Abstract
Swimming movements in the leech and lamprey are highly analogous, and lack homology. Thus, similarities in mechanisms must arise from convergent evolution rather than from common ancestry. Despite over forty years of parallel investigations into this annelid and primitive vertebrate, a close comparison of the approaches and results of this research is lacking. The present review evaluates the neural mechanisms underlying swimming in these two animals and describes the many similarities that provide intriguing examples of convergent evolution. Specifically, we discuss swim initiation, maintenance and termination, isolated nervous system preparations, neural-circuitry, central oscillators, intersegmental coupling, phase lags, cycle periods and sensory feedback. Comparative studies between species highlight mechanisms that optimize behavior and allow us a broader understanding of nervous system function.
1. Introduction
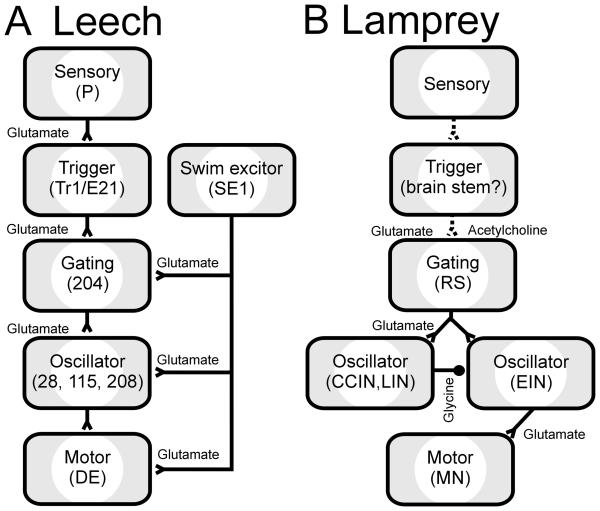
The central goal of neuroethologists is to understand the neural underpinnings of animal behavior. This broad research endeavor requires comparative research on a comprehensive set of animals and their behaviors (Pearson, 1994; Marder and Calabrese, 1996). Since most individual researchers focus on the behaviors of one species, the effort is necessarily a communal one. Reviews that directly compare results from studies on similar behaviors in different species are essential for drawing broad conclusions from these undertakings. Rhythmic behaviors are studied in a wide variety of species (Delcomyn, 1980; Marder and Calabrese, 1996); such behaviors occur in nearly all animals and the repetition inherent to the behavior permits detailed study of the mechanisms which underlie it. Swimming is one such rhythmic behavior. Similarities in swimming locomotion are seen across many species including the leech, crayfish, lamprey and tadpole (Skinner and Mulloney, 1998). Our review closely compares the neuronal mechanisms underlying the swimming undulations in two distantly related animals, leeches and lampreys, for the purpose of illustrating general principles important to the generation of locomotion (Fig. 1).
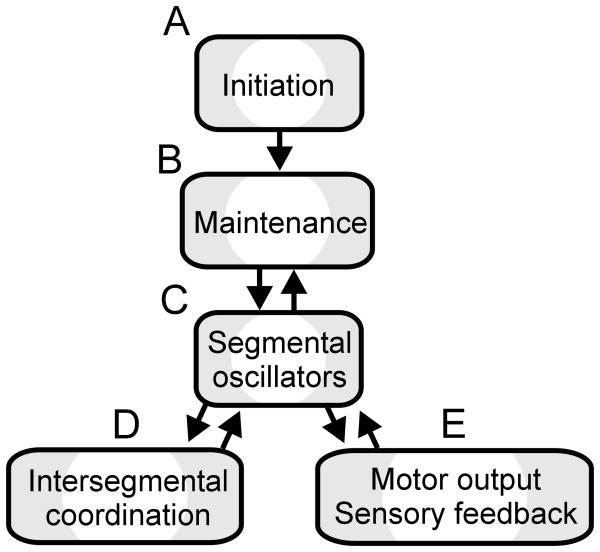
Figure 1.
Block diagram of leech and lamprey systems that control swimming. Arrows indicate the bidirectionality of all interactions but swim initiation.
The neural circuits underlying swimming in the leech and lamprey are among the best understood systems that generate complex behaviors and they produce remarkably similar rhythmic swimming movements (Fig. 2). Leeches and lampreys had their last common ancestor over 560 million years ago (Kumar and Hedges, 1998). Their disparate evolutionary lineages since that common ancestor gave rise to unrelated CNS morphologies, yet the nervous systems of the two animals share many features. For these reasons, a comparison of swimming behaviors between the leech and lamprey is particularly apt.
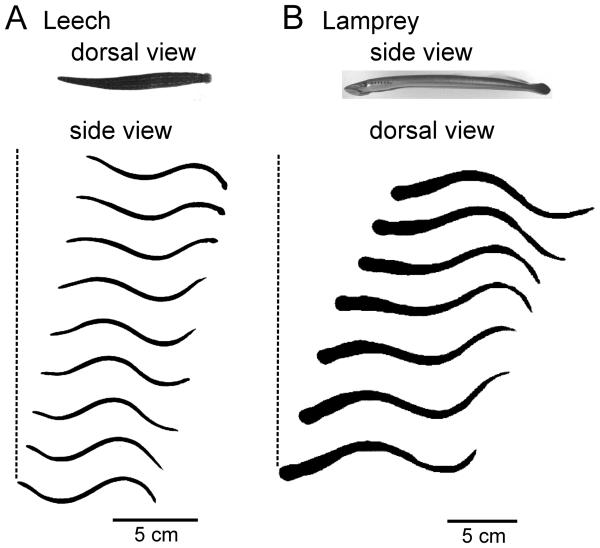
Figure 2.
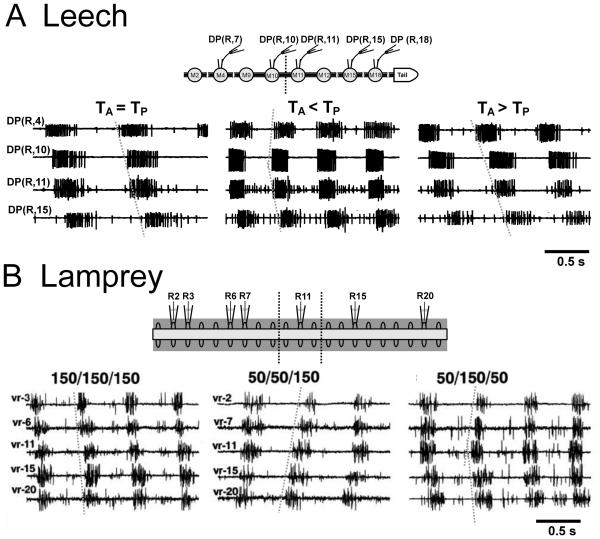
Body undulations in swimming leeches and lampreys. A Video frames of a swimming leech (Hirudo verbana). Dorsal view show shows the elongated body from above; side view shows the body undulations. Profiles were captured at 100 fps, with every fourth frame shown, for one complete cycle. The dashed reference line indicates forward progression during the 0.35 s cycle period. B Video frames of a lamprey (Petromyzon marinus; young adult). Side view shows the body profile from the side, at rest; dorsal view shows the swimming undulations viewed from above. Swimming profiles were captured at 30 fps, with every second frame shown. The dashed reference line indicates forward progression during the 0.4 s cycle period. Rostral is to the left.
Research on the nervous systems of the leech and lamprey has an extensive and rich history. Research on the neuronal substrates of leech behavior began in the 19th century with anatomic and embryologic observations, continued with behavioral and physiological studies in the first half of the 20th century, and now continues with numerous studies that also include development, pharmacology, evolution and ecology (Muller et al., 1981; Kristan et al., 2005; Siddall et al., 2007). Studies of the lamprey nervous system date back to at least 1840 and continue unabated (Rovainen, 1979; McClellan, 1987; Buchanan 2001; Grillner, 2006; Dubuc et al., 2008). The lamprey holds a special position as “primitive” vertebrate; it shares many features with higher species, including humans, but is more tractable than other vertebrate systems. Neuroethological research in both animals is facilitated by their relatively simple nervous systems, comprised of relatively few, but often large neurons. The leech CNS comprises about 104 neurons, most of which are sufficiently large and distinct for identification as individual cells and delineation of circuit interactions. By comparison, the lamprey CNS is considerably more complex, comprising approximately 105 cells in the spinal cord alone; it is nevertheless amenable to cell-class identification and circuit mapping.
This review summarizes the parallel experimental approaches applied to swimming locomotion in leeches and lampreys and the findings from those studies. It is our hope that evaluation of these independent research programs will lead to a greater understanding of each species, as well as inform locomotion research in other animals. In particular, differences in results should highlight species-specific mechanisms and expand our understanding of which neural elements are essential and which are incidental for generating rhythmic movements.
We first address the establishment and justification of using isolated spinal cord and ventral nerve cord preparations, which are fundamental to the study of swimming. Comparisons of the mechanisms behind initiation, maintenance and termination of swimming follow. Finally, origins of rhythm generation, intersegmental coupling and sensory feedback are examined. This review focuses on the neurobiology of swimming behavior; although occasionally mentioned, details of studies on development, regeneration, swim mechanics, and modeling are not presented. Finally, only a fraction of the large amount of research on the neuromodulation of swimming is discussed in this review.
1.1 A note on language
Although the leech and lamprey literatures often share a common vocabulary, differences do exist. For example, leech researchers tend to use the term “cycle period” when referring to the repetition interval of swimming movement cycles, whereas scientists studying the lamprey more often use “burst frequency,” the reciprocal of cycle period. To avoid confusion, we adopted the terminology of the leech literature, cycle period, and its reciprocal “cycle frequency,” while using the term “burst impulse frequency” to denote the frequency of impulses within individual bursts. Intersegmental phase lags are typically normalized as a percentage (or a fraction) of the cycle period by lamprey researchers, whereas the leech literature reports phase relationships in units of degrees. To allow easy comparisons between species, this review presents phases and phase lags as a percentage of the cycle period. In leeches, the terms “ganglion” and “segment” interchangeably denote the repeating units of the nerve cord; lamprey spinal segments are simply given as “segments.” Lamprey literature refers to neuronal projections from the brain to spinal cord as “descending,” while “ascending” projections are the reverse. Although the leech has both a rostral and caudal brain, “descending projections” refer to those extending rearward, while “ascending projections” convey information towards the rostral brain. In both literatures, animals described as “intact” may have experienced minimally invasive procedures, such as electrode implantation for EMG recording in the lamprey. Finally, when referring to behaviors of isolated or semi-intact preparations, the terms “fictive swimming” and “swimming” are used interchangeably.
2. Morphology: Body and CNS
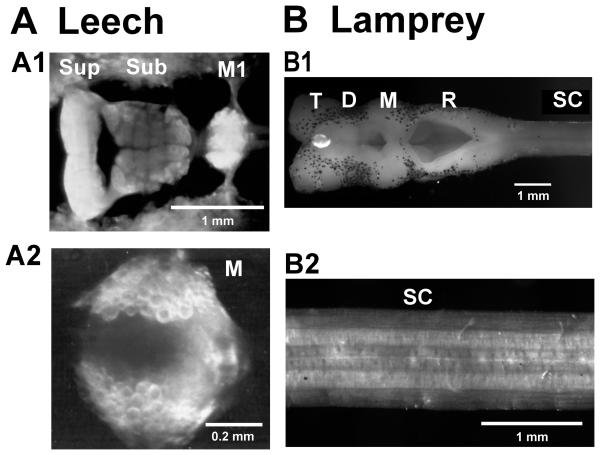
Nearly all studies reported in this review were conducted on the adult medicinal leech, Hirudo verbana. (Until recently H. verbana was thought to be Hirudo medicinalis (Siddall et al., 2007).) Leeches emerge from their cocoon with the adult body form and locomotion patterns (Weisblat, 1981, French et al., 2005). The size of the adult leech varies; they can weight 1-3+ g, and, when elongated, have a length of around 3-12+ cm and a width of about 0.5 cm. The nervous system of H. verbana comprises a rostral brain (often called a head brain, H), a caudal (or tail) brain (T), and 21 midbody ganglia (M1-M21; Payton, 1981). The rostral brain includes the subesophageal ganglion, developed from four fused neuromeres, and a superesophageal ganglion, which is not of segmental origin (Fig. 3A1), while the caudal brain arises from seven fused neuromeres (Stent et al., 1992). Hence, the complete CNS includes 32 units that are homologous, although highly differentiated at both ends. Two lateral connectives, containing approximately 2,800 axons each, and one medial connective, which contains around 100 axons, link the ganglia (Wilkinson and Coggeshall, 1975). The medial connective is often called Faivre's Nerve.
Figure 3.
Gross neuroanatomy. A Leech CNS comprises the rostral brain (A1 – ventral view of supra- and subesophageal ganglia), a concatenated series of 21 segmental ganglia (A1 –ventral view of M1; A2 – dorsal view of midbody ganglion) and the caudal brain (not shown). Round profiles seen in darkfield illumination are the somata of individually identifiable neurons. Sup – supraesophageal ganglion; Sub – subesophageal ganglion; M – one of 21 midbody ganglia. B Lamprey (Petromyzon; young adult) CNS comprises the brain and brainstem (B1, dorsal view) and the spinal cord (B2 – 3 segments). T – telencephalon; D – diencephalon; M – mesencephalon; R – rhombencephalon; SC – spinal cord. Rostral is to the left in all photomicrographs.
Most midbody ganglia contain around 400 neurons (Macagno, 1980) and exhibit a high degree of morphological and physiological similarity. The remarkable stereotyped nature of this system means many segmental neurons are easily individually identifiable through a combination of location, size, and electrical properties. The neuronal somata, which are mostly paired, are located on the ventral or dorsal surface surrounding the neuropile (Fig. 3A2). Leech neurons are monopolar, like most invertebrate neurons, with axons and neurites extending from a single process that exits the cell body (Fig. 4A). Because the neurons are robust and survive well in dissected preparations and in tissue culture, much is known about their physiological properties (Muller et al., 1981).
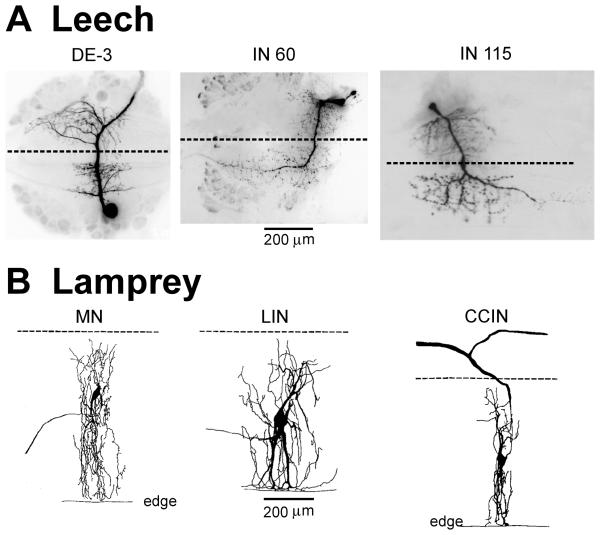
Figure 4.
Microanatomy. A Morphology of the dorsal longitudinal excitor, DE-3 motor neuron (MN; impulses in the axon of this cell are prominent in DP nerve records) and two interneurons (INs). DE-3 projects to local muscle. The neurite of interneuron, IN 60 crosses the midline and projects to rostral ganglia via the contralateral intersegmental lateral connective. IN 115 has a similar morphology but projects caudally. B Lamprey spinal neurons project to local muscle (MN) or to local neurons, and project intersegmentally in the ipsilateral hemicord (lateral interneurons [LIN]) or cross the midline and project rostrally and caudally (contralaterally and caudally projecting interneurons [CCIN]). Dashed lines indicate the midline of leech ganglia (A) and lamprey spinal cord (B). The lateral edge of the spinal cord is denoted by “edge.” Calibrations apply to all leech photographs and lamprey drawings, respectively. Leech microphotographs are abstracted from Fan et al. (2005; DE-3), Friesen (1985; IN 60) and Friesen (1989b; IN 115). Lamprey drawings are from Buchanan (2001).
The lamprey belongs to the primitive vertebrate class, Cyclostomata. Three species of lampreys commonly used for locomotion studies are Petromyzon marinus, the sea lamprey, Ichthyomyzon unicuspis, the silver lamprey, and Lampetra fluviatilis, the river lamprey. All discussions on lampreys in this review refer to one of these three species. Adult lampreys used in locomotor studies tend to be 150-350 mm long depending on the species and age. Lampreys spend a large portion of their lives, 3-12 or more years, as larvae, or ammocoetes, before undergoing a remarkable transformation to adults. As ammocoetes, they are filter feeders that burrow in the mud and grow to be 100-200 mm, depending on the species, just prior to transformation (Hardisty and Potter, 1971a). Following this metamorphosis, which takes many months, they live another 1-2 years in a parasitic phase, feeding on blood. Once they reach full maturity they stop feeding, migrate, spawn, and eventually die (Hardisty and Potter, 1971b).
The lamprey brain (Fig. 3B) is attached to a flexible spinal cord which lies atop of a notochord. In the ammocoete (16 cm long) the spinal cord is about 800 μm wide and 160 μm thick (Rovainen, 1967a); in an adult (35 cm long) the spinal cord enlarges to about 1800 μm wide and 300 μm thick (Fig. 3B; Brodin et al. 1988a). It has around 100 segments with approximately 1000 neuronal somata each. More than one thousand cells project from the brain into the spinal cord in the ammocoete (Zhang et al., 2002) and more than two thousand in the adult (Dubuc et al., 2008). As in the leech, iterated spinal segments exhibit a high degree of serial homology, with similar neuronal morphologies and interaction patterns. Unlike the leech, however, the lamprey nervous system shares major homologies with the nervous systems of higher vertebrates, including the telencephalon, diencephalon and basal ganglia, the mesencephalon, rhombencephalon, cranial nerves, and descending reticulospinal pathways (Nieuwenhuys et al., 1998). Further, typical of vertebrates, most lamprey neurons are multipolar, with multiple dendrites and the axon originating from the cell body or a proximal dendrite (Fig. 4B), although they are unmyelinated. Similar to leeches, lamprey neurons are robust and experimentally accessible.
2.1. Swimming movements
Swimming undulations in the leech and lamprey share many important features, although some aspects of the movements are fundamentally different. To initiate swimming, leeches flatten and elongate their body via tonic contraction of dorso-ventral muscles to generate a semi-rigid hydroskeleton (Kristan et al., 1974). In this state, the caudal end of their body is wider than the rostral end (Fig. 2A). Waves of active contractions and relaxations of longitudinal muscle propagate along the body, producing caudally directed body undulations (Fig. 2A) with cycle periods of 0.35 – 1.1 s (Gray et al., 1938; Kristan et al., 1974). Lampreys, due to their rigid notochord, do not change their body dimensions when they commence swimming undulations, and have roughly uniform rostral and caudal body height, while the width of their body tapers toward the caudal end (Fig. 2B). Their movements occur in the lateral plane (Fig. 2B) through rhythmic alternations of muscle contractions and relaxations. Aided by midline dorsal and caudal fins, these rearward traveling lateral body waves propel them through the water with cycle periods, in adults, that range from 0.13 – 0.66 s, (Wallén and Williams, 1984; Williams et al., 1989). Electromyogram (EMG) recordings show anti-phasic activation of ipsilateral fin muscle and myotomal muscle within segments (Mentel et. al., 2006). Swimming is more stereotyped in leeches than in lampreys, as lampreys can swim backwards as well as forwards (Paggett et al., 1998; Islam et al., 2006), but leeches cannot. In both animals, undulation amplitude increases with caudal progression (Fig. 2; Gray et al., 1938; Paggett et al., 1998, French et al., 2005). Also in both animals, temporal delays in muscle activation along the body generate nearly constant intersegmental phase lags that are appropriate for the expression of an energetically favorable approximate single cycle of the body wave (Williams et al., 1989; Kristan et al., 1974). In addition to swimming, leeches can locomote by two types of crawling, veriform or “inchworm” (Kristan et al., 2005). Lampreys can exhibit crawling when stuck in tight places (Archambault et al., 2001; Zelenin, 2005) while ammocoetes also engage in burrowing behavior (Hardisty and Potter, 1971a; Paggett et al., 1998).
