceride mobilization and conversion to ketones. J Biol Chem. 1981 Nov 25;256(22):11724–11733. [PubMed] [Google Scholar]
- Sabesin S. M., Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977 Jul;18(4):496–511. [PubMed] [Google Scholar]
- Tso P., Balint J. A., Bishop M. B., Rodgers J. B. Acute inhibition of intestinal lipid transport by Pluronic L-81 in the rat. Am J Physiol. 1981 Dec;241(6):G487–G497. doi: 10.1152/ajpgi.1981.241.6.G487. [DOI] [PubMed] [Google Scholar]
- Tso P., Balint J. A., Rodgers J. B. Effect of hydrophobic surfactant (Pluronic L-81) on lymphatic lipid transport in the rat. Am J Physiol. 1980 Nov;239(5):G348–G353. doi: 10.1152/ajpgi.1980.239.5.G348. [DOI] [PubMed] [Google Scholar]
- Tso P., Barrowman J. A., Granger D. N. Importance of interstitial matrix hydration in intestinal chylomicron transport. Am J Physiol. 1986 Apr;250(4 Pt 1):G497–G500. doi: 10.1152/ajpgi.1986.250.4.G497. [DOI] [PubMed] [Google Scholar]
- Tso P., Buch K. L., Balint J. A., Rodgers J. B. Maximal lymphatic triglyceride transport rate from the rat small intestine. Am J Physiol. 1982 Apr;242(4):G408–G415. doi: 10.1152/ajpgi.1982.242.4.G408. [DOI] [PubMed] [Google Scholar]
- Tso P., Drake D. S., Black D. D., Sabesin S. M. Evidence for separate pathways of chylomicron and very low-density lipoprotein assembly and transport by rat small intestine. Am J Physiol. 1984 Dec;247(6 Pt 1):G599–G610. doi: 10.1152/ajpgi.1984.247.6.G599. [DOI] [PubMed] [Google Scholar]
- Tso P., Gollamudi S. R. Pluronic L-81: a potent inhibitor of the transport of intestinal chylomicrons. Am J Physiol. 1984 Jul;247(1 Pt 1):G32–G36. doi: 10.1152/ajpgi.1984.247.1.G32. [DOI] [PubMed] [Google Scholar]
- Tso P., Pitts V., Granger D. N. Role of lymph flow in intestinal chylomicron transport. Am J Physiol. 1985 Jul;249(1 Pt 1):G21–G28. doi: 10.1152/ajpgi.1985.249.1.G21. [DOI] [PubMed] [Google Scholar]