3. Types of preparations: Intact, nearly-intact, semi-intact and isolated nervous system
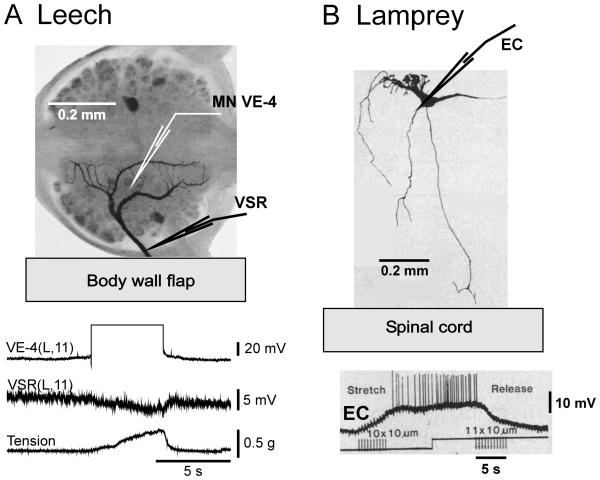
The rhythmic axial bending movements that characterize swimming behavior are caused by anti-phasic contractions of dorsal and ventral longitudinal muscles in leeches (Kristan et al., 1974; Ort et al., 1974) and left-right myotomal muscles in lampreys (Buchanan and Cohen, 1982). Segmental leech motoneurons (MNs) that are excitatory to the dorsal (DE) or ventral (VE) longitudinal muscle burst in anti-phase. However, bilateral homologs in each segment oscillate in-phase with each other (Fig. 5A). Moreover, leeches have inhibitory MNs as well as the excitors; these directly inhibit both the excitatory MNs and longitudinal muscle and oscillate in anti-phase to their excitatory counterparts (Ort et al., 1974). All lamprey MNs are excitatory; consistent with the pattern of muscle activation, bilateral recordings reveal that contralateral myotomal MNs are out-of-phase with each other (Fig. 5B; Buchanan and Cohen, 1982).
Figure 5.
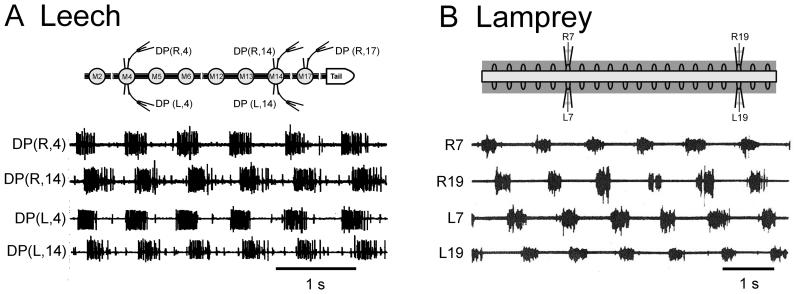
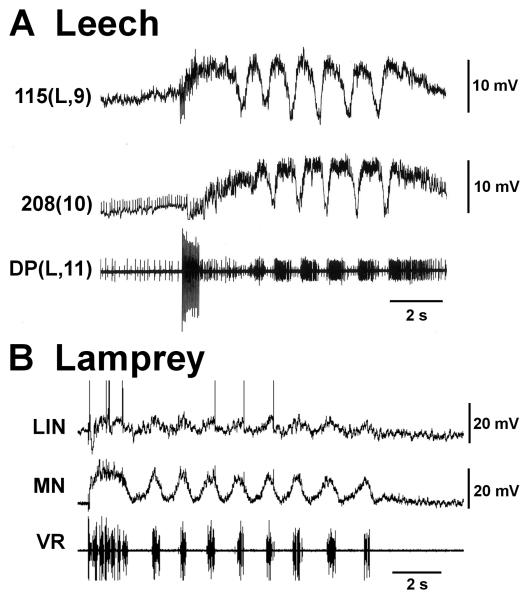
MN activity during fictive swimming. A Leech nerve cord preparation. The inset at top illustrates the M2 – T (midbody ganglion number 2 through tail [caudal] brain) preparation. Extracellular recording are made from suction electrodes on dorsal-posterior (DP) nerves. During fictive swimming DP nerves exhibit synchronized MN impulse bursts on left (L) and right (R) sides of any segment with rostro-caudal phase lags. B Lamprey spinal cord preparation. The inset at top illustrates a 20-segment-long section of the spinal cord with four extracellular suction electrodes attached to ventral roots. During fictive swimming anti-phase MN impulse bursts are recorded from left and right ventral roots of any segment; during forward swimming there is rostro-caudal phase lag. DP(R/L,“X”) – recording from dorsal posterior nerve on the right/left aspect of midbody segment “X”; R/L“X” – recording from right/left ventral root “X” of the spinal cord piece. Traces in B are redrawn from Fig. 2, Cohen and Wallén, (1980).
The development of suitable animal preparations has been critical for the successful study of animal locomotion. Detailed studies of neuronal mechanisms are feasible only if neuronal activity and movement expression can be observed simultaneously and also if stable intracellular membrane potential recordings can be obtained. Numerous experiments in leeches and lampreys are directed towards the development of nearly-intact (allowing some electrophysiological recording with minimal restriction of movements), semi-intact (allowing limited movements and electrical recording) and isolated CNS preparations. In semi-intact preparations, some body wall is removed, allowing the experimenter to observe body wall movements, muscle contractions and sensory input while simultaneously recording CNS neuronal activity. In isolated preparations, all muscle and organ tissue is removed from the nervous system, making it particularly accessible for intra- and extracellular recordings. However, use of dissected preparations raises the issue of whether the inevitable disruptions of normal sensory inputs, including sensory feedback, alter the activity patterns generated by central oscillator circuits. For this reason, measurements of cycle period and intersegmental phase lags among different preparations are of particular interest.
Semi-intact leech preparations were developed by Gray and coworkers (1938) and perfected by Stent and coworkers (Kristan et al., 1974; Ort et al., 1974). Isolated preparations of the leech nerve cord were successfully implemented by Kristan and Calabrese (1976). Semi-intact and isolated lamprey spinal cord preparations were established, respectively, by Rovainen (1979) and by Poon (1980) and Cohen and Wallén (1980). In nearly-intact lamprey preparations, swimming activity is monitored by EMG recordings while the neuronal activity characteristic of fictive swimming in both species is recorded from peripheral nerves. Fictive swimming is so-called because the neuronal activity recorded from the completely isolated nervous system clearly resembles motor patterns present during swimming in nearly-intact animal preparations (also designated by “in situ;” Kristan and Calabrese, 1976; Cohen and Wallén, 1980; Pearce and Friesen, 1984; Yu et al., 1999). This motor activity consists of high frequency bursts of impulses separated by quiescence (Fig. 5); the interval between burst onsets defines the cycle period. In the leech, fictive swimming is monitored by extracellular recordings from the dorsal-posterior (DP) nerve, which is marked by the large axon spikes of the dorsal longitudinal excitor MN, cell DE-3 (Fig 5A; Ort et al., 1974). In the lamprey, such recordings are obtained from the ventral roots (VR; Fig. 5B) which show axon spikes from the tens of MN axons contained there (Teräväinen and Rovainen, 1971).
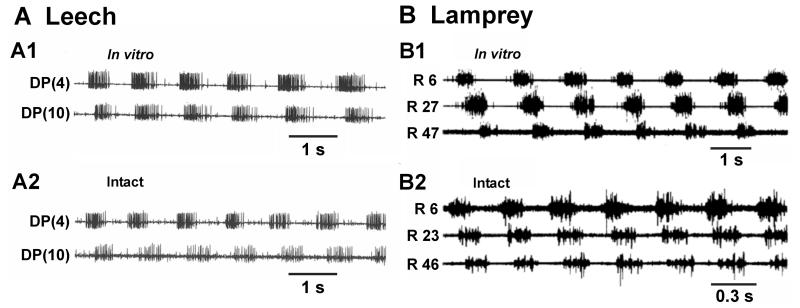
Quantitative comparisons of swimming properties in the isolated nerve cord to those in nearly-intact preparations revealed that fictive swimming approximates, with some discrepancies, MN activity patterns in nearly-intact animals (Fig. 6A; Pearce and Friesen, 1984; Yu et al., 1999). In these experiments a nearly-intact leech preparation had its most anterior and posterior ganglion disconnected and some DP nerves freed for recording, but the rest of the body remained intact. Suction electrodes recorded from the DP nerves through small slits in the body wall in two midbody segments while the leech produced swim oscillations. The DP nerve motor patterns obtained from this preparation were qualitatively similar to those obtained from an isolated nerve cord; however, importantly, phase lags were smaller in the isolated preparation (2.4%/segment vs. 4.1%/segment) and the cycle period was longer than in the nearly-intact preparation. An intact leech displaying phase lags of only 2.4%/segment would not generate a full body wavelength during a swim cycle. Also, bursts in the isolated nerve cord exhibited higher impulse frequencies and longer durations than those in nearly-intact preparations. It is thought that the presence of muscle and associated receptors in intact leeches provides feedback that increases the intersegmental phase lag and decreases cycle period.
Figure 6.
In vitro versus intact swimming in leech and lamprey. A Leech: A1 Motor neuron (MN) bursts recorded during fictive swimming in an isolated nerve cord closely resemble those obtained from the same nerve cord in the nearly-intact preparation (A2). B Lamprey: MN bursts recorded from ventral roots (VR) during fictive swimming (B1) have a similar pattern to electromyograms obtained during swimming in an intact animal (B2). Traces in A are redrawn from Fig. 2, Friesen (2009). Traces in B are from Wallén and Williams (1984). Extracellular records from nerves and roots are as noted in Fig. 5.
An early comparison of swimming activity in the isolated lamprey spinal cord and intact lampreys found many measures of swim characteristics to be statistically identical (Fig. 6B; Wallén and Williams, 1984). EMGs recorded from nearly-intact lampreys in a swim mill and ventral root recordings in isolated preparations yielded a constant phase lag of approximately 1%/segment for both conditions, albeit with greater variability in the isolated condition. More recent studies report mixed results regarding the influence of sensory feedback on phase lag (see Section 8.1; Boyd and McClellan, 2002; Guan et al., 2001). The duty cycle, or burst proportion (ratio of burst duration to cycle period), was similar in intact and isolated preparations, although cycle frequencies during fictive swimming in isolated preparations were lower (Wallén and Williams, 1984). Cycle frequencies of 0.5 – 1.4 Hz were seen in the isolated preparation in response to varying bath concentrations of D-glutamate or N-methyl-D,L-aspartate (NMDA; for convenience, the D,L mixture as well as the D isomer will be referred to as NMDA). In contrast, by varying the speed of the water current in the swim mill, intact animals produced swim frequencies ranging over 1.5 – 7.6 Hz. A third preparation in the study, intact except for a transection between the spinal cord and brain, produced swims with intermediate cycle frequencies, ranging from 0.8 – 4.1 Hz. These experiments suggest that sensory inputs and descending brainstem inputs increase the cycle frequency of lamprey swims.
In both the leech and lamprey, phase lag was nearly a constant proportion of the cycle period within a given experimental condition, intact, nearly-intact, or isolated (Wallén and Williams, 1984; Pearce and Friesen, 1984). A constant phase lag allows the intact animal to maintain the same body form, approximately one complete body wave, at any cycle frequency. Overall, both the leech and lamprey isolated CNS preparations generate, to a good approximation, the neuronal activity that occurs during swimming in the intact animal. Although quantitative differences were found in impulse frequency, burst duration and intersegmental phase lags in the leech, and in the cycle period in the lamprey, the recordings taken from the isolated preparations clearly demonstrate the occurrence of a “fictive” swim. Therefore, neurons that generate rhythmic, swim-like activity can be studied in the isolated CNS. Importantly, these experiments demonstrated that the isolated nervous systems of lampreys and leeches, devoid of descending brain inputs and sensory feedback, contain sufficient central motor programs to generate the rhythmic swimming cycle.
4. Control of swimming behavior
4.1. Initiation
Of the three stages of a swim episode, initiation, maintenance and termination, swim initiation is the most studied. Development of a wide range of methods for swim initiation in the leech and lamprey has greatly facilitated detailed investigation of locomotor behavior in these animals and has broadened our understanding of rhythmic behavior generally.
Intact leeches and lampreys swim in response to a variety of stimuli. Mechanical stimulation to the caudal or rostral end elicits swimming in both animals, although in the leech caudal inputs are more effective (Kristan, 1982; McClellan and Grillner, 1983). Surface water waves can initiate swimming in the leech via sensillar movement receptors (SMR) located on the body wall (Brodfuehrer and Friesen, 1984). Leeches are more likely to swim in deep, rather than shallow water (Esch et al., 2002; Puhl and Mesce, 2010). In lampreys, swim initiation can occur in response to water waves, vestibular stimulation, illumination the eyes and illumination of caudal dermal photoreceptors (Currie, 1991; Ullén et al., 1993; Orlovsky et al., 1992).
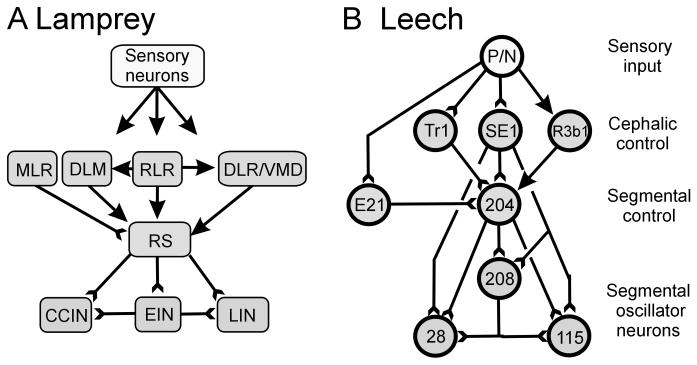
In both animals, sensory inputs active cephalic pathways whose descending outputs initiate swimming (Fig. 7; Kristan et al., 2005, Dubuc et al., 2008). In the lamprey, two routes of sensory input have been identified. Dorsal cells in the spinal cord respond to pressure (P-cells) and touch (T-cells; Rovainen, 1967b; Martin and Wickelgren, 1971; Chistenson et al., 1988) and their fibers travel through dorsal columns to the brainstem (Dubuc et al., 1993a,b), while mechanical inputs to the head are relayed by the trigeminal nerve (Viana Di Prisco et al., 1995). These sensory inputs provide indirect input to the reticulospinal (RS) neurons in the brainstem (Dubuc et al., 1993b; Viana Di Prisco et al., 1995; 2005). The RS neurons make up the main descending system to the spinal cord and provide the excitatory drive to initiate swimming (Fig. 7A; Rovainen, 1979; Brodin et al., 1988b; Dubuc et al., 2008). The RS system is made up of four main nuclei, the mesencephalic reticular nucleus (MRN), and the anterior, middle and posterior rhombencephalic reticular nuclei (ARRN, MRRN and PRRN, respectively). Bilateral pharmacological stimulation of the MRRN, PRRN and ARRN (with D-glutamate and D-asparate) in in vitro and semi-intact larval lampreys elicited swimming activity (Paggett et al., 2004; Jackson et al, 2007). Also, bilateral injection of acetylcholine onto the reticulospinal MRRN in larval and adult lampreys sometimes elicited swimming activity (Le Ray et al., 2003). Further, RS neurons were depolarized in response to swim-initiating stimuli prior to onset of an evoked swim, implying a causative function (Viana Di Prisco et al., 1997). Reticulospinal Müller and Mauthner cells and neurons in the PRRN have direct excitatory connections to excitatory and inhibitory spinal interneurons, as well as to spinal MNs (Rovainen, 1974b; Buchanan, 1982; Buchanan et al., 1987; Ohta and Grillner, 1989). Application of NMDA antagonists or Ca2+-free solutions that block chemical transmission revealed these connections to be both chemical and electrical; the excitatory chemical components are mediated by both NMDA and non-NMDA receptors (Ohta and Grillner, 1989). The RS system of the lamprey can be thought of as driving swimming behavior; this system is the final source of cephalic control for this behavior.
Figure 7.
Circuits that control swim initiation. A Brainstem structures that control swim initiation in lamprey. Areas were identified in either adult or larval lampreys. B Identified interactions in the leech. Lines ending in “Y's” ( ) indicate monosynaptic connections; arrows indicate excitatory polysynaptic pathways that are not identified. RLR – rostrolateral rhombencephalon ; MLR – mesencepalic locomotor region; DLM – dorsolateral mesencepalon ; VMD – ventromedial diencepalon; DLR – diencepalic locomotor region ; RS – reticulospinal.
) indicate monosynaptic connections; arrows indicate excitatory polysynaptic pathways that are not identified. RLR – rostrolateral rhombencephalon ; MLR – mesencepalic locomotor region; DLM – dorsolateral mesencepalon ; VMD – ventromedial diencepalon; DLR – diencepalic locomotor region ; RS – reticulospinal.
Many higher order brain areas have been shown to elicit excitation in RS neurons (Fig. 7A). The most studied higher order brain area is the mesencephalic locomotor region (MLR), a region also found in higher vertebrates, including mammals (Jordan, 1998; Sirota et al., 2000; Dubuc et al., 2008). Unilateral electrical stimulation of the MLR in larval and young adult sea lampreys initiated swimming and produced EPSPs in RS neurons (Sirota et al., 2000). Similar results were also seen following unilateral stimulation of the diencephalic locomotor region (DLR; El Manira et al., 1997; Ménard and Grillner, 2008). The MLR is thought to have monosynaptic excitatory connections to RS neurons that are mediated through glutamatergic and nicotinic receptors (Le Ray et al., 2003; Brocard and Dubuc, 2003). Moreover, this excitation is shown to bilaterally excite RS neurons, accounting for the ability of stimulation of one side of the MLR to elicit coordinated swimming (Brocard et al., 2010). It is not entirely known how MLR and DLR become activated, however it appears that its release from tonic GABA inhibition is important (Ménard et al., 2007; Ménard and Grillner, 2008). Bilateral pharmalogical or electrical stimulation in the larval lamprey of three other areas, the ventromedial diencephalon (VMD; which is near the identified DLR in the adult and may be part of the same region), the dorsolateral mesencephalon (DLM), and the rostrolateral rhombecephalon (RLR), have also been found to elicit swimming (Paggett et al., 2004, Jackson et al., 2007). Unilateral stimulation of these regions tended to cause asymmetrical rhythmic movements (Jackson et al., 2007). The VMD and DLM initiate swimming through activation of RS neurons, as “blocking” a portion of RS neurons through a GABA, glycine, kyurenic acid and zero-Ca2+ solution could block or greatly attenuate swimming during stimulation of the VMD and DLM (Paggett et al., 2004). Meanwhile, blocking the VMD and DLM attenuated RLR-initiated swimming, indicating that the RLR activates the RS system indirectly. It is likely that some of these higher order pathways are independent of each other; EPSPs elicited in RS neurons by MLR stimulation had a different shape than those elicited by trigeminal nerve stimulation. Further, stimulation of one area did not affect the EPSPs elicited by stimulation of the other (Sirota et al., 2000). Despite many regions capable of initiating swimming in the lamprey, these inputs all converge on the RS system, the final descending pathway to the spinal cord. More studies are needed to elucidate how these regions interact, and the inputs they receive.
In the leech, intracellular current injection into touch (T), pressure (P) and nociceptive (N) sensory cells evokes swim episodes in an isolated nerve cord (Debski and Friesen, 1987). Many of these sensory neurons have direct excitatory synapses with trigger neurons cells Tr1 and Tr2, whose somata are located in the subesophageal head brain (Fig 7B; Brodfuehrer and Friesen, 1986c,e). These neurons are designated as “triggers” because their brief depolarization evokes swim episodes with durations independent of the length or intensity of the stimulus (Brodfuehrer and Friesen, 1986c). These trigger neurons appear to elicit swimming largely through monosynaptic glutamatergic excitation of the gating command neuron, cell 204, an unpaired cell whose excitation initiates and maintains swimming (see Section 4.2; Weeks and Kristan, 1978; Weeks, 1981; Brodfuehrer and Friesen, 1986c,d). Since the identification of the original trigger neurons (Brodfuehrer and Friesen, 1986c), several other cells have been identified that also can elicit swimming. These are cell SE1 (Brodfuehrer et al., 1995), cell R3b1 (Esch et al., 2002) and cell E21 (Mullins et al., 2011). Cells SE1 and R3b1 are located in the head brain and, unlike cell Tr1, depolarize during swimming, and so may have maintenance as well as trigger functions. Cell SE1 directly excites cell 204; this connection has not been examined for cell R3b1 (Fig. 7B). Cell R3b1 excitation can also elicit crawling; experiments in semi-intact preparations showed that this choice depended on the water level, with swimming elicited only in deep (> 10mm) water (Esch et al. 2002). The cell most similar to Tr1 is cell E21 which is located in the most caudal midbody ganglia. In addition to receiving direct input from sensory neurons and sending direct output to cell 204 homologs, cell E21 exhibits only a modest increase in firing frequency during swimming and therefore, like cell Tr1, its excitation is not necessary for maintaining the swim episode (Mullins et al., 2011). Identification of this neuron demonstrated that neurons with triggering properties are also located outside the rostral brain.
These intracellular studies on leeches have identified cell-to-cell swim-initiation pathways from sensory input to motor output (Fig. 7B; Brodfuehrer and Friesen, 1986a). Sensory stimuli activate the T, P and N sensory cells, which directly excite cells Tr1, SE1 and E21. (Cell R3b1 is also excited by sensory inputs, but its specific circuitry is unknown). These cells have monosynaptic excitatory inputs to cell 204 homologs; cell 204 then excites oscillator interneurons throughout the nerve cord and thereby drives the swimming rhythm. Output from these segmental oscillator interneurons controls the activity of excitatory and inhibitory MNs, which provide the final common path to longitudinal muscles in the body wall, and hence swimming undulations. In the intact animal, sensory feedback plays a crucial role in this pathway.
Several approaches can be used to elicit swimming for experimental purposes. In isolated leech preparations, swim initiation is commonly produced via electrical stimulation of the DP nerve (Kristan and Calabrese, 1976). A single 5 ms pulse is sufficient to evoke a swim episode (Friesen et al., 2011). Swimming is also sometimes initiated by tactile inputs in semi-intact preparations or by intracellular stimulation of identified cells.
In the lamprey, stimulation of the ventral root or the intact spinal cord does not reliably produce swim episodes (Cohen and Wallén, 1980; Wallén and Lansner, 1984). Swimming can be elicited by microinjection of excitatory amino acids (EAA) or acetylcholine into the brainstem as well as by electrical microstimulation of certain brainstem regions (McClellan, 1994; Le Ray et al., 2003, Paggett et al., 2004). In isolated spinal cord preparations, swim activation via brainstem inputs is circumvented by EAA bath application. NMDA and D-glutamate are most frequently used; additionally, swimming can be elicited by 3,4-dihydroxy-L-phenylalanine (L-DOPA) and kainate (Poon, 1980; Cohen and Wallén, 1980; Wallén and Williams, 1984; Brodin et al., 1985). Although activation of multiple receptor types can induce swimming, co-activation is not essential. For example, bursting was still elicited in an isolated preparation following bath application of both kainate and an NMDA antagonist (Brodin et al., 1985).
While EAA application to the lamprey spinal cord reliably elicits swimming, drug application to the leech nerve cord has merely been found to increase the likelihood of a swim episode occurring. Both serotonin (5-HT) and octopamine (OA) application to the nerve cord increase the probability of “spontaneous” swims (swim episodes that occur without an acute stimulus), without affecting other aspects of the swim (Willard, 1981; Hashemzadeh-Gargari and Friesen, 1989). Monoamine depletion by reserpine treatment in an isolated leech nerve cord blocked swim initiation; swimming was restored with the addition of 5-HT or OA (Hashemzadeh-Gargari and Friesen, 1989). Thus, in the leech, 5HT and OA are important contributors to swim initiation, but neither alone seems to be essential for swim generation. These modulators also have different effects on circuitry in the rostral brain, as their focal application to this region inhibits swimming (Crisp and Mesce, 2003).
In the lamprey, the presence of serotonin seems to be necessary for swimming behavior. In the river lamprey, which contains a small spinal 5-HT nerve plexus (Zhang et al., 1996), NMDA application alone often elicits either bursting with irregular cycle periods or tonic ventral root activity, while concurrent NMDA and 5-HT application produce normal swimming (Brodin et al., 1985; Zhang and Grillner, 2000). In the sea lamprey, which contains a larger spinal 5-HT nerve plexus, NMDA application elicits normal swimming (Zhang and Grillner, 2000). The addition of spiperone, a 5-HT antagonist, often abolished swimming. Thus, in both the leech and lamprey, serotonin is not the primary transmitter associated with the initiation of swimming, but in both acts as an important, perhaps critical, neuromodulator.
To summarize, there are a variety of ways to initiate swimming in both leeches and lampreys. Sensory stimulation, electrical nerve stimulation and drug application all elicit swimming in either preparation. Intracellular current injection into several cells in the leech can cause a swim episode. Activation of individual cells is not adequate for swim production in the lamprey (Rovainen, 1974a), however the RS system along with several higher order brain regions serve functions comparable to trigger cells and cell 204 in leeches. The techniques described above are useful for the study of swimming, but caution must be exercised in drawing conclusions about the physiologically relevant stimuli in freely behaving animals. DP shock in the leech stimulates sensory axons, whose excitation can elicit swimming (Wilkinson and Coggeshall, 1975, Debski and Friesen, 1987). However, many other cells are stimulated concurrently that would not be activated by ordinary sensory stimuli in intact leeches. Similarly, there is uncertainty about whether EAA application to an isolated lamprey spinal cord mimics swim initiation in the intact animal. RS activation of swimming is mediated by several types of transmitters and by electrical synapses (Ohta and Grillner, 1989), whereas usually only one EAA is applied to the isolated spinal cord during an experiment. Nonetheless, the similar motor activity observed during fictive swimming and actual swimming suggests that our various means of swim initiation activate the same mechanisms to generate swim oscillations in these different preparations.
4.2. Maintenance
Although many aspects of swim initiation in the leech and lamprey swimming are relatively well-described, the mechanisms that maintain this behavior, despite recent progress, remain more enigmatic. How is a transient stimulus transformed into a prolonged behavior? What determines the duration of this behavior?
A number of studies suggest that swim initiation and swim maintenance are driven by two distinct systems in the leech. First, the length of an evoked swim episode is independent of the strength of the initiating stimulus; that is, neither stimulus intensity nor stimulus duration significantly affect swim duration (Brodfuehrer and Friesen, 1986c, Mullins et al., 2011). Second, habituation of swim initiation is independent of the habituation of swim maintenance (Debski and Friesen, 1985). Repeatedly initiating swim episodes caused a decrease in swim duration, showing the maintenance system, and not the initiation system, was habituating. Then swim initiation failed abruptly even though swim episode duration had remained at an average of 50 % of the controls in the episodes just prior to swim-failure. That is, swim initiation failed prior to the maintenance system.
Durations of lamprey swim episodes in isolated preparations are often strongly modulated by the swim-initiating stimuli; for example, bursting is usually coterminous with electrical or pharmalogical stimulation of the brainstem or drug bath application (e.g. Cohen and Wallén, 1980; McClellan and Grillner, 1984; McClellan, 1994), making it difficult to discern if the initiation and maintenance systems are distinct. However, in semi-intact preparations, swim episodes sometimes outlasts the stimulus by tens of seconds (Jackson et al., 2007; Ménard and Grillner, 2008). Ménard and Grillner (2008) found that in these semi-intact preparations a longer initiating stimulus resulted in longer swim duration. This might imply that the initiation and maintenance systems are more intertwined in the lamprey than in the leech, however more experiments are needed to explore this issue.
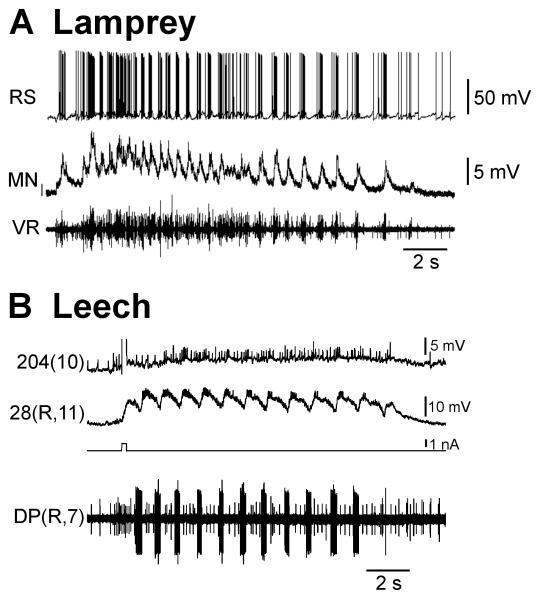
There is substantial evidence showing that RS neurons in the lamprey brainstem are important in gating, or driving, swimming behavior. As discussed above, pharmacological and electrical stimulation of this region, as well as several others, elicits swimming. However, the other brain regions that can elicit swimming (the MLR, DLR/VMD, DLM and RLR) are thought to do so through activation of the RS system (Paggett et al., 2004), while the RS neurons activate swimming through direct projections to the spinal swim oscillator neurons (Rovainen, 1974b; Buchanan, 1982; Buchanan et al., 1987; Ohta and Grillner, 1989). Importantly, there has not been a method of swim initiation tested that does not activate the RS neurons (e.g. Viana Di Prisco et al., 1997; Sirota et al., 2000; Deliagina et al., 2000; Deliagina and Fagerstedt, 2000). Intracellular and extracellular recordings show that the majority of RS neurons depolarize just prior to swim onset, remain depolarized for the duration of the swim and oscillate in-phase with ipsilateral VR roots in both isolated and intact behaving animals (Fig. 8A; Kasicki et al., 1989; Deliagina et al., 2000; Einum and Buchanan, 2005). Further, a positive correlation was seen between the intensity of swimming and the level of mass RS activity (Deliagina et al., 2000), suggesting that RS activity controls these locomotor features.
Figure 8.
Excitatory drive. A Excitation to drive swimming is provided in lampreys by reticulospinal neurons (RS; upper trace), leading to prolonged depolarization with superimposed oscillations in motor neurons (MN; middle trace). Many RS neurons oscillate in phase with motor bursts, which is thought to be a result of feedback from spinal neurons. The locomotor activity was initiated by a dimming of the lights. B Injection of a brief (0.22 s) pulse of depolarizing current (third trace) into swim-gating cell 204 (upper trace) can elicit swimming activity that is driven by prolonged cell 204 depolarization and maintains the depolarization of oscillator interneuron IN 28 (second trace). Preparation was superfused with saline containing 50 μM serotonin. IN 28 was slightly hyperpolarized by continuous current injection. VR – ventral nerve root recording; DP – dorsal-posterior nerve recording; (R/L, X), R/L refers to the left or right side, X is the ganglion number.
Other evidence for a maintenance role of RS neurons is seen in their response to mechanical sensory stimuli. In semi-intact preparations, cutaneous inputs to the head that were sub-threshold for swimming caused RS cell depolarization, but not spiking, for the duration of the stimulus (Viana Di Prisco et al., 1997). Stronger cutaneous input that did evoke RS spiking activity also elicited swimming, and the RS spiking lasted for the duration of the swim episode. This suggests that RS neurons mediate the transformation from a sensory input to a prolonged motor output. These excitatory effects were shown to arise from both synaptic inputs and from intrinsic RS cell properties that sustain depolarization. In regard to intrinsic cell mechanisms, local application of an NMDA antagonist, injection of a Ca2+ chelator, and blockage of the calcium-activated nonselective cation currents (ICAN) all blocked sustained depolarization in RS neurons (Viana Di Prisco et al., 2000). This suggests that NMDA activation increases the concentration of intracellular Ca2+, which activates an ICAN current that supports sustained depolarization in RS neurons, and thus maintains swimming behavior. The blockers did not affect swim duration, perhaps because local application of the drugs affected only a few out of thousands of RS neurons in the brainstem. The synaptic inputs that sustain RS depolarization are thought to arise from spinal central pattern generator (CPG) feedback onto RS neurons. When spinal cord feedback to RS neurons was blocked via xylocaine application to the rostral spinal segments, the duration of the sustained depolarizations in RS neurons in response to cutaneous head inputs was significantly decreased (Antri et al., 2009). One potential source of this swim-prolonging feedback is spinobulbar neurons. Spinobulbar cell somata reside in the rostral spinal cord and send axonal projections into the brainstem which can directly inhibit or excite RS neurons to modulate their activity during swimming (Vinay et al., 1998a,b; Einum and Buchanan, 2004, 2005). Spinobulbar cells also receive excitatory or inhibitory input from RS neurons, and mutual excitation between an RS neuron and a spinobulbar neuron has been observed (Einum and Buchanan, 2006). Although this feedback loop certainly modulates other aspects of swimming, it may contribute to swim maintenance as well.
Cell 204 is an unpaired cell whose homologs are located in midbody ganglia M10-M16 (Weeks, 1982) and has a similar function to lamprey RS neurons. Another homolog, cell 205, is present in M9. Like RS neurons, cell 204 is depolarized prior to swim initiation and remains depolarized for the duration of a swim episode; its decay coincides with swim termination (Fig. 8B). Long-lasting depolarization of cell 204 is capable of producing over 100 BPE, the only continuous bursting seen in the isolated leech nerve cord that parallels the continuous swimming observed in the lamprey (Weeks and Kristan, 1978). Further, in a two-ganglion chain, swimming was maintained by continuous depolarizing current injection into two cell 204s (Weeks, 1981). Simultaneously hyperpolarizing two of the eight cell 204s decreased swim duration in a full nerve cord (Brodfuehrer et al., 2008). It is known that cell 204 receives excitatory input from higher order trigger neurons (Brodfuehrer and Friesen, 1986c,d;Mullins et al., 2011). However, after a triggering input has ceased, the firing rate in cell 204 continues to increase. The source of this prolonged excitation is unknown. One possibility is that cell 204 has intrinsic membrane properties similar to those in lamprey RS neurons. Pressure ejection of glutamate agonists onto cell 204 produced sustained depolarizations and sometimes elicited swimming (Brodfuehrer and Cohen, 1990). Further, bath application of an ICAN antagonist reduced the level of sustained depolarization in cell 204 in response to nerve shock in an isolated ganglion (Brodfuehrer et al., 2008). However, these results are not incompatible with another potential mechanism for sustained activity in cell 204, reciprocal excitation between cell 204 and other excitor neurons. Brief excitation of cell 204 elicits excitation in other cell 204 homologs, and then, with some delay, further excitation in itself (Friesen et al., 2011). These connections are known to be indirect (Weeks and Kristan, 1978) and indicate the presence of self-sustaining polysynaptic excitatory feedback. Further, the functional removal of posterior ganglia during a swim episode with a stream of sodium-free sucrose over an intersegmental connective decreased swim duration (Friesen et al., 2011) indicating the importance of continual synaptic communication between ganglia for maintaining swim episodes. Some of these intersegmental synaptic contacts may come from cells SE1, Tr3 (originally identified as BN) and R3b1, which are all depolarized during swim episodes and can initiate swimming (Friesen and Brodfuehrer, 1984; Esch et al., 2002; Brodfuehrer et al., 1995). More work will be required to determine if these cells contribute substantially to swim maintenance.
Other manipulations in the leech and lamprey affect swim duration, and hence, the maintenance system. In the leech, the presence of the rostral and caudal brains influences swim duration (Brodfuehrer and Friesen, 1986a; Brodfuehrer et al., 1993, Puhl and Mesce, 2010). A full-length isolated nerve cord with both H and T brains attached produced swim durations that were almost twice those seen in a nerve cord with the caudal brain removed. A preparation with both brains removed actually had longer swim durations than controls, indicating that the influence of the rostral brain on swim duration is inhibitory. Further, in leeches that were intact posterior to M4, functional removal of the head ganglion by isotonic sucrose increased swim duration almost seven-fold when the leech was in deep water (Puhl and Mesce, 2010). While the circuitry responsible for these effects is unknown, it is clear that that the presence of the caudal brain prolongs swim duration, while the presence of the rostral brain shortens it.
In the intact lamprey, depletion of dopamine (DA) from the forebrain, brainstem, and spinal cord shortens the duration of swim episodes from approximately 150 s to 10 s. (Thompson et al., 2008). This effect appeared to be due to supraspinal DA depletion because an isolated cord with DA depletion produced normal swimming. Other interesting clues about swim maintenance in the lamprey can be found in experiments on hemicords. Recordings from VR roots in the hemicords, which are generated by a longitudinal cut along the spinal cord, show episodes of high frequency bursting that appear to arise from the swim central pattern generator (CPG; Cangiano and Grillner, 2003, 2005; for discussion of the validity of hemicords as a swim model, see Section 9). These experiments suggest that some maintenance mechanisms are also present in the spinal cord networks. Experiments on maintenance in hemicords show many similarities to the leech. Above a certain intensity, electrical stimulation of the hemicord elicited episodes that lasted minutes, and further increases in the stimulation intensity had little additional effect on the burst count (Cangiano and Grillner, 2005). These results suggest that the spinal maintenance system is at least partially independent of initiation networks, like that of the leech. In the leech, shortening the nerve cord reduces swim duration (Friesen et al., 2011) as does shortening the lamprey hemicord. Finally, reducing the interval between swim episodes reduces swim duration in both the leech nerve cord and the lamprey hemicord (Friesen et al., 2011; Cangiano and Grillner, 2005). Although these similarities are intriguing, the lamprey hemicord is lacking all contralateral inputs as well as descending RS inputs from the brainstem. It remains to be seen how well the results in the hemicord will reveal mechanisms of the complete nervous system.
In both species, important neurons in the maintenance system have been identified. However, our knowledge of the systems that determine swim duration remain incomplete. Further research on the mechanisms and circuit interactions that sustain depolarizations in the RS neurons and cells 204 will greatly aid our understanding of the transformation through which brief sensory input gives rise to prolonged motor output.
4.3. Termination
Termination of swim episodes in leeches and lampreys is the least studied stage of swimming. Perhaps it is difficult to discover terminating mechanisms when the mechanisms maintaining a behavior are poorly understood.
Excitation of two neurons in the leech rostral brain terminates an on-going swim episode. One, cell Tr2, is located in the subesophageal ganglia. Tr2 was originally identified as a trigger neuron; depolarizing current injections initiated swims in 30 % of preparations (Brodfuehrer and Friesen, 1986c). However, O'Gara and Friesen (1995) subsequently found that depolarizing current injection into cell Tr2 during a swim episode reliably terminated the episode. Two post-synaptic targets of Tr2, midbody cells 54 and 256, are capable of terminating swim episodes (Taylor et al., 2003). The underlying mechanism is unknown, but Tr2 stimulation does cause hyperpolarization, although weak, to a wide range of cells, including cells 204. Depolarizing current injection into swim-inhibitory neuron 1 (SIN1) in the rostral brain also reliably terminates ongoing swim episodes (Brodfuehrer and Burns, 1995), perhaps because SIN1 activation hyperpolarizes cells 204.
Some information regarding swim-terminating mechanisms in lampreys comes from experiments on isolated spinal hemicords (Cangiano and Grillner, 2005). Intracellular recordings of MNs during swimming showed that MNs fired one spike per ventral root burst for most of the episode. Near the end of the episode, however, MNs were often observed to “skip” a spike during a ventral root burst. Although caution must be taken in interpreting hemicord results, these data suggest that termination of lamprey swim episodes involves weakening of excitatory interneuron (EIN) oscillations leading to a progressive de-recruitment of MNs. One higher-level mechanism might include decreased glutamate release from RS neurons or activation of a subset of RS neurons specifically associated with cessation of swimming (Juvin and Dubuc, 2009). Because of these rather limited results, termination of swimming in the leech and lamprey remains an open area for more research. Progress in the area of swim maintenance seems likely to inform further experiments and new ideas on how maintenance processes are terminated.
5. Cycle periods
In a natural environment, leeches and lampreys must react to stimuli with varying swimming velocities. It follows that cycle periods in the leech and lamprey should be malleable. Despite the extensive similarity in cell type and organization from segment to segment, there is an intrinsic gradient in the cycle period of segments along the neuroaxis (Pearce and Friesen, 1985a; Cohen, 1987; Hagevik and McClellan, 1999; Hocker et al., 2000). In the leech, experiments were conducted on nearly isolated ganglia or on ganglion chains of various lengths to test whether there are regional differences in cycle period (Hocker et al., 2000). These short chains or nearly isolated ganglia were driven to generate swimming through connections to the remaining nerve cord by the small, medial Faivre's Nerve connective. Although this connective carries only about 2 % of intersegmental connective axons (Wilkinson and Coggeshall, 1975) it includes the axons of gating cells 204 and provides sufficient excitatory drive to induce swimming without transmitting coordinating information (Weeks, 1982). Preparations containing short chains of ganglia anterior to M12 exhibited intrinsic cycle periods that decreased progressively as ganglion origin became more caudal. However, short chains that included ganglia posterior to M12 had longer cycle periods than the more rostral ones, suggesting that the rostro-caudal changes in period have a “U-shape” that is, intrinsic cycle period is shortest in mid-cord (Hocker et al., 2000). In fact, individual ganglia or even short ganglion chains comprising segments caudal to M12 appear to be nearly incapable of rhythm generation. Similarly, isolated rostral ganglia M2, M3, and M4 (M1 was not tested) produced erratic bursting with long (>2 s) cycle periods. Nearly isolated ganglia from M5-M12 produced the strongest bursting. Another set of experiments demonstrated that nearly-intact leech segments embody a bias towards longer intrinsic anterior cycle periods. Of eight whole leeches cut in half, the rostral halves of six “swam” with longer cycle periods than the caudal halves (Yu et al., 1999). Results in the remaining two leeches were reversed. Thus in the leech, the properties of the local CPGs along the neuroaxis vary in a non-linear manner.
The distribution of period gradients in segmental swim oscillations in lampreys is simpler, with rostral segments usually exhibiting shorter cycle periods than those more caudal. When 17 isolated lamprey spinal cords were cut in half, twelve had shorter rostral cycle periods, four had shorter caudal periods and three exhibited no detectable differences between the two ends (Cohen, 1987). When spinal cords were cut into thirds, the cycle period of the middle piece was always either intermediate to the ends or similar to one of the ends. These data suggest that an intrinsic cycle period gradient exists along the spinal cord without abrupt transitions. Further support for this conclusion comes from experiments on functionally isolated rostral halves of the spinal cord (generated by Ca2+-free saline at the caudal end), which had shorter cycle periods than similar functionally isolated caudal halves (Hagevik and McClellan, 1999).
Cycle periods for swimming leeches and lampreys can be altered by various manipulations. Cooling the isolated leech nerve cord from 25°C to 16°C increased the cycle period almost two-fold (Pearce and Friesen, 1985a). Moreover, cycle period of leech fictive swimming was controlled by varying the impulse frequency of the gating cell 204 through current injection (Debski and Friesen, 1986). A similar phenomenon was observed in the lamprey when swimming was initiated via electrical stimulation of brainstem locomotor regions. Increased stimulation intensity resulted in higher cycle frequencies (and higher burst impulse frequencies; McClellan and Grillner, 1984, Sirota et al., 2000; Ménard and Grillner, 2008). Also, cycle period is affected by the concentration of agents in the bath of the isolated lamprey spinal cord, increased concentrations of L-DOPA (Poon, 1980), or NMDA or kainite (Brodin et al., 1985) producing higher cycle frequencies. Bath application of 5-HT to a lamprey isolated spinal cord, where fictive swimming was induced with NMDA, increased the cycle period (Harris-Warrick and Cohen, 1985; Zhang and Grillner, 2000). This result differs from those obtained from leeches, where 5-HT has no effect on cycle periods (Hashemzadeh-Gargari and Friesen, 1989). Further, in the lamprey, application of acetylcholine to the isolated spinal cord significantly decreased cycle period (Quinlan et al., 2004). Nonuniform cycle periods are important for establishing phase delays (Skinner and Mulloney, 1998; Hill et al., 2003). Thus, the differing gradients in intrinsic cycle periods in leeches and lampreys, which nevertheless give rise to similar intersegmental phase lags, should alert us to expect different mechanisms for generating these phase lags in these species.
6. Rhythm generation
6.1. Oscillations in short chains
A fundamental question in the field of neuronal rhythmicity concerns the location and extent of the neuronal interactions that give rise to the oscillations. Clearly, reduced preparations of the isolated CNS can generate at least the rudiments of fictive swimming in leeches and lampreys. Hence, we might ask: Is every segment capable of producing oscillations, or are intersegmental interactions essential for generating the basic swim rhythm? In other words, does every segment contain one, or, given bilateral symmetry, even two CPGs?
To produce reliable fictive swimming in leeches, nerve cord preparations that comprise a chain of at least six or seven ganglia are needed (Kristan and Calabrese, 1976). However, the rudiments of swimming can be induced even in nearly isolated ganglia by excitatory drive via the median connective (Weeks, 1981), or in completely isolated ganglia when 50 μM 5HT is added to the bath (Hashemzadeh-Gargari and Friesen, 1989). Although such rudimentary swim episodes were often brief, cycle periods were appropriate (0.7 – 2.0 s) for swimming.
In the lamprey spinal cord, it has likewise been reported that chains of three segments produced fictive swimming with appropriate phase lags (Grillner et al., 1991). Bursting was also elicited in one or two segments, although the burst pattern was more variable (Buchanan 1999b). The ability to produce swim-like oscillations in a single segment shows that each segment has at least one rudimentary oscillator within it. Therefore, the functional swim CPG in the leech nerve cord and lamprey spinal cord may be viewed as a series of local oscillators coupled by intersegmental interconnections.
6.2. Central pattern generator circuitry and burst generation
Intensive research over many decades has succeeded in elucidating the mechanisms by which neural networks, the CPGs, generate swimming oscillations. These neuronal circuits are critical for generating rhythmic movements in species ranging from jellyfish to humans (Orlovsky et al., 1999; Butt et al., 2002; Marder and Calabrese, 1996; Kiehn, 2006). The vast number of neurons in mammalian nervous systems has hampered full descriptions of neuronal circuits underlying rhythmic locomotory behaviors via standard electrophysiological and anatomical techniques. These techniques are, however, well-suited for the simpler nervous systems of leeches and lampreys. Currently, the swim CPG in the leech CNS is described by numerous synaptic interactions between individually identifiable neurons. The nervous system of the lamprey has an intermediate complexity; although a few uniquely identifiable neurons are described in the CNS, such as the Müller and Mauthner neurons, none of these are components of the swim CPG (Buchanan, 2001). However, distinct classes of lamprey neurons are identified by their morphology and physiology. Intracellular recordings from members of different classes allowed researchers to generate circuit diagrams similar to those derived for identified neurons in leeches. Comparisons of these circuits can illuminate aspects of CPG function that apply to rhythm-generating systems generally, including mammalian locomotory circuits.
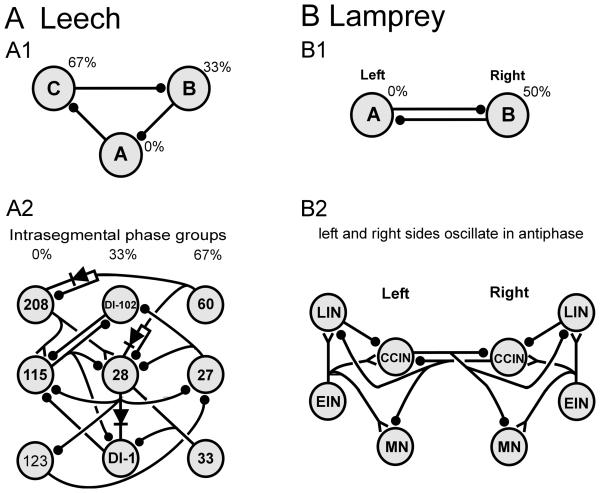
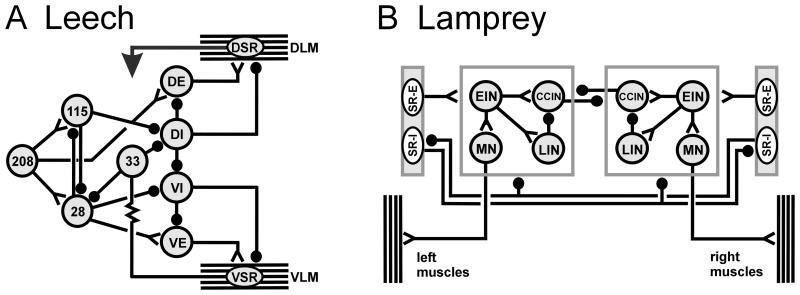
As described earlier, individual ganglia within the leech appear to contain competent CPG subunits, which are sufficient for rhythmic bursting. Intersegmental connections strengthen the locally weak oscillations, either through rhythmic synergistic intersegmental inputs or simply from additional excitatory input (Friesen and Hocker, 2001). A cell is considered to be a candidate swim oscillator neuron if (1) its membrane potential oscillations are phase-locked to the swimming rhythm and (2) current injections into the cell shift the swimming rhythm (Friesen et al., 1978). Moreover, to function in a CPG, members must have synaptic interactions with other CPG members and some of these neurons must drive MN output. At least six paired and one unpaired intersegmental interneurons that meet these criteria are identified in most, and perhaps all, segmental ganglia of the leech nerve cord (Fig. 9A; Friesen et al., 1976, 1978; Weeks, 1982; Friesen, 1985, 1989b). These 13 neurons receive input from swim-initiating and swim- maintaining cells, including gating cell 204 homologs (Nusbaum et al., 1987). Interactions among the oscillator cells include a large set of intra- and interganglionic synapses, as well as many synaptic and electrical interactions with MNs (Friesen et al., 1978; Poon et al., 1978). The synapses are largely inhibitory, with the exception of the unpaired cell 208, which has excitatory outputs to two oscillator interneurons, among others (Fig. 10A; Weeks, 1982; Nusbaum et al., 1987).
Figure 9.
Intracellular potentials during fictive swimming. A Leech. Membrane potentials in interneurons (IN) 115 and 208 (upper two traces), both swim oscillator interneurons, compared to extracellular motor bursts (bottom trace). Swimming was evoked by brief stimulation (at large artifacts) of a segmental nerve. Because the midpoint of the dorsal-posterior (DP) nerve impulse bursts occur concurrently with the peak of the IN oscillations, they are designated with the same activity phase (0%). B Lamprey. Membrane potentials in a lateral IN (LIN) and a motor neuron (MN) occur phase-locked to MN impulse bursts recorded from a ventral root (VR) in a brainstem-spinal cord preparation. Both LIN and the MN are depolarized during ventral root bursts and hence have a phase of 0%. Swimming activity in the lamprey preparation was elicited by electric shock of the spinal cord (large artifacts).
Figure 10.
Neuronal circuits for generating swim oscillations. A Leech circuits. A1 The current minimal model for swim generation is a circuit of three inhibitory INs that form an inhibitory ring. Such a circuit generates oscillations that have three phases without strong dependence on cellular properties. A2 Summary of many of the segmental interactions between MNs and INs. The numbers denote individually identified INs; DI-102 and DI-1 are inhibitory MNs. Note that inhibitory MNs are strongly interconnected with the INs and may contribute significantly to rhythm generation. Phase values for the three columns of neurons in the CPG are indicated at the top. B Lamprey circuits. B1 “Half-center” model for spinal interactions leading to vertebrate locomotion. Two neurons oscillate in anti-phase because of reciprocal inhibitory interactions and because of critical cellular properties. B2 Circuit summary for the segmental CPG in lamprey. Crossed inhibitory interactions ensure that when one side is active, the other is inhibited. Abbreviations: MN, motor neuron; DI, dorsal longitudinal inhibitor; CCIN, contralaterally and caudally projecting interneuron; EIN, excitatory interneuron; LIN, lateral interneuron. Lines ending in filled circles ( ) denote inhibitory synapses; those terminating with a Y (
) denote inhibitory synapses; those terminating with a Y ( ) are excitatory; diode symbols denote rectifying electrical junctions.
) are excitatory; diode symbols denote rectifying electrical junctions.
The neuronal circuits comprising the CPG, when provided with a source of tonic excitation, such as the excitatory input from cells 204, can generate continuous multiphasic oscillations. Recurrent cyclic inhibition (RCI) was proposed as a mechanism by which leech swim oscillations might arise (Friesen and Stent, 1977). Modeling shows that identified circuit properties can account for the observed membrane oscillations and intersegmental phase relationships (Friesen and Stent, 1977; Zheng et al., 2007). The CPG neurons (cells 33, 27, 28, 123, 60, 115 and 208) can be divided into three groups based on their activity phases which are near 0%, 33% and 67% (where the cycle phase of cell DE-3 is arbitrarily assigned 0%: Figs. 9A, 10A). Using the RCI principle, the oscillator circuit was grouped into these three phase sets; membrane potential rhythms with a verisimilitude to swimming membrane rhythms were successfully generated (Zheng et al., 2007). There is no evidence that any of the oscillator circuit neurons are intrinsic bursters or can generate plateau potentials.
Because the lamprey CNS contains numerous neurons, lamprey CPG circuitry is described at the cell-class level. As noted, the lamprey has at least one functional CPG per segment; its CPG interactions are both intra- and intersegmental. The normal source of excitation for the CPG appears to be RS neurons in the brainstem that provide excitatory input to excitatory interneurons (EINs) and to inhibitory interneurons in the spinal cord (Ohta and Grillner, 1989). Spinal interneurons exhibit oscillations that are phase-locked to ventral root bursts (Fig. 9B; Buchanan and Cohen, 1982). EINs excite other EINs along with ipsilateral contralaterally and caudally projecting interneurons (CCIN) and lateral interneurons (LIN; Buchanan and Grillner, 1987; Parker and Grillner, 2000). CCINs inhibit each other and the LINs (Fig. 10B; Buchanan, 1982). Although CCINs have often been modeled as inhibiting contralateral EINs, there is no direct evidence that these connections exist (Parker and Grillner, 2000). LINs are glycinergic and inhibit ipsilateral CCINs (Fig. 10B; McPherson et al., 1994).
There are two major differences between lamprey and leech locomotor CPG function. First, in the lamprey intrinsic cell properties as well as circuit properties are known to be responsible for neuronal oscillations. During bath application of NMDA, oscillations persisted in some CPG neurons following the addition of tetrodotoxin to block action potential evoked inputs (Sigvardt et al., 1985; Grillner and Wallén, 1985). Thus individual CPG neurons have pacemaker properties. Briefly, the conceptual model posits that excitation from the RS neurons and EINs cause the oscillators cells to fire and promotes calcium entry into CPG neurons. Each burst is terminated largely by a calcium-activated potassium current (KCa) that hyperpolarizes the cell (El Manira, 1994). A sodium-activated potassium channel (KNa) may also contribute to the burst termination (Wallén et al., 2007). Second, in the leech, inhibitory synaptic connections are thought to be necessary for oscillations to occur in individual CPG neurons, whereas in the lamprey it is thought that excitation combined with membrane properties is sufficient for rhythm generation (Grillner, 2003). There is some debate on this latter issue (see Section 9), which is based on studies of hemicords, which, devoid of crossed inhibitory input produce rhythmic activity even after the addition of the glycine antagonist, strychnine (Cangiano and Grillner, 2003, 2005). GABA antagonists were not tested on the hemicords. However, although there are ipsilaterally projecting GABAeric spinal neurons (Brodin et al., 1990; Mahmood et al., 2009) bursting still occurred following bath application of GABA antagonists to the intact spinal cord (Tegnér et al., 1993; Schmitt et al., 2004). It is clear that inhibition in the lamprey is crucial 1) to cause the alternating left-right bursting required for swimming and 2) and for the generation of normal cycle periods. Blocking glycinergic synapses causes high frequency, inappropriate L-R bursting in the VR, although rhythmic bursting remains (Cohen and Harris-Warwick, 1984). Bath application of GABA antagonists also elicited higher than normal burst frequencies (Tegnér et al., 1993; Schmitt et al., 2004).
As in the leech, the extensive interconnections in the lamprey CPG require modeling studies to test the rhythm-generating capabilities of these circuits. These studies range from highly detailed biophysical models to ones that are highly conceptual, and like in the leech, generate physiologically realistic results (Grillner et al., 2007). These studies have provided valuable insights into the mechanisms of rhythm generation and into the origins of intersegmental coordination.
6.3. Relationship of the CPG to MNs
Leech oscillatory interneurons connect with appropriate MNs to establish the exquisitely timed muscle contractions that propel the leech through water. The MNs, which are extensively interconnected within their segment of origin, activate or inhibit body wall muscle in a phase-delayed manner to generate the traveling body wave (Poon et al., 1978; Friesen, 1989a; Fan et al., 2005). The dorsal inhibitor MN, cell DI-1, for example, inhibits the ipsilateral cell DE-3 and contralateral ventral inhibitor, cell VI-2, and is electrically coupled to both its contralateral homolog and the ipsilateral inhibitory MN, cell DI-102. It is also, seemingly paradoxically, electrically coupled to the anti-phasic ipsilateral cell VI-2, a connection most likely necessary for a non-swimming behavior in which dorsal and ventral muscles are co-activated rather than antagonistic.
The excitatory MNs in leeches appear to have no role in generating the underlying rhythm. The leech inhibitor MNs, by way of contrast, do contribute to rhythm generation as shown by their interactions with the oscillatory interneurons (INs) (Fig. 10A2) and by the phase-shift of the swim rhythm following current injection into their somata (Kristan and Calabrese, 1976; Friesen, 1989a). Because their processes are limited to their segment of origin, the inhibitory MNs have only intraganglionic interactions. In contrast, INs have intersegmental projections with a span of five segments in either direction. The MNs, therefore, make no direct contributions to intersegmental phase lags.
Lamprey motor neurons receive excitatory inputs from RS neurons, as well as from local EINs (Fig. 10B; Ohta and Grillner, 1989; Buchanan et al., 1989). This latter excitation can account for much of the MN depolarization. Activated EINs produce monosynaptic glutamatergic EPSPs in ipsilateral MNs (Buchanan and Grillner, 1987). MNs then activate ipsilateral myotomal muscle (Teräväinen and Rovainen, 1971). Some CCINs produce monosynaptic glycinergic inhibition in contralateral MNs, and therefore contribute to the rhythmic hyperpolarizations of MN oscillations (Buchanan, 1982; McPherson et al., 1994). The LINs only rarely inhibit ipsilateral MNs (Rovainen, 1974a), although small local inhibitory interneurons that inhibit ipsilateral motoneurons have been described (Buchanan and Grillner, 1988).
As in leeches, MNs in lampreys have processes that are local and hence do not participate in intersegmental coordination. Stimulation of the ventral roots, which carries some 60 to 80 MN axons, did not affect the swim rhythm, suggesting that MNs have no important role as rhythm generators for swimming (Wallén and Lanser, 1984). However, VR stimulation does reveal some synaptic interactions of lamprey motoneurons with other motoneurons and interneurons (Quinlan and Buchanan, 2008).
In summary, common features of rhythm generation in the leech and lamprey systems include a source of tonic excitation that drives iterated segmental neuronal circuits. Although the core units of the lamprey CPG may not require synaptic inhibition, inhibitory interneurons are necessary in both circuits for the generation of the complete swim pattern. Some components of both systems remain undiscovered, such as the source of inputs to INs cells 60 and 208 in the leech and refinements of the functions of EINs, CCINs and LINs in the lamprey. There surely remain many unidentified neurons in both systems, especially in the lamprey, that may make substantial contributions to rhythm generation and other aspects of swimming.
7. Intersegmental coordination
The CPGs within individual body segments of leeches and lampreys must be coupled with one another for coordinated swimming to occur. Cycle periods in all segments must be equal, and appropriate phase lags must be maintained between the segments to produce efficient swimming. Because leeches and lampreys contain at least one CPG in most body segments, and because isolated systems are capable of producing coordination without sensory input, the complete CPG in these animals can be viewed as a chain of coupled unitary oscillators (Fig. 11). Understanding the properties of intersegmental neuronal connections is essential to understanding how coordination occurs.
Figure 11.
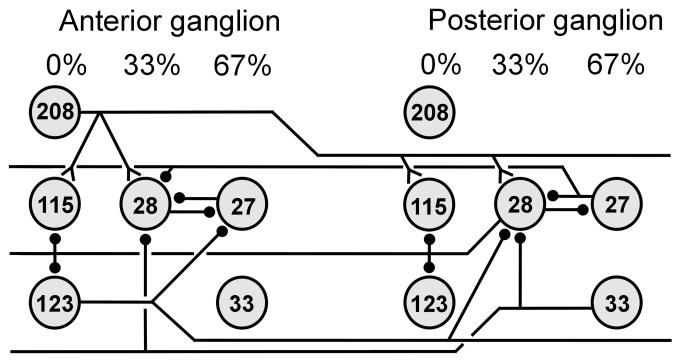
Intersegmental coordinating interactions in the leech. The intersegmental interactions shown extend both in the rostral and caudal directions for about 5 segments. There is only one identified excitatory oscillator neuron, IN 208.. The interactions shown quantitatively account for intersegmental phase lags during fictive swimming. Symbols are as in Fig. 10.
7.1. Strength of intersegmental coordinating projections
In leeches, following initiation, fictive swimming activity arises nearly synchronously throughout the nerve cord. That is, once the swim is underway, all ganglia or segments generate MN impulse bursts with appropriate rostro-caudal phase delays. An intact leech nerve cord, therefore, almost always acts as a whole when generating fictive swimming. In contrast, bursting in lamprey ventral roots in an intact spinal cord may be restricted to a limited number of segments. For example, near threshold current injected into locomotor brainstem regions activates only a few segments; subsequent increases in current intensity elicit bursting in previously inactive roots (McClellan and Grillner, 1984). Furthermore, NMDA application to only the rostral half of the spinal cord with the serotonin blocker spiperone on the caudal half elicits bursting in the rostral ventral roots, but only tonic activity in the posterior ventral roots (Zhang and Grillner, 2000). Lamprey segments, therefore, are capable of bursting independently of each other in an intact spinal cord. One source for this difference in leech and lamprey performance might be weaker intersegmental coupling in the lamprey than in the leech.
7.2. Extent of coordinating projections
The longitudinal extent of intersegmental neuronal connections must be known to fully comprehend intersegmental coupling and coordination. Blocking experiments (lesions and low-Ca2+) were used to investigate these connections. Application of low- or zero-Ca2+ or high-magnesium (Mg2+) saline over a portion of the isolated nervous system has the effect of blocking synaptic connections while allowing passage of nerve impulses (Nicholls and Purves, 1970). With this manipulation it is possible to test the extent of functional intersegmental connections. When four or five consecutive segments of an isolated M2-M19 leech nerve cord were bathed in saline containing elevated Mg2+, the ends on either side of the high-Mg2+ bath still exhibited 1:1 coupling, although with five segments blocked swim initiation often failed (Pearce and Friesen, 1985b). Swimming could not be elicited when six segments were blocked. These experiments show that direct intersegmental coupling connections span at least five segments, and possibly further, and implicate long-ranged fibers as being important for coordination. Further, in a preparation in which long-ranged projections were destroyed by cuts to contralateral lateral connectives on either side of an individual midbody ganglion, a “Z-cut” preparation (Fig. 12B), coordination was greatly reduced between the anterior and posterior ends (Friesen and Hocker, 2001). Coordination within the ends was normal. Complementary evidence for a five segment coupling span was seen in the five segment reach of two oscillator interneurons, cell 33 and cell 28 (Friesen et al., 1976; Poon et al., 1978). A coupling span of five segments in the leech nerve cord, therefore, appears to be a good estimate.
Figure 12.
Elimination of long-range interactions. A Lamprey preparation. Long-distance axons in the spinal cord are interrupted through contralateral hemisections separated by several segments. B Leech Z-cut preparation. To interrupt through-going interactions, the right lateral intersegmental connective nerve is cut rostral to ganglion M10 and the left connective is cut caudal to M10. Schematic in A from Guan et al., 2001.
In brain/spinal cord larval sea lamprey preparations, phase-locked ventral root bursts occurred in either end of the spinal cords when up to 40 consecutive medial segments (out of 100) were blocked by low-Ca2+/high-Mn2+ Ringers solution (McClellan and Hagevik, 1999). However, in an earlier study on isolated spinal cords of silver lampreys, swimming was not elicited when 20 consecutive segments were blocked with zero-Ca2+ saline; with only 10 segments blocked coupling was obtained (Cohen, 1987). The experimental discrepancies seen here could be due to the method of swim initiation (brainstem microinjection vs. EAA bath), the presence or absence of the brain, or an age or species difference. Another experiment performed on isolated silver lamprey spinal cords revealed a high degree of coordination between two ends when 16 segments were blocked by a low-Ca2+/high-Mn2+saline (Miller and Sigvardt, 2000), similar to the results seen by Cohen (1987). In any event, out of 100 total body segments, long-ranged fibers sufficient for intersegmental coordination span a minimum of 10 and a maximum of 40 segments. Further evidence for the importance of long-ranged coupling in intersegmental coordination in lampreys comes from lesion experiments of Guan et al. (2001). A spinal cord preparation was created with two contralateral hemisections five segments apart, similar to the Z-cut preparation in the leech (Fig. 12A). Because fibers cross the spinal cord only once, near their segment of origin (Rovainen, 1985), this dissection severed most of the long-ranged connections responsible for directly coupling the intact segments located distally to the lesions, with only minor lesioning of short-ranged connections. Following this manipulation, recordings from either side of the lesion showed significantly reduced, though not abolished, coupling between these ventral roots, further implicating direct long-ranged fibers as significant contributors to intersegmental coordination.
Although there is no direct evidence in support of the involvement of specific classes of spinal neurons in intersegmental coupling, several classes are candidates. Coupling that spans up to 40 segments (McClellan and Hagevik, 1999) may be mediated by propriospinal neurons with long axonal projections that have been demonstrated with retrograde labeling (Rouse and McClellan, 1997; Vinay et al., 1998b). CCINs with a 14-20 segment reach (Buchanan, 1982) may also contribute to long- or intermediate-ranged coupling. Neurons with shorter axons that may be candidates for short-ranged intersegmental coupling include other CCINs (<5 segments), EINs (up to 9 segments) and other inhibitory INs (<5 segments; Ohta et al., 1991; see also Buchanan, 2001). Blocking glycinergic inhibition does not disrupt intersegmental coupling or phase lags during fictive swimming (Hagevik and McClellan, 1994) suggesting that excitatory interneurons are sufficient for intersegmental coupling while not ruling out a contribution from inhibitory interneurons.
In the leech and lamprey, location of important intersegmental coordinating information has been identified. In the leech, cutting both lateral connectives between two ganglia (leaving only the median connective) nearly abolishes coordination between the chains on either side of the cuts (Weeks, 1981; Hocker et al., 2000). Further, transection of the median connective alone leaves intersegmental coordination intact (Weeks, 1981). In the lamprey, lesioning the lateral fascicles in conjunction with blocking synaptic interactions in 10 segments abolished coupling (Cohen, 1987). Lesions of the medial fascicle in this condition degraded coupling but did not block it. It appears, therefore, that long-ranged coordinating fibers in lampreys predominantly travel through the lateral fascicles.
We have described studies suggesting that for both the leech and lamprey, direct connections from long-ranged axons are required for strong intersegmental coupling between distant segments, whereas local coupling can also be maintained via short-ranged fibers because short chains of segments still display coordination (Wallén and Williams, 1984; Pearce and Friesen, 1985b),. In the lamprey, short-ranged fibers are also capable of transmitting long-ranged coupling information through indirect connections if the long-ranged fibers are destroyed, but under this condition coordination is weakened. Approximating a long-distance coupling span of 20 segments for the lamprey (intermediate between the minimum and maximum observed coupling range) and 5 segments for the leech, long-ranged fibers in both animals project about 20 % of the CNS length (20/100 segments and 5/21 segments, respectively, in lampreys and leeches). Investigations of this fractional coordinating fiber span in other species could determine whether these findings illustrate a common feature of intersegmental coordination.
7.3. Intersegmental phase lags
In order to express a single complete body wave at various swimming speeds, intact leeches and lampreys maintain nearly constant intersegmental phase lags relative to cycle period. Lampreys have approximately 100 segments and phase lags of around 1.0 %/segment. With cycle frequencies ranging from 0.25 – 10 Hz, the absolute intersegmental time delays between bursts vary 40-fold (Wallén and Williams, 1984; Williams et al., 1989). In leeches, 18 segments are most important for swim production; using cinematographic analysis, intersegmental phase lags from 4.4 – 10.0 %/segment in intact animals have been reported (Kristan et al., 1974; Pearce and Friesen, 1984). Leech phase lags increase toward the caudal end of isolated preparations (Pearce and Friesen, 1985b). Similarly, Miller and Sigvardt (2000) found that phase lags at the rostral end were slightly less than 1.0 % in isolated spinal cord of adult silver lampreys, whereas those at the caudal end were slightly greater than 1.0 %.
Experimental manipulations can influence intersegmental phase lags. Blocking five consecutive midbody ganglia in the leech with high-Mg2+ increased the local phase lags on either side of the block (Pearce and Friesen, 1985b). Similarly, reducing the length of an isolated nerve cord increased intersegmental phase lags (Pearce and Friesen, 1985b). However, the phase lags between the ends of the shortened chain changed little from the control; that is, a full wave would still be expressed in a shortened leech. Indeed, ‘whole’ leeches cut in two displayed almost one full wavelength in the caudal half, whereas rostral halves did not reliably generate a traveling wave (Yu et al., 1999). These studies suggest that the removal of long-ranged interactions increases phase lags between segments in the leech. Unlike the leech, the lamprey maintained 1.0 %/segment phase lags in shortened isolated spinal cords with as few as five segments (Wallén and Williams, 1984). Further, blocking synaptic transmission in middle spinal segments in the lamprey with low-Ca2+/high-Mn2+ decreased the phase lags between the ends of the spinal cord while leaving the phase lags within compartments intact (Miller and Sigvardt, 2000). A similar manipulation caused the phase lags on either side of a medial block to vary as a function of cycle period in the larval lamprey, while normally phase lags are independent of cycle period (McClellan and Hagevik, 1999). These studies suggest that short-ranged fibers are primarily responsible for maintaining proper segment-to-segment phase delays in lampreys. However, like the leech, and unlike the isolated lamprey system, the rostral half of a decapitated “whole” lamprey had increased phase lags and swam with almost a full cycle of the body wave (Guan et al., 2001). It seems likely that sensory feedback in the lamprey experiments modulated CNS intersegmental coupling to produce a full body wave.
7.4. Asymmetries establish intersegmental phase lags
The creation of rostro-caudal intersegmental phase lags in a chain of coupled segmental oscillators requires some combination of asymmetries in the intersegmental interactions between unit segmental oscillators and asymmetric or nonuniform intrinsic cycle periods in the unit oscillators (Skinner and Mulloney, 1998). Both of these components, intersegmental asymmetries and nonuniform intrinsic cycle periods, have been investigated extensively in leeches and lampreys.
Suggestions that leech intersegmental phase lags are caused by a gradient of increasing cycle periods toward the caudal end of the leech nerve cord were proved wrong by establishing that the anterior segments have a higher cycle frequency than the medial segments. (Pearce and Friesen, 1985a). Thus, asymmetric intersegmental interactions must counter the intrinsic differences in cycle periods to generate the observed rostro-caudal phase lags. It should be noted that uniform changes in cycle periods alter phase relationships little in the leech and even less in lamprey. For example, the ranges of cycle periods in isolated and intact leech preparations overlap, whereas the phase lag ranges (measured as a percentage of cycle period) do not (Peace and Friesen, 1984). In the lamprey, systematic examinations of cycle periods and phase lags found no correlation between the two (Wallén and Williams, 1984; Boyd and McClellan, 2002).
There is evidence that local, intrinsic cycle periods influence phase lags. Based on the knowledge that cooling a nerve cord increased the cycle period, a leech preparation was generated with the rostral portion of the nerve cord in 16°C saline and the caudal portion in 24°C saline (Pearce and Friesen, 1985a). The phase lag along the nerve cord significantly decreased when compared to controls due to the temperature effect on local cycle periods (Fig. 13A). When the posterior half was cooled with respect to the anterior, the phase lag between the chains, as well as within the chains, increased. When one lateral connective was lesioned to decrease intersegmental coupling strength between the two chains, increasing intrinsic cycle periods in the anterior nerve cord via cooling often reversed the phase lag between the chains. Likewise, cooling the posterior end in this condition dramatically increased the cycle period between the chains.
Figure 13.
Manipulation of intersegmental phase lags through changes in local, segmental cycle period. A Period changes controlled by saline temperature superfusing the leech nerve cord. Splitting the recording chamber (vertical dashed line) allowed independent control of rostral (TA) and caudal (TP) nerve cord temperature, and hence intrinsic local cycle periods. Intersegmental phase lags were decreased when TA was less than TP and increased when TA was greater than TP. B Period changes controlled by NMDA concentrations in the lamprey spinal cord. The recording chamber was split into three compartments allowing independent control of cycle period in rostral, middle and caudal portions of the spinal cord. Inset shows the recording arrangement for the ventral root traces. The numbers above each set of traces indicate the NMDA concentrations in μM. Decreasing cycle period in caudal segments by elevating NMDA led to a reversal of the normal phase lag, with caudal segments now leading rostral ones. Decreasing cycle period in the middle chamber caused this portion to phase-lead the rostral and caudal compartments. Trace in B is from Matsushima and Grillner (1992).
Manipulation of intrinsic cycle periods in portions of the lamprey spinal cord affects phase lags throughout the whole cord. Like the intact leech nerve cord, these manipulations affect the within-compartment and between-compartment phase lags roughly equally. Because higher concentrations of EAAs cause lower cycle periods, sections of the lamprey spinal cord were exposed to differing D-glutamate concentrations, causing differences in local intrinsic cycle periods (Fig. 13B). When the rostral portion of the spinal cord was bathed in the higher D-glutamate concentration (and therefore had the highest intrinsic cycle periods), phase lags increased both within and between the spinal cord sections (Matsushima and Grillner, 1992). Further, in contrast to the leech, the spinal cord segments exposed to the highest D-glutamate concentration became the “leader” in the chain of segments regardless of the segment location. That is, the shortest intrinsic cycling segments phase-led the other segments. An intersegmental phase lag gradient also occurred within saline compartments; if the most-caudal chain was the “leader”, every segment within the caudal chain phase-led its rostral neighbor. Therefore, the locally decreased cycle periods in the caudal segments in the lamprey spinal cord reversed the direction of the phase lag throughout the spinal cord. This plasticity in phase relationships likely accounts for the ability of lampreys to swim backwards (Grillner, 1974; Paggett et al., 1998; Islam et al., 2006). Such plasticity has not been observed in leeches. To conclude, both lamprey and leech intersegmental phase lags are affected by local changes in intrinsic cycle periods, but leech intersegmental connections prevent a phase lag reversal (i.e. negative phase lags), whereas lampreys can exhibit phase lags of either sign.
One obvious asymmetry in the leech nerve cord and lamprey spinal cord is the direction of axonal projections. Eight inhibitory leech oscillator INs in each segment project rostrally, while five INs (one excitatory, four inhibitory) send axons in the caudal direction (Fig. 11). In the lamprey, EINs which project 4-6 segments caudally, but only 2-3 segments rostrally, provide one source of asymmetry (Dale, 1986). Asymmetry is even greater in CCINs, which project up to 20 segments caudally and have, at most, short ascending axons (Buchanan, 1982). One can reasonably ask, “Do these asymmetries produce differing effective coupling strengths?”
To examine this issue in the leech, a “Z-cut” preparation was constructed with the contralateral lateral connectives severed on either side (Fig. 12B). This manipulation eliminated coupling between ganglia on opposing sides of the lesions without altering coordination at other locations. At the same time, the Z-cut ganglion continued to receive half-strength coordinating inputs from both sides (Friesen and Hocker, 2001). Spectral analysis of the motor bursts in the Z-cut ganglion during fictive swimming demonstrated that ascending and descending coupling strengths are nearly equal. Thus, in leeches, asymmetries in coupling interactions, not asymmetries in coupling strength, appear to underlie intersegmental phase lags. In lampreys, however, there is evidence for differences in ascending and descending coupling strengths. Application of the glycine antagonist, strychnine, to the middle third of the larval lamprey spinal cord caused synchronous bilateral ventral root bursting in this spinal cord section, as well as the caudal ventral roots, where the strychnine was not applied (Hagevik and McClellan, 1994). However, rostral ventral roots, also in normal Ringers, maintained approximately normal anti-phasic bursts, providing evidence that the descending coupling strength is stronger than the ascending. Changing local cycle periods through sensory entrainment supports this view. In such experiments, cycle period changes were generated through lateral movement of one end of the spinal cord during fictive swimming (Grillner et al., 1981; see Section 8.3). Entrainment by the caudal end of the spinal cord induced larger changes in the phase lags at various cycle periods than entrainment through movement of the rostral end, indicative of stronger descending coupling. While some modeling studies have stressed the importance of these asymmetric coupling interactions in establishing phase lags (Cohen et al., 1992), it remains unclear whether they are essential for generating the observed intersegmental phase lags in the lamprey system.
In summary, during swimming in intact animals, approximately one full body wave is maintained by period-independent intersegmental phase lags. Specific neuronal interactions that establish these segmental phase delays in lampreys are not well understood, but of great importance. In the leech, increasingly more sophisticated modeling studies have established that the identified intersegmental synaptic interactions between oscillator interneurons (Fig. 11) provide an adequate explanation for the origins of the phase lags (Friesen and Stent, 1977; Pearce and Friesen, 1988; Friesen and Pearce, 1993; Friesen and Cang, 2001; Zheng et al., 2007). Currently available data suggest that short-ranged interactions, particularly in the descending direction, are especially important in setting appropriate phase lags in the lamprey, but not in the leech. In intact leech nerve cords, manipulations of local cycle periods alter the magnitude, but not the sign of phase lags; phase reversals are only seen when intersegmental coupling strength is reduced. Manipulation of cycle periods in the intact lamprey spinal cord, however, can change both the magnitude and the sign of phase lags. In the lamprey especially, it would be interesting to learn about interaction asymmetries that contribute to intersegmental phase lags in forward and backward swimming.
8. Sensory feedback
It is well-established that isolated leech nerve cords and lamprey spinal cords approximate the neuronal activity that underlies swimming in intact animals. However, intact swimming animals receive many environmental inputs that affect their behavior (Friesen, 2009). Numerous studies of animal locomotion have demonstrated that such sensory inputs strongly influence several aspects of swimming, including cycle period as well as inter-limb and intersegmental coordination.
8.1. Effects of sensory feedback on intersegmental phase lag
Comparisons of intact and fictive swimming show that sensory feedback can strongly influence intersegmental phase lags. In the leech, sensory feedback increases phase delays. Phase lags of 3.3 %/segment were recorded from DP nerves in otherwise intact leeches (phase lags are longer when measured from cinematographic records of swimming animals) whereas 0.5 – 2.8 %/segment phase lags occur in isolated nerve cords (Pearce and Friesen, 1984). Otherwise intact leeches with nerve cords transected between M10 and M11 can still generate coordinated swimming activity, however, en passant DP nerve recordings reveal a post-transection increase in phase lag across this area from 3.8 %/segment to 5.6 %/segment (Yu et al., 1999).
Data concerning the influence of sensory feedback on setting lamprey intersegmental phase lags are mixed. It was originally reported that phase lags computed from EMG recordings in intact animals and ventral root extracellular recordings in isolated spinal cords were not significantly different (Wallén and Williams, 1984). Other researchers reported that phase lags obtained from EMG records from intact larval animals were greater than those computed from ventral root recordings during fictive swimming in in vitro brain/spinal cord preparations (Boyd and McClellan, 2002). However, Guan and coworkers (2001) provided evidence contradicting these studies through an examination of phase lags in the isolated spinal cord and a semi-intact “muscle” condition. All preparations were decapitated. In the muscle condition, all skin along with some muscle was removed, and EMGs recorded from remaining muscle. In all cases the phase lag was greater in the isolated condition (~1.5 %/segment) than in the muscle condition (~0.6 %/segment).
There are a variety of explanations for these mixed results. Wallén and Williams (1984) recorded from an intact animal, whereas Guan and coworkers (2001) removed the skin and some muscle. The differing results obtained by Guan and coworkers and Boyd and McClellan (2002) could be due to Boyd and McClellan's use of D-tubocurarine in the in vitro condition, as Ach blockers have been shown to increase phase lags in lampreys (Quinlan et al., 2004). Alternatively, some remaining muscle fibers in the Guan and coworkers study could have affected phase lags. Also the age (larval vs. adult), species, type of in vitro preparation (brain vs. no brain), and method of swim initiation differed in these studies.
In both leeches and lampreys, the presence of body wall and muscle modulates the magnitude of intersegmental phase lags. Sensory inputs increase the intersegmental phase lags in the leech, however it is not clear if phase lags are increased or decreased in lampreys. As phase lags are strongly influenced by intrinsic cycle periods, the sometimes opposing effects of sensory input on the magnitude of phase lags may be partially attributed to opposing effects of cycle period gradients in the two species. We can conclude that the isolated nervous system in both species can approximate the phase lags expressed during intact swimming, but sensory inputs help to coordinate the body for highly efficient swimming.
8.2. Coordination in preparations with transected nerve cords or spinal cords
When expressing fictive swimming, intersegmental interactions within lamprey spinal cords and leech nerve cords are sufficient to coordinate rhythmic activity along the neuroaxis. During swimming in intact animals, another coordinating mechanism is present: sensory feedback to the CPG circuits induced by body wall bending. In fact, parallel experiments in both animals revealed that sensory feedback is sufficient to ensure coordinated swimming undulations. In an otherwise intact leech, the ventral nerve cord was transected at midbody, between segmental ganglia M10 and M11. In these animals, in which all direct neuronal intersegmental interactions were eliminated between the two ends, swimming behavior often appeared normal and was well-coordinated (Yu et al., 1999). One deficiency that was observed concerned the body waveform, which included somewhat more than one body cycle. There were also some instances of failure of coordination between the two halves of the body, particularly immediately following swim initiation. Comparison of control and post-transection cycle periods in these leeches produced mixed results. Two preparations had similar periods prior to and after nerve cord transection, while in one the period increased, and in two others the period decreased. Also, swim velocity was lower in operated animals. Despite these minor alterations in behavior, the experiment demonstrated that coordination of undulatory movements in the rostral and caudal halves in the leech can occur without intersegmental neuronal communication.
Similarly surprising results were obtained from lampreys. Otherwise intact larval lampreys with midbody spinal cord transections (as well as rostral transections to eliminate voluntary movement) were able to swim following intraperitoneal injections of NMDA (McClellan, 1990). Rhythmic activity in EMG recordings obtained on either side of the lesion was occasionally uncoordinated, but usually 1:1 coupling was observed. Cycle periods were longer in the post-transection animals and phase lags were more variable as compared to controls. Although the control animals did not receive NMDA injections, it is unlikely that the increase in cycle periods seen in the transected animals was due to the presence of NMDA. While EAA injections into intact animals may affect the cycle period, it might be expected that they would shorten rather than lengthen the period. These studies show that sensory feedback aids in intersegmental coordination during rhythmic locomotion.
In the lamprey, sensory feedback appears to modify swimming activity generated by CPG circuits, but to a lesser degree than in the leech. Leeches were able to initiate, maintain and coordinate swimming with their nerve cords cut in half. Lampreys were only able to initiate swimming after this same procedure with the injection of EAAs. Once swimming had begun, undulations were near normal in both animals. The expression of coordinated swimming movements between two halves of an animal in the absence of continuous neuronal interactions is remarkable. The two ends act as semi-autonomous phase-locked oscillators linked, not directly by interactions within the CNS, but by the mechanical wave transduced by segmental sensory feedback circuits.
8.3. Mechanoreceptors and entrainment
In addition to responding to stimuli from the external environment, swimming leeches and lampreys use a proprioceptive sense to monitor body contours, and thereby to alter their undulations as appropriate. Receptors which respond either to local body length or to muscle tension are found in both animals (Grillner et al., 1984; Blackshaw and Thomas, 1988). These receptors differ between the two animals in their locations and their sensory modalities; nevertheless, their effects on the CPGs are similar in the leech and lamprey.
Proprioceptive sensory input in leeches arises from mechanoreceptors located in the body wall. The most fully characterized of these is the paired ventral stretch receptor (VSR) associated with ventral longitudinal muscle in many, and perhaps all, segments of the body wall. The VSR comprises a peripheral 10 μm soma, with processes that interact with longitudinal muscle fibers, and a giant axon (about 25 μm diameter, 3-5 mm long), which terminates within the ipsilateral neuropile of the segmental ganglion (Fig. 14A; Blackshaw and Thompson, 1988; Fan and Friesen, 2006). Originally designated simply as “stretch receptors,” it was later shown that VSRs respond specifically to increases in body wall tension (rather than to length) by hyperpolarization. They convey this tension information (which causes tonic membrane polarization changes) via their non-spiking giant axons to the segmental ganglion and, through a strong electrical synapse, onto an oscillator IN, cell 33 (Fig. 15A; Cang et al., 2001). VSRs likely exert their influence on swimming exclusively through interactions with the local CPG; searches for direct interactions between the VSR and MNs have yielded only negative results (Cang et al., 2001). In body wall-CNS preparations, VSR oscillations are phase-locked with DP swim-bursts; strong evidence that the VSR conveys information from the body wall is seen in the decreased amplitude of VSR oscillations in the isolated nervous system. The remaining low amplitude VSR oscillations in isolated preparations reflect the identified interactions with cell 33 and potentially other undiscovered connections with the CPG (Cang and Friesen, 2000; Yu and Friesen, 2004). Square-pulse current injection into the VSR shifts the phase of ongoing swimming activity and, more importantly, injection of continuous sinusoidal or triangle wave currents, within limits, entrained the swim rhythm to the frequency of the of the injected current (Yu and Friesen, 2004). Finally, appropriately timed rhythmic pulses injected into the VSR can alter intersegmental phase lags in the leech nerve cord in a phase-dependent manner (Cang and Friesen, 2000). Although not fully described, another proprioceptor, the dorsal stretch receptor, is associated with dorsal longitudinal muscle (Fan and Friesen, 2006).
Figure 14.
Form and function of stretch receptors. A Leech. The terminals of stretch receptors in the leech terminate broadly within segmental ganglia. Recordings are from the giant axons near the ganglion edge (VSR electrode). The records in A arose from an experiment in which an excitatory MN (upper trace, VE-4) was excited via intracellular current injection to induce increased tension in a fixed-length flap piece of body wall (bottom trace). The increased isometric tension induced a hyperpolarization in the giant axon of the ventral stretch receptor (VSR, middle trace). B Lamprey. The edge cell (EC) has processes that terminate near the lateral edge of the spinal cord and an ipsilaterally projecting axon. Stretching the margin of the spinal cord depolarizes the edge cell and gives rise to sustained impulse activity. The small upward and downward deflections in the lower trace indicated step increases (stretch) and decreases (release) in length, respectively. A is constructed from Fig. 2 of Cang et al. (2001). B is constructed from Fig. 3 in Grillner et al. (1984).
Figure 15.
Interactions of stretch receptors with the CPG. A Leech circuit. Muscle tension is detected by the dorsal (DSR) and ventral (VSR) stretch receptors. The VSR neuron has strong non-rectifying electrical interactions with IN 33 and hence is directly interconnected the CPG. Interactions between the DSR and the segmental neurons are unknown. B Lamprey circuit. There are two classes of stretch receptors (a.k.a., edge cells). One (SR-E) excites most neurons in the ipsilateral CPG. The second (SR-I) makes inhibitory contacts with contralateral CCINs and LINs and inhibits the contralateral SR-I as well. Both types of stretch receptors have processes near the lateral margin of the spinal cord and detect changes in spinal cord length caused either by imposed bending or by contraction of segmental muscles. A is redrawn from Fig. 5, Friesen and Kristan, 2007; B is redrawn from Viana Di Prisco et al. 1990. Note that only a subset of CPG interactions are shown. Symbols are as in Fig. 10. The resistor symbol denotes a nonrectifying electrical connection.
In the lamprey, segmentally located “edge cells” mediate proprioceptive information (Rovainen, 1967b; Grillner et al., 1984). Edge cells are located in the lateral edge of the white matter in the lamprey spinal cord and depolarize in response to stretch along the longitudinal margin. These cells have a unique morphology, with fine nest-like processes that branch off of otherwise blunt dendritic processes (Fig. 14B). One class of edge cells, the SR-Es, have excitatory, apparently direct connections to ipsilateral MNs, CCINs, LINs and perhaps EINs (Viana Di Prisco et al., 1990). The inhibitory edge cells, SR-Is, directly inhibit their contralateral homologs as well as contralateral CCINs and LINs (Fig 15B). Unlike leech mechanoreceptors, edge cells use spike-mediated transmission. Lamprey and leech proprioceptive sensors therefore differ in several ways, the location (spinal cord or body wall), their sensory modality (length or tension changes), their direct outputs (to CPG neurons and motor neurons or just CPG neurons) and their mode of transmission (spike-mediated or non-spike mediated).
Despite major differences in their morphology and physiology, the VSR and edge sensory neurons are functionally very similar. For example, McClellan and Sigvardt (1988) found rhythmic bending of the isolated spinal cord entrained the CPG oscillations in lampreys. As in the leech, the amplitude and frequency of the entraining movement mattered; the minimum displacement to achieve entrainment was around 2 mm. Larger movements to the caudal end entrained the CPG to 40 % above and 10 % below its “natural” frequency. Similar to the effects of step current pulses injected into the VSR in the leech, “step” bends (non-oscillatory) of the spinal cord reset the swim rhythm. Moreover, bends timed to occur during ipsilateral bursting phase-advanced the next burst while those timed to occur at the opposite phase caused phase delays. Lesions of the lateral or medial fascicles did not affect sensory entrainment. However, in split bath preparations, blocking fictive locomotor activity near the site of the bend with a low-Ca2+ solution abolished entrainment (McClellan and Sigvardt, 1988). These data suggest that, like the leech, lamprey mechanoreceptors entrain local CPG networks, which then interact with other CPGs at a distance.
Although isolated spinal cord and nerve cord preparations are capable of producing fictive swimming, sensory feedback plays a major role in molding the CPG for effective swimming by the intact animals. Leeches and lampreys can sense their own body movements and adjust the CPG-initiated cycle periods and intersegmental phase lags. One striking example of the importance of sensory feedback is that for both animals, the removal of their fluid environment changes their undulations profoundly. Lampreys “swimming” on a wet bench and leeches “swimming” in air generate standing, rather than traveling waves (Bowtell and Williams, 1991; Friesen et al., 2007). Thus, fluid resistance forces provided by water, whose effects are sensed by proprioceptors, are clearly essential to establish appropriate intersegmental phase lags and thereby establish the traveling waves required for locomotion.
9. Segmental unit oscillators
Most segments in the leech and lamprey contain at least one swim oscillator. In the lamprey, reciprocal inhibition between neurons in bilaterally symmetric hemisegments might be essential for generating CPG oscillations; if not, each segment might comprise two CPGs. The leech the same issue arises, does each ganglion contain one or two oscillators?
Experiments performed on lamprey fictive swimming decades ago (Cohen and Harris-Warrick, 1984), demonstrated that the reciprocally inhibitory interactions between left and right segmental homologs are necessary for generating left-right anti-phasic bursting. They found that the application of the glycine antagonist, strychnine, to an isolated spinal cord preparation led to inappropriate synchronous bursting in the ventral roots of a given segment. In addition to the switch from anti-phasic to synchronous bursting, the cycle frequency increased from 1.0 to 3.8 Hz. This study suggested that the reciprocal inhibition across the midline was not essential for rhythm generation and suggested that a hemicord might be sufficient to generate oscillations. Further experiments examining whether connections between hemisegments are essential led to the opposite conclusion. These experiments were carried out by Buchanan (1999a), who created hemisegments by making longitudinal cuts along approximately 40 % of an isolated spinal cord and then induced swimming by the application of NMDA. In thirteen of nineteen experiments no rhythmicity occurred in hemisegments five or more segments distant from the intact portion of the cord. The remaining six preparations exhibited bursting, but with a cycle frequency nearly triple that of controls. The quality of the rhythm in intact segments adjacent to those hemisected was very low, but increased with distance from the transection. Intersegmental axonal projections might account for the bursting seen in hemisegments near the intact region, for it is known that contralateral inhibition of the ventral roots spans 5-9 segments along the spinal cord (Fagerstedt et al., 2000).
More recently, Jackson et al. (2005) created midline lesions in the spinal cord of otherwise intact animals, as well as in isolated brainstem-spinal cord preparations, from larval lampreys. The “whole” animals with short lesions were able to swim, albeit with small deficits. In the in vitro preparations, swimming was induced via EAA injection into the brainstem. The caudal hemisections of in vitro preparations that were connected to intact rostral section of the spinal cord often exhibited rhythmic activity, however when the rostral activity was blocked or when the brain was detached, bursting activity was no longer seen in the caudal hemicord, suggesting that caudal hemisegments are unable to elicit swimming rhythms on their own. Similarly, rostral hemisections attached to an intact isolated brainstem-spinal cord nervous system produced only tonic activity while isolated rostral hemisections had some rhythmic activity with long cycle periods (> 4s) that was apparently unrelated to swimming. These experiments led to the conclusion that crossed inhibition is necessary for rhythm generation and that isolated hemicords are incapable of producing rhythmic behavior.
Experiments performed by Cangiano and Grillner (2003, 2005) led to a different conclusion. Small segments of the spinal cord were cut along the midline, and activity was evoked by bath application of D-glutamate or NMDA, or by electrical stimulation of the hemicords. Rhythmic activity with higher cycle frequencies (2 – 12 Hz) than controls (1 – 3 Hz), was induced by all three methods. NMDA application, however, produced this rhythm in less than a third of the preparations, similar to the results seen in Buchanan (1999a), whereas D-glutamate elicited fast bursting in a chain as short as 2.5 hemisegments. Electrical stimulation also produced “fast” bursting in a single hemisegment. This fast bursting appeared to be produced by the CPG that underlies swimming because spinal cords with partial hemisections that were gradually lengthened also gradually increased their cycle frequencies. Furthermore, increasing the concentration of D-glutamate in the bath increased the cycle frequency in the hemicord as it did in an intact spinal cord. The source of the increase in cycle frequency appeared to be the removal of the crossed inhibition, as the burst frequencies are similar to those seen with glycine transmission blocked (Cohen and Harris-Warwick, 1984). Further, blockage of glycine receptors in the hemisections had no effect on cycle frequency (Cangiano and Grillner, 2003). These results must be interpreted with caution because cellular and synaptic properties are altered by the lesion to the spinal cord. Such changes were seen in hemisected preparations 30-60 minutes after the lesion, including increases in the slow after-hyperpolarization potential in excitatory interneurons (EINs) and motor neurons (MNs), increased excitability of EINs and MNs and perhaps stronger connections between EINs (Hoffman and Parker, 2010). The authors suggested that these changes might account for the delay in bursting induced by NMDA bath application following spinal lesion. However, bursting occurs immediately when hemicords are stimulated electrically or with D-glutamate bath application. Whether these changes contribute to hemicord rhythmicity is an open question.
The experiments of Cangiano and Grillner strongly suggest that hemisections of lamprey spinal cord are in fact capable of producing swim-like bursting, and therefore contain a functional CPG. Thus, evidence suggests that full segments comprise two CPGs that are coupled by reciprocal glycinergic inhibitory connections; however, NMDA application does not reliably evoke swim-related bursting in split-cord preparations. In addition, the immature nervous system of larval lampreys may be unable to sustain rhythmic activity in hemisections without sensory feedback (Jackson et al., 2005). Alternatively, the method of swim initiation may be important for observing bursting in hemicords.
Because of the extensive intraganglionic connections in the neuropile, sagittal section of the leech nervous system produces major damage. Hence, to determine the number of CPGs per ganglion, the Z-cut preparation was used (Fig. 12B). In this preparation, one side of a segmental ganglion receives exclusively ascending inputs and the other side receives exclusively descending inputs (Friesen and Hocker, 2001). Because the rostral and caudal ends of this preparations generate swimming activity with differing cycle periods, the two sides of the Z-cut ganglion are driven at differing cycle frequencies. Spectral analyses of the impulse bursts revealed nearly equal power contributed by the two ends. Importantly, left and right activity recorded from bilateral DP nerves in the Z-cut ganglion was nearly identical, with synchronous bursting, despite the differing ascending and descending inputs. Thus, either there is only one oscillator unit per segment, or, if there are two, they are so tightly coupled that strong asynchronous drive cannot disassociate their activity. That is, each segment behaves as though it contains only one functional CPG. The possibility that there are two, though tightly coupled, CPGs per segment seems unlikely for several reasons. (1) Each segment includes an unpaired IN, cell 208 (Weeks, 1982) that interacts with both homologs of several other oscillator INs (Nusbaum et al., 1987). (2) Some of the oscillator INs make strong intraganglionic oscillator interactions across the midline (Friesen et al., 1978, 1989b). (3) No drug application or manipulation uncouples the bilateral DP bursting in a given segment. And, (4) leech locomotion is based on synchronous left-right motion; hence, there would appear to be strong selection pressures against independent bilateral segmental CPGs in the leech.
10. Comparisons between species and age groups
Most research on leech swimming behavior is performed on adults of one species, Hirudo verbana (unless development is specifically being examined). Lamprey studies, however, are performed on several species, and in both larvae and adults. There is little discussion in the literature regarding differences in experimental results arising from these species and age differences. However, there is reason to believe that these differences should not be dismissed, especially when contradictory results are obtained in different labs.
Lamprey studies are commonly performed on Petromyzon marinus, the sea lamprey, Ichthyomyzon unicuspis, the silver lamprey, and Lampetra fluviatilis, the river lamprey. There are no reported differences in the anatomy or activity of CCINs and MNs in the sea and silver lampreys, or between fin MN and myotomal MN interactions in the sea and river lampreys (Buchanan, 1982; Buchanan and Cohen, 1982; Mentel et al., 2006). In other measures, however, notable species differences were found. Application of NMDA or kainate to isolated spinal cord preparations caused irregular ventral root bursting in the river lamprey and normal ventral root bursting in the silver lamprey (Brodin et al., 1985). Bath application of D-glutamate also caused rhythmic bursting in the silver lamprey, whereas it only produced tonic ventral root activity in the river lamprey (Cohen and Wallén, 1980). One explanation for these differences is that the 5-HT nerve plexus is smaller in the river lamprey than in sea and silver lampreys (Zhang et al., 1996). Supporting evidence for this idea comes from the rhythmic ventral root bursting produced in the river lamprey preparations excited by concomitant NMDA and 5-HT application (Zhang and Grillner, 2000). Addition of a 5-HT antagonist converted bursting back to tonic spiking.
Many studies on lamprey reveal that there are several differences between the adult and the larval lamprey, or ammocoetes. For example, intact adult lampreys swim more efficiently than larvae (Cohen et al., 1990). Likewise, ventral root bursting in isolated adult spinal cords is more stable. Differences in the ammocoete and adult CPG could explain, for instance, why Jackson and coworkers (2005) were unable to induce bursting in larval lamprey hemisegments. Cohen and coworkers (1990) also found species and life-stage dependant differences in cycle frequency. Both sea and silver lamprey larvae exhibited slow bursting (~5 s periods) in response to D-glutamate application, but such output was more pronounced in silver lampreys. Adult sea lamprey rarely produced slow bursting, whereas it was often observed in the adult silver lamprey.
Although the span of research on leeches encompasses a wide range of sizes, from about 0.05 g to about 15 g (2.3 cm to 17 cm in length), these animals are at the same life stage. The leech develops inside its “egg,” and, once hatched, has the basic morphology and physiology of an adult (Weisblat, 1981). Some leech studies, performed on both Hirudo verbana and Macrobdella decora point to some modest differences between the species, although the general physiology of the two species is highly similar. For example, the synaptic strength of the inhibitory input from oscillator cell 28 to cell 208 was found to be stronger in the Macrobdella (Nusbaum et al., 1987). Another difference was found in the ability of some command cells to initiate swimming. In the Macrobdella, current injection into cells 21 and 61 often induced fictive swims (Nusbaum, 1986), whereas in the Hirudo, attempts to initiate swimming by these means was largely ineffective (W. O. Friesen, unpublished data). Most other experimental results were indistinguishable in the two species, including the effectiveness of cell 204 depolarization in driving swimming (Weeks and Kristan, 1978).
General conclusions for some aspects of lamprey neurobiology remain elusive because conflicting results are generated by experiments conducted on several species and at differing life stages. Consistency in the preparations employed might reduce this difficulty. However, focusing on a single, adult model animal would reduce the potential for discovering differences in CPG mechanisms over development and also would prevent evolutionary insights gained from comparisons between species.
11. Overview and Conclusions
11.1 The historical context
Research on the control of rhythmic movements was shaped by the clash of two opposing theories. Promulgated by Charles Scott Sherrington (1910) at the beginning of the 20th century, one theory held that coordinated chains of reflexes generate the underlying rhythms of neuronal activity patterns. Concurrently, Graham Brown (1911) proposed that innate neuronal circuits within the cat spinal cord generate rhythmic movement. The chain-of-reflexes view prevailed for many decades. However, studies on the crayfish swimmeret system (Hughes and Wiersma, 1960) and on flight in deafferented locusts (Wilson, 1961) as well as other studies on a wide variety of rhythmic preparations (Delcomyn, 1980; Selverston and Moulins, 1985; Marder and Calabrese, 1996; Orlovsky et al., 1999) showed convincingly that neuronal circuits in the central nervous system underlie rhythmic movement. It is now widely understood that complex movements require both central oscillators and peripheral feedback (Pearson, 2000). In the medicinal leech, physiological properties of swimming were studied by Gray and coworkers (1938) during the 1930s, but conclusive evidence for a central oscillator in this system had to await studies by Kristan and Calabrese (1976). Fictive swimming was first described in lamprey several years later (Cohen and Wallén, 1980; Poon, 1980). Since those seminal studies of fictive locomotion in leech and lamprey, researchers have focused on 1) identification of the neurons that contribute to swim initiation, modulation and rhythm generation, 2) the mechanisms underlying rhythmicity and intersegmental phase relationships and 3) contributions of sensory feedback.
11.2 Why study swimming in leeches and lampreys?
The numerous features of leech and lamprey behavior and anatomy that make these animals favorable for neuroethological research have been described in some detail (Grillner et al., 1991; Kristan et al. 2005). Briefly, in both species, 1) swim undulations involve the whole body, 2) the nervous system is relatively simple and robust, 3) neurons are relatively large and readily identifiable (as individual cells or as cell classes), 4) fictive behavior is easily detected and 5) the non-cephalic nervous system is formed of nearly identical segments that have homologous neurons. These common felicitous features have lead to highly successful investigations into the origins of swimming locomotion in both animals. Although the lamprey nervous system is more complex than that of the leech, it is considerably simpler than that of other vertebrates. At the same time, the lamprey CNS shares the vertebrate homologies. For instance, the MLR and RS neurons are both present in mammals (Garcia-Rill and Skinner, 1987a,b; Jordan et al., 2008). Due to the accessible and robust nervous systems of the leech and lamprey, our understanding of these systems is particularly advanced and has lead to great gains in understanding rhythmic behaviors, generally.
11.3 Convergent evolution
In two species as evolutionarily distant as the leech and lamprey, major differences in the expression of behavior are to be expected. The similarities in leech and lamprey behaviors and their neural control are therefore remarkable. Why do these similarities arise? Although one can readily comprehend that convergent evolution would dictate that elongated aquatic creatures sport flattened, streamlined body shapes that minimize drag and maximize speed, it is not intuitively obvious that the controlling neuronal circuits would likewise be so constrained. It seems likely that the requirement for efficient, yet flexible undulations led to some mechanisms evolving more than once (Moroz, 2009). What neuronal features give rise to fast and malleable locomotor systems that are efficient for chasing prey and escaping predators? Our comparison of swim circuits in leech and lamprey suggests such emerging principles (Fig. 16).
Figure 16.
Simplified systems overview. A A cell-to-cell pathway has been identified in leeches from sensory inputs to motor output. B The reticulospinal (RS) spinal system that drives swimming and the oscillator interneurons in the lamprey are relatively well characterized, but other aspects are less well understood. It is not clear what neurons serve trigger functions. Many of the synaptic interactions in the excitatory cascades that drive swimming in leeches (A) and lampreys (B) are mediated by glutamatergic receptors. Neuromodulators and sensory feedback (not shown) are also important to the swim systems. P, pressure cell; Tr1, trigger neuron 1; SE1, swim excitor neuron 1; RZ - Retzius cell ; DE, dorsal excitor ; MLR, mesencephalic locomotor region; RS, reticulospinal cell; CCIN, caudal and contralaterally projecting interneuron; LIN, lateral interneuron; EIN, excitatory interneuron; MN, motor neuron. Symbols as in Fig. 7 and Fig. 10.
A major feature in both animals is the local-distributed nature of the nervous circuits. The oscillator kernel is found in individual segments, repeated 18-20 times in the leech and 100 times in the lamprey. These local units generate the rhythm and, through motor neuron activity, drive rhythmic contraction of segmental muscles. They also are the targets of sensory inputs from stretch receptors, which can alter segmental phase relationships and cycle period (Fig. 15). Extensive intersegmental interactions among these kernels, spanning roughly 20% of the neuroaxis, coordinate segmental output to generate phase-delayed activity that is independent of cycle period. The distributed system of oscillators can generate cord-wide output that approximates movements expressed in intact animals without input from the brain or sensory feedback. Such coordinated oscillations arising from local oscillators are also in control of swimmeret movements in crayfish (Murchison et al., 1993) and walking in stick insects (Büschges, 2005) and are thought to underlie rhythmic movement patterns in other vertebrates, as indicated in research on chicks, turtles and rodents (see Kiehn, 2006).
Other parallels abound in the motor control of these two species. Gating neurons in leeches (cells 204) and lampreys (the RS neurons) have nearly identical roles. These cells can initiate and maintain swimming, are excited by all inputs that initiate swimming, project to oscillator neurons, and receive feedback from the oscillator system (Weeks and Kristan, 1978; Weeks, 1982b; Dubuc et al., 2008). These similarities occur despite differences in the location and number of the cell somas; there are eight of the segmental cells 204 and thousands of the cephalic RS cells. Gating neurons that drive segmental oscillators are clearly important and, in higher vertebrates, that function also is served by reticulospinal neurons (Jordan et al., 2008). These parallels between the leech and lamprey systems that are often present in other species alert us to mechanisms fundamental for generation of rhythmic behavior.
11.4 Species differences
Differences between the leech and lamprey alert us to mechanisms that may be species specific, or that may be different as a species become more complex. General principles inform us that intersegmental coordination results from two processes: the specific intersegmental connections between segmental oscillator neurons and the intrinsic cycle periods of the component oscillators (Skinner and Mulloney, 1998). However, the contributions of these two elements differ between the leech and lamprey. In the leech intrinsic cycle periods vary non-monotonically, with the shortest cycle periods found in the middle segments (Pearce and Friesen, 1985a; Hocker et al., 2000) whereas in the lamprey there is a monotonic increase in cycle period along the rosto-caudal axis (Cohen, 1987; Hagevik and McClellan, 1999). In addition, intersegmental coupling appears weaker in lamprey because manipulations of their local cycle periods lead to large changes phase lags changes that included phase lag reversals (Matsushima and Grillner, 1992). In leeches, these reversals occurred only if intersegmental interactions were artificially reduced (Pearce and Friesen, 1985a). Although this difference may seem simply quantitative, it does help illuminate why lampreys, but not leeches, can swim backwards.
Mechanisms that generate the fundamental oscillations underlying swimming also differ in leech and lamprey. CPG units in both species were once thought to arise largely from inhibitory interactions among the component neurons. This is still the model for the source of oscillations in the leech. However, a more recent model in lamprey relies on excitatory circuit connections combined with intrinsic cell properties, without the need of any inhibitory synapses (Grillner et al., 2000). Such a model has been proposed for oscillations in other vertebrates, including mammals (Butt et al., 2002; Hägglund et al., 2010) and shares features of rhythm generation by individual neurons in invertebrates (Marder and Calabrese, 1996). Inhibitory synaptic interactions are, of course, critical in lamprey and leech - perhaps in all animals - for generating anti-phasic and multi-phasic output (Marder and Calabrese,1996; McCrea and Rybak, 2008; Kiehn et al., 2010).
11.5 Future directions
A fundamental assumption that guided the studies described in this review is that results obtained from experiments on isolated or semi-intact preparations are applicable to understanding the origins of movements in intact animals. Powerful arguments supporting this assumption are derived from electrophysiological experiments on a series of increasingly less dissected preparations that provide transition between the isolated segment and the intact animal. However, in many experiments, results have not been verified in semi-intact or nearly-intact preparations. Further, the brain is often detached in studies on both animals resulting, at least in the leech, in preparations with altered swim patterns (Brodfuehrer et al., 1986a; Puhl and Mesce, 2010; Mullins et al., 2010). More broadly, ethological field experiments are needed to establish the expression and roles of swimming under natural conditions. A further issue is the unavoidable fact that conclusions are based on identified neurons and neuronal classes. In both leeches and lampreys there are numerous neurons that remain unidentified and unstudied. Surely, incorporating finding on these as yet unidentified neurons into our circuits and models will alter our understanding of how these systems function.
The well-studied leech and lamprey swim systems provide excellent preparations for further research. One major problem is that the mechanisms by which higher-order swim-initiating neurons in the brain select and initiate specific swimming modes are largely unknown. In the lamprey, for example, it remains unclear whether areas such as the MLR and DLR contribute to swim-maintenance as well as swim-initiation. In the leech, there is a major gap in our understanding of how trigger neuron activation leads to the sustained excitation of swim-gating neurons. Even at the level of the segmental oscillators there are unidentified inputs to the oscillator neurons in the leech. In the lamprey, the mechanism leading to oscillations of CPG neurons, as explored in hemicords, remain under active investigation (Cangiano and Grillner, 2003; 2005; Jackson et al., 2007). Resolution of these issues is sure to lead to further insights into the neuronal control of animal behavior.
Acknowledgements
Funding was provided by a grant from the NSF (IOB-0615631 to WOF and JTH) and by grants from the NIH (NS40755 to JTB and NRSA GC11999 to OJM).
Abbreviation List
- BPE
(bursts per episode) – one measure of swim episode duration
- CPG
(central pattern generator)
- EAA
(excitatory amino acid)
- DLM
(dorsolateral mesencephalon)
- DLR
(diencephalic locomotor region)
- DP
(dorsal posterior nerve) – in the leech
- H, T
(head or tail) refers to the head (rostral) or tail (caudal) brain in the leech
- IN
(interneuron)
lamprey INs
- EIN
(excitatory interneuron)
- CCIN
(contralaterally and caudally projecting interneuron)
- LIN
(lateral interneuron)
- MN
(motor neuron)
leech MNs
- DE
(excitor of dorsal longitudinal muscle)
- DI
(inhibitor of dorsal longitudinal muscle)
- VE
(excitor of ventral longitudinal muscle)
- VI
(inhibitor of ventral longitudinal muscle)
- MX
(midbody ganglion X) – refers to a particular midbody ganglion, numbering starts at the anterior end.
- MLR
(mesencephalic locomotor region)
- MRRN
(middle rhombencephalon reticular nuclei)
- PRRN
(posterior rhombencephalon reticular nuclei)
- RCI
(recurrent cyclic inhibition)
- RLR
(rostrolateral rhombecephalon)
- RS
(reticulospinal)
- SR-E
(excitatory stretch receptor) – in the lamprey
- SR-I
(inhibitory stretch receptor) – in the lamprey
- T, P, and N cells
(touch, pressure and nociceptive cells) – sensory cells in the leech
- VMD
(ventromedial diencephalon)
- VR
(ventral root) – in the lamprey
- VSR
(ventral stretch receptor) – in the leech
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antri M, Fenelon K, Dubuc R. The contribution of synaptic inputs to sustained depolarizations in reticulospinal neurons. J. Neurosci. 2009;29:1140–1151. doi: 10.1523/JNEUROSCI.3073-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault PS, Deliagina TG, Orlovsky GN. Non-undulatory locomotion in the lamprey. Neuroreport. 2001;12:1803–1807. doi: 10.1097/00001756-200107030-00009. [DOI] [PubMed] [Google Scholar]
- Blackshaw SE, Thompson SW. Hyperpolarizing responses to stretch in sensory neurones innervating leech body wall muscle. J. Physiol. 1988;396:121–37. doi: 10.1113/jphysiol.1988.sp016954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell G, Williams TL. Anguilliform body dynamics: modelling the interaction between muscle activation and body curvature. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1991;334:385–390. doi: 10.1007/BF00163025. [DOI] [PubMed] [Google Scholar]
- Boyd MR, McClellan AD. Changes in locomotor activity parameters with variations in cycle time in larval lamprey. J. Exp. Biol. 2002;205:3707–3716. doi: 10.1242/jeb.205.23.3707. [DOI] [PubMed] [Google Scholar]
- Brocard F, Dubuc R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J. Neurophysiol. 2003;90:1714–1727. doi: 10.1152/jn.00202.2003. [DOI] [PubMed] [Google Scholar]
- Brocard F, Ryczko D, Fenelon K, Hatem R, Gonzales D, Auclair F, Dubuc R. The transformation of a unilateral locomotor command into a symmetrical bilateral activation in the brainstem. J. Neurosci. 2010;30:523–533. doi: 10.1523/JNEUROSCI.3433-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodfuehrer PD, Burns A. Neuronal factors influencing the decision to swim in the medicinal leech. Neurobiol. Learn. Mem. 1995;63:192–199. doi: 10.1006/nlme.1995.1020. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Cohen AH. Initiation of swimming activity in the medicinal leech by glutamate, quisqualate and kainate. J. Exp. Biol. 1990;154:567–572. doi: 10.1242/jeb.154.1.567. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Friesen WO. Control of leech swimming activity by the cephalic ganglia. J. Neurobio. 1986a;17:697–705. doi: 10.1002/neu.480170612. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Friesen WO. From Stimulation to Undulation: A Neuronal Pathway for the Control of Swimming in the Leech. Science. 1986b;234:1002–1004. doi: 10.1126/science.234.4779.1002. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Friesen WO. Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. I. Output connections of Tr1 and Tr2. J. Comp. Physiol. A. 1986c;159:489–502. doi: 10.1007/BF00604169. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Friesen WO. Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. II. Role of segmental swim-initiating interneurons. J. Comp. Physiol. A. 1986d;159:503–510. doi: 10.1007/BF00604170. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Friesen WO. Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. III. Sensory inputs to Tr1 and Tr2. J. Comp. Physiol. A. 1986e;159:511–519. doi: 10.1007/BF00604171. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Kogelnik AM, Friesen WO, Cohen AH. Effect of the tail ganglion on swimming activity in the leech. Behav. Neural Biol. 1993;59:162–166. doi: 10.1016/0163-1047(93)90912-2. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Parker HJ, Burns A, Berg M. Regulation of the segmental swim-generating system by a pair of identified interneurons in the leech head ganglion. J. Neurophysiol. 1995;73:983–992. doi: 10.1152/jn.1995.73.3.983. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Bryant A, Brady R. Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2008. Mechanisms underlying swim maintenance in the leech. Program No. 371.9. [Google Scholar]
- Brodfuehrer PD, Friesen WO. A sensory system initiating swimming activity in the medicinal leech. J. Exp. Biol. 1984;108:341–355. doi: 10.1242/jeb.108.1.341. 341. [DOI] [PubMed] [Google Scholar]
- Brodin L, Buchanan JT, Hokfelt T, Grillner S, Rehfeld JF, Frey P, Verhofstad AA, Dockray GJ, Walsh JH. Immunohistochemical studies of cholecystokininlike peptides and their relation to 5-HT, CGRP, and bombesin immunoreactivities in the brainstem and spinal cord of lampreys. J. Comp. Neurol. 1988a;271:1–18. doi: 10.1002/cne.902710103. [DOI] [PubMed] [Google Scholar]
- Brodin L, Dale N, Christenson J, Storm-Mathisen J, Hokfelt T, Grillner S. Three types of GABA-immunoreactive cells in the lamprey spinal cord. Brain Res. 1990;508:172–175. doi: 10.1016/0006-8993(90)91134-3. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S, Dubuc R, Ohta Y, Kasicki S, Hokfelt T. Reticulospinal neurons in lamprey: transmitters, synaptic interactions and their role during locomotion. Arch. Ital. Biol. 1988b;126:317–345. [PubMed] [Google Scholar]
- Brodin L, Grillner S, Rovainen CM. N-Methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Res. 1985;325:302–306. doi: 10.1016/0006-8993(85)90328-2. [DOI] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc. R. Soc. Lond. B. Biol. Sci. 1911;84:308–319. [Google Scholar]
- Buchanan JT. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J. Neurophysiol. 1982;47:961–975. doi: 10.1152/jn.1982.47.5.961. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Commissural interneurons in rhythm generation and intersegmental coupling in the lamprey spinal cord. J. Neurophysiol. 1999a;81:2037–2045. doi: 10.1152/jn.1999.81.5.2037. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. The roles of spinal interneurons and motoneurons in the lamprey locomotor network. Prog. Brain Res. 1999b;123:311–21. doi: 10.1016/s0079-6123(08)62866-6. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog. Neurobiol. 2001;63:441–466. doi: 10.1016/s0301-0082(00)00050-2. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Brodin L, Dale N, Grillner S. Reticulospinal neurones activate excitatory amino acid receptors. Brain Res. 1987;408:321–325. doi: 10.1016/0006-8993(87)90397-0. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Cohen AH. Activities of identified interneurons, motoneurons, and muscle fibers during fictive swimming in the lamprey and effects of reticulospinal and dorsal cell stimulation. J. Neurophysiol. 1982;47:948–960. doi: 10.1152/jn.1982.47.5.948. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. Newly identified ‘glutamate interneurons’ and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. A new class of small inhibitory interneurones in the lamprey spinal cord. Brain Res. 1988;438:404–407. doi: 10.1016/0006-8993(88)91373-x. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S, Cullheim S, Risling M. Identification of excitatory interneurons contributing to generation of locomotion in lamprey: structure, pharmacology, and function. J. Neurophysiol. 1989;62:59–69. doi: 10.1152/jn.1989.62.1.59. [DOI] [PubMed] [Google Scholar]
- Büschges A. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J. Neurophysiol. 2005;93:1127–1135. doi: 10.1152/jn.00615.2004. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Lebret JM, Kiehn O. Organization of left-right coordination in the mammalian locomotor network. Brain Res. Rev. 2002;40:107–117. doi: 10.1016/s0165-0173(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Cang J, Friesen WO. Sensory modification of leech swimming: rhythmic activity of ventral stretch receptors can change intersegmental phase relationships. J. Neurosci. 2000;20:7822–7829. doi: 10.1523/JNEUROSCI.20-20-07822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Yu X, Friesen WO. Sensory modification of leech swimming: interactions between ventral stretch receptors and swim-related neurons. J. Comp. Physiol. A. 2001;187:569–579. doi: 10.1007/s003590100229. [DOI] [PubMed] [Google Scholar]
- Cangiano L, Grillner S. Fast and slow locomotor burst generation in the hemispinal cord of the lamprey. J. Neurophysiol. 2003;89:2931–2942. doi: 10.1152/jn.01100.2002. [DOI] [PubMed] [Google Scholar]
- Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord. J. Neurosci. 2005;25:923–935. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson J, Lagerback PA, Grillner S. The dorsal cell, one class of primary sensory neuron in the lamprey spinal cord. II. A light- and electron microscopical study. Brain Res. 1988;440:9–17. doi: 10.1016/0006-8993(88)91153-5. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Dobrov TA, Li G, Kiemel T, Baker MT. The development of the lamprey pattern generator for locomotion. J. Neurobiol. 1990;21:958–969. doi: 10.1002/neu.480210703. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Ermentrout GB, Kiemel T, Kopell N, Sigvardt KA, Williams TL. Modelling of intersegmental coordination in the lamprey central pattern generator for locomotion. Trends Neurosci. 1992;15:434–438. doi: 10.1016/0166-2236(92)90006-t. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Harris-Warrick RM. Strychnine eliminates alternating motor output during fictive locomotion in the lamprey. Brain Res. 1984;293:164–167. doi: 10.1016/0006-8993(84)91464-1. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Wallén P. The neuronal correlate of locomotion in fish. “Fictive swimming” induced in an in vitro preparation of the lamprey spinal cord. Brain Res. 1980;41:11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- Cohen AH. Intersegmental coordinating system of the lamprey central pattern generator for locomotion. J. Comp. Physiol. A. 1987;160:181–193. 181. [Google Scholar]
- Crisp KM, Mesce KA. To swim or not to swim: regional effects of serotonin, octopamine and amine mixtures in the medicinal leech. J. Comp. Physiol. A. Neuroethol Sens. Neural Behav. Physiol. 2003;189:461–470. doi: 10.1007/s00359-003-0424-0. [DOI] [PubMed] [Google Scholar]
- Currie SN. Vibration-evoked startle behavior in larval lampreys. Brain Behav. Evol. 1991;37:260–271. doi: 10.1159/000114364. [DOI] [PubMed] [Google Scholar]
- Dale N. Excitatory synaptic drive for swimming mediated by amino acid receptors in the lamprey. J. Neurosci. 1986;6:2662–2675. doi: 10.1523/JNEUROSCI.06-09-02662.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debski EA, Friesen WO. Habituation of swimming activity in the medicinal leech. J. Exp. Biol. 1985;116:169–188. doi: 10.1242/jeb.116.1.169. [DOI] [PubMed] [Google Scholar]
- Debski EA, Friesen WO. Role of central interneurons in habituation of swimming activity in the medicinal leech. J. Neurophysiol. 1986;55:977–994. doi: 10.1152/jn.1986.55.5.977. [DOI] [PubMed] [Google Scholar]
- Debski EA, Friesen WO. Intracellular stimulation of sensory cells elicits swimming activity in the medicinal leech. J. Comp. Physiol. A. 1987;160:447–457. doi: 10.1007/BF00615078. [DOI] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Fagerstedt P. Responses of reticulospinal neurons in intact lamprey to vestibular and visual inputs. J. Neurophysiol. 2000;83:864–878. doi: 10.1152/jn.2000.83.2.864. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Fagerstedt P, Grillner S, Orlovsky GN. Activity of reticulospinal neurons during locomotion in the freely behaving lamprey. J. Neurophysiol. 2000;83:853–63. doi: 10.1152/jn.2000.83.2.853. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Bongianni F, Ohta Y, Grillner S. Anatomical and physiological study of brainstem nuclei relaying dorsal column inputs in lampreys. J. Comp. Neurol. 1993a;327:260–270. doi: 10.1002/cne.903270208. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Bongianni F, Ohta Y, Grillner S. Dorsal root and dorsal column mediated synaptic inputs to reticulospinal neurons in lampreys: involvement of glutamatergic, glycinergic, and GABAergic transmission. J. Comp. Neurol. 1993b;327:251–259. doi: 10.1002/cne.903270207. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fenelon K, Gariepy JF, Smetana R, Ménard A, Le Ray D, Prisco VD, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res. Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Einum JF, Buchanan JT. Reticulospinal neurons receive direct spinobulbar inputs during locomotor activity in lamprey. J. Neurophysiol. 2004;92:1384–1390. doi: 10.1152/jn.00625.2003. [DOI] [PubMed] [Google Scholar]
- Einum JF, Buchanan JT. Membrane potential oscillations in reticulospinal and spinobulbar neurons during locomotor activity. J Neurophysiol. 2005;94:273–81. doi: 10.1152/jn.00695.2004. [DOI] [PubMed] [Google Scholar]
- Einum JF, Buchanan JT. Spinobulbar neurons in lamprey: cellular properties and synaptic interactions. J. Neurophysiol. 2006;96:2042–55. doi: 10.1152/jn.01331.2005. [DOI] [PubMed] [Google Scholar]
- El Manira A, Pombal MA, Grillner S. Diencephalic projection to reticulospinal neurons involved in the initiation of locomotion in adult lampreys Lampetra fluviatilis. J. Comp. Neurol. 1997;389:603–616. [PubMed] [Google Scholar]
- El Manira A, Tegner J, Grillner S. Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. J. Neurophysiol. 1994;72:1852–1861. doi: 10.1152/jn.1994.72.4.1852. [DOI] [PubMed] [Google Scholar]
- Esch T, Mesce KA, Kristan WB., Jr. Evidence for sequential decision making in the medicinal leech. J. Neurosci. 2002;22:11045–11054. doi: 10.1523/JNEUROSCI.22-24-11045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstedt P, Zelenin PV, Deliagina TG, Orlovsky GN, Grillner S. Crossed reciprocal inhibition evoked by electrical stimulation of the lamprey spinal cord. Exp. Brain Res. 2000;134:147–154. doi: 10.1007/s002210000455. [DOI] [PubMed] [Google Scholar]
- Fan RJ, Friesen WO. Characterization of central axon terminals of putative stretch receptors in leeches. J. Comp. Neurol. 2006;494:290–302. doi: 10.1002/cne.20818. [DOI] [PubMed] [Google Scholar]
- Fan RJ, Marin-Burgin A, French KA, Otto Friesen W. A dye mixture (Neurobiotin and Alexa 488) reveals extensive dye-coupling among neurons in leeches; physiology confirms the connections. J. Comp. Physiol. A. Neuroethol. Sens. Neural Behav. Physiol. 2005;191:1157–1171. doi: 10.1007/s00359-005-0047-8. [DOI] [PubMed] [Google Scholar]
- French KA, Chang J, Reynolds S, Gonzalez R, Kristan WB, 3rd, Kristan WB., Jr. Development of swimming in the medicinal leech, the gradual acquisition of a behavior. J. Comp. Physiol. A. Neuroethol. Sens. Neural Behav. Physiol. 2005;191:813–821. doi: 10.1007/s00359-005-0003-7. [DOI] [PubMed] [Google Scholar]
- Friesen WO, Brodfuehrer PD. Identification of neurones in the leech through local ionic manipulations. J. Exp. Biol. 1984;113:455–460. [Google Scholar]
- Friesen WO. Neuronal control of leech swimming movements. I. Inhibitory interactions between motor neurons. J. Comp. Physiol. A. 1989a;166:195–203. 195. [Google Scholar]
- Friesen WO. Neuronal control of leech swimming movements. II. Motor neuron feedback to oscillator cells 115 and 28. J. Comp. Physiol. A. 1989b;166:205–215. 205. [Google Scholar]
- Friesen WO. Central pattern generators: Sensory feedback. In: Squire LR, editor. Encyclopedia of Neuroscience. Academic Press; Oxford: 2009. pp. 701–799. [Google Scholar]
- Friesen WO, Chen J, Iwasaki T. Intl. Soc. for Neuroethol. Congress. Vancouver; Canada: 2007. Environmental factors affect locomotion: swimming in air. Abstract, P0226. [Google Scholar]
- Friesen WO, Pearce RA. Mechanisms of intersegmental coordination in leech locomotion. Sem. Neurosci. 1993;5:41–47. 41. [Google Scholar]
- Friesen WO. Neuronal control of leech swimming movements: interactions between cell 60 and previously described oscillator neurons. J. Comp. Physiol. A. 1985;156:1432–1351. [Google Scholar]
- Friesen WO, Cang J. Sensory and central mechanisms control intersegmental coordination. Curr. Opin. Neurobiol. 2001;11:678–683. doi: 10.1016/s0959-4388(01)00268-9. [DOI] [PubMed] [Google Scholar]
- Friesen WO, Hocker CG. Functional analyses of the leech swim oscillator. J. Neurophysiol. 2001;86:824–835. doi: 10.1152/jn.2001.86.2.824. [DOI] [PubMed] [Google Scholar]
- Friesen WO, Poon M, Stent GS. An oscillatory neuronal circuit generating a locomotory rhythm. Proc. Natl. Acad. Sci. U. S. A. 1976;73:3734–3738. doi: 10.1073/pnas.73.10.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WO, Poon M, Stent GS. Neuronal control of swimming in the medicinal leech. IV. Identification of a network of oscillatory interneurones. J. Exp. Biol. 1978;75:25–43. doi: 10.1242/jeb.75.1.25. [DOI] [PubMed] [Google Scholar]
- Friesen WO, Stent GS. Generation of a locomotory rhythm by a neural network with recurrent cyclic inhibition. Biol. Cybern. 1977;28:27–40. doi: 10.1007/BF00360911. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987a;411:1–12. doi: 10.1016/0006-8993(87)90675-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 1987b;411:13–20. doi: 10.1016/0006-8993(87)90676-7. [DOI] [PubMed] [Google Scholar]
- Gray J, Lissmann HW, Pumphrey RJ. The mechanism of locomotion in the leech (Hirudo Medicinalis Ray) J. Exp. Biol. 1938;15:408–430. 408. [Google Scholar]
- Grillner S. On the generation of locomotion in the spinal dogfish. Exp. Brain Res. 1974;20:459–470. doi: 10.1007/BF00238013. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Grillner S. Neuronal networks in motion from ion channels to behaviour. An. R. Acad. Nac. Med. (Madr) 2006;123:297–298. [PubMed] [Google Scholar]
- Grillner S, Cangiano L, Hu G, Thompson R, Hill R, Wallén P. The intrinsic function of a motor system - from ion channels to networks and behavior. Brain Res. 2000;886:224–236. doi: 10.1016/s0006-8993(00)03088-2. [DOI] [PubMed] [Google Scholar]
- Grillner S, Kozlov A, Dario P, Stefanini C, Menciassi A, Lansner A, Hellgren Kotaleski J. Modeling a vertebrate motor system: pattern generation, steering and control of body orientation. Prog. Brain Res. 2007;165:221–234. doi: 10.1016/S0079-6123(06)65014-0. [DOI] [PubMed] [Google Scholar]
- Grillner S, Matsushima T. The neural network underlying locomotion in lamprey - synaptic and cellular mechanisms. Neuron. 1991;7:1–15. doi: 10.1016/0896-6273(91)90069-c. [DOI] [PubMed] [Google Scholar]
- Grillner S, McClellan A, Perret C. Entrainment of the spinal pattern generators for swimming by mechano-sensitive elements in the lamprey spinal cord in vitro. Brain Res. 1981;217:380–386. doi: 10.1016/0006-8993(81)90015-9. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P. The ionic mechanisms underlying N-methyl-D-aspartate receptor-induced, tetrodotoxin-resistant membrane potential oscillations in lamprey neurons active during locomotion. Neurosci. Lett. 1985;60:289–294. doi: 10.1016/0304-3940(85)90592-0. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P, Brodin L, Lansner A. Neuronal network generating locomotor behavior in lamprey: circuitry, transmitters, membrane properties, and simulation. Annu. Rev. Neurosci. 1991;14:169–199. doi: 10.1146/annurev.ne.14.030191.001125. [DOI] [PubMed] [Google Scholar]
- Grillner S, Williams T, Lagerback PA. The edge cell, a possible intraspinal mechanoreceptor. Science. 1984;223:500–503. doi: 10.1126/science.6691161. [DOI] [PubMed] [Google Scholar]
- Guan L, Kiemel T, Cohen AH. Impact of movement and movement-related feedback on the lamprey central pattern generator for locomotion. J. Exp. Biol. 2001;204:2361–2370. doi: 10.1242/jeb.204.13.2361. [DOI] [PubMed] [Google Scholar]
- Hagevik A, McClellan AD. Coupling of spinal locomotor networks in larval lamprey revealed by receptor blockers for inhibitory amino acids: neurophysiology and computer modeling. J. Neurophysiol. 1994;72:1810–1829. doi: 10.1152/jn.1994.72.4.1810. [DOI] [PubMed] [Google Scholar]
- Hagevik A, McClellan AD. Coordination of locomotor activity in the lamprey: role of descending drive to oscillators along the spinal cord. Exp. Brain Res. 1999;128:481–490. doi: 10.1007/s002210050871. [DOI] [PubMed] [Google Scholar]
- Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat. Neurosci. 2010;13:246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat. Neurosci. 2010;13:246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- Hardisty MW, Potter IC. Hardisty MW, Potter IC, editors. The behavior, ecology and growth of larval lampreys. The Biology of Lampreys. 1971a:85–126. [Google Scholar]
- Hardisty MW, Potter IC. Hardisty MW, Potter IC, editors. The general biology of adult lampreys. The Biology of Lampreys. 1971b:127–206. [Google Scholar]
- Harris-Warrick RM, Cohen AH. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J. Exp. Biol. 1985;116:27–46. doi: 10.1242/jeb.116.1.27. [DOI] [PubMed] [Google Scholar]
- Hashemzadeh-Gargari H, Friesen WO. Modulation of swimming activity in the medicinal leech by serotonin and octopamine. Comp. Biochem. Physiol. 1989;94:295–302. doi: 10.1016/0742-8413(89)90182-5. [DOI] [PubMed] [Google Scholar]
- Hill AA, Masino MA, Calabrese RL. Intersegmental coordination of rhythmic motor patterns. J. Neurophysiol. 2003;90:531–538. doi: 10.1152/jn.00338.2003. [DOI] [PubMed] [Google Scholar]
- Hocker CG, Yu X, Friesen WO. Functionally heterogeneous segmental oscillators generate swimming in the medical leech. J. Comp. Physiol. A. 2000;186:871–883. doi: 10.1007/s003590000140. [DOI] [PubMed] [Google Scholar]
- Hoffman N, Parker D. Lesioning alters functional properties in isolated spinal cord hemisegmental networks. Neurosci. 168:732–743. doi: 10.1016/j.neuroscience.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Hughes GM, Wiersma CAG. The co-ordination of swimmeret movements in the crayfish, Procambarus clarkii (Giard) J. Exp. Biol. 1960:657–670. [Google Scholar]
- Islam SS, Zelenin PV, Orlovsky GN, Grillner S, Deliagina TG. Pattern of motor coordination underlying backward swimming in the lamprey. J. Neurophysiol. 2006;96:451–60. doi: 10.1152/jn.01277.2005. [DOI] [PubMed] [Google Scholar]
- Jackson AW, Horinek DF, Boyd MR, McClellan AD. Disruption of left-right reciprocal coupling in the spinal cord of larval lamprey abolishes brain-initiated locomotor activity. J. Neurophysiol. 2005;94:2031–2044. doi: 10.1152/jn.00039.2005. [DOI] [PubMed] [Google Scholar]
- Jackson AW, Pino FA, Wiebe ED, McClellan AD. Movements and muscle activity initiated by brain locomotor areas in semi-intact preparations from larval lamprey. J. Neurophysiol. 2007;97:3229–3241. doi: 10.1152/jn.00967.2006. [DOI] [PubMed] [Google Scholar]
- Jordan LM. Initiation of locomotion in mammals. Ann. N. Y. Acad. Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Juvin L, Dubuc R. Neuroscience Meeting Planner. Society for Neuroscience; Chicago, IL: 2009. Patterns of activity of reticulospinal neurons during locomotion in lampreys. Program No. 565.10. [Google Scholar]
- Kasicki S, Grillner S, Ohta Y, Dubuc R, Brodin L. Phasic modulation of reticulospinal neurones during fictive locomotion and other types of spinal motor activity in lamprey. Brain Res. 1989;484:203–216. doi: 10.1016/0006-8993(89)90363-6. [DOI] [PubMed] [Google Scholar]
- Kemenes G. Behavioral choice: a novel role for presynaptic inhibition of sensory inputs. Curr. Biol. 2009;19:R1087–8. doi: 10.1016/j.cub.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Dougherty KJ, Hägglund M, Borgius L, Talpalar A, Restrepo CE. Probing spinal circuits controlling walking in mammals. Biochem. Biophys. Res. Commun. 2010;396:11–18. doi: 10.1016/j.bbrc.2010.02.107. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr., Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog. Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kristan WB., Jr. Sensory and motor neurones responsible for the local bending response in leeches. J. Exp. Biol. 1982;96:161–180. 161. [Google Scholar]
- Kristan WB, Jr., Calabrese RL. Rhythmic swimming activity in neurones of the isolated nerve cord of the leech. J. Exp. Biol. 1976;65:643–68. doi: 10.1242/jeb.65.3.643. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr., Stent GS, Ort CA. Neuronal control of swimming in the medicinal leech. I. Dynamics of the swimming rhythm. J. Comp. Physiol. A. 1974;94:97–119. 97. [Google Scholar]
- Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Le Ray D, Brocard F, Bourcier-Lucas C, Auclair F, Lafaille P, Dubuc R. Nicotinic activation of reticulospinal cells involved in the control of swimming in lampreys. Eur. J. Neurosci. 2003;17:137–148. doi: 10.1046/j.1460-9568.2003.02417.x. [DOI] [PubMed] [Google Scholar]
- Macagno ER. Number and distribution of neurons in leech segmental ganglia. J. Comp. Neurol. 1980;190:283–302. doi: 10.1002/cne.901900206. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Restrepo CE, El Manira A. Transmitter phenotypes of commissural interneurons in the lamprey spinal cord. Neurosci. 2009;164:1057–1067. doi: 10.1016/j.neuroscience.2009.08.069. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol. Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Martin AR, Wickelgren WO. Sensory cells in the spinal cord of the sea lamprey. J. Physiol. 1971;212:65–83. doi: 10.1113/jphysiol.1971.sp009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima T, Grillner S. Neural mechanisms of intersegmental coordination in lamprey: local excitability changes modify the phase coupling along the spinal cord. J. Neurophysiol. 1992;67:373–388. doi: 10.1152/jn.1992.67.2.373. [DOI] [PubMed] [Google Scholar]
- McClellan AD. In vitro CNS preparations: unique approaches to the study of command and pattern generation systems in motor control. J. Neurosci. Methods. 1987;21:251–264. doi: 10.1016/0165-0270(87)90120-8. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Locomotor recovery in spinal-transected lamprey: regenerated spinal coordinating neurons and mechanosensory inputs couple locomotor activity across a spinal lesion. Neurosci. 1990;35:675–685. doi: 10.1016/0306-4522(90)90338-5. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Time course of locomotor recovery and functional regeneration in spinal cord-transected lamprey: in vitro preparations. J. Neurophysiol. 1994;72:847–860. doi: 10.1152/jn.1994.72.2.847. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Grillner S. Initiation and sensory gating of ‘fictive’ swimming and withdrawal responses in an in vitro preparation of the lamprey spinal cord. Brain Res. 1983;269:237–50. doi: 10.1016/0006-8993(83)90133-6. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Grillner S. Activation of ‘fictive swimming’ by electrical microstimulation of brainstem locomotor regions in an in vitro preparation of the lamprey central nervous system. Brain Res. 1984;300:357–361. doi: 10.1016/0006-8993(84)90846-1. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Hagevik A. Coordination of spinal locomotor activity in the lamprey: long-distance coupling of spinal oscillators. Exp. Brain Res. 1999;126:93–108. doi: 10.1007/s002210050719. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Sigvardt KA. Features of entrainment of spinal pattern generators for locomotor activity in the lamprey spinal cord. J. Neurosci. 1988;8:133–145. doi: 10.1523/JNEUROSCI.08-01-00133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson DR, Buchanan JT, Kasicki S. Effects of strychnine on fictive swimming in the lamprey: evidence for glycinergic inhibition, discrepancies with model predictions, and novel modulatory rhythms. J. Comp. Physiol. A. 1994;175:311–321. doi: 10.1007/BF00192990. [DOI] [PubMed] [Google Scholar]
- Ménard A, Auclair F, Bourcier-Lucas C, Grillner S, Dubuc R. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. J. Comp. Neurol. 2007;501:260–273. doi: 10.1002/cne.21258. [DOI] [PubMed] [Google Scholar]
- Ménard A, Grillner S. Diencephalic locomotor region in the lamprey--afferents and efferent control. J. Neurophysiol. 2008;100:1343–1353. doi: 10.1152/jn.01128.2007. [DOI] [PubMed] [Google Scholar]
- Mentel T, Krause A, Pabst M, El Manira A, Büschges A. Activity of fin muscles and fin motoneurons during swimming motor pattern in the lamprey. Eur. J. Neurosci. 2006;23:2012–26. doi: 10.1111/j.1460-9568.2006.04738.x. [DOI] [PubMed] [Google Scholar]
- Miller WL, Sigvardt KA. Extent and role of multisegmental coupling in the Lamprey spinal locomotor pattern generator. J. Neurophysiol. 2000;83:465–476. doi: 10.1152/jn.2000.83.1.465. [DOI] [PubMed] [Google Scholar]
- Moroz LL. On the independent origins of complex brains and neurons. Brain Behav. Evol. 2009;74:177–190. doi: 10.1159/000258665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KJ, Nicholls JG, Stent GS. Neurobiology of the Leech. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1981. p. 320. [Google Scholar]
- Mullins OJ, Hackett JT, Friesen WO. Local-distributed integration by a novel neuron ensures rapid initiation of animal locomotion. 2011 doi: 10.1152/jn.00507.2010. [DOI] [PubMed] [Google Scholar]
- Mullins OJ, Hackett JT, Friesen WO. Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2010. Descending brain inputs cause variability in the behavioral responses to a swim excitatory neuron Program No. 290.5. [Google Scholar]
- Murchison D, Chrachri A, Mulloney B. A separate local pattern-generating circuit controls the movements of each swimmeret in crayfish. J. Neurophysiol. 1993;70:2620–2631. doi: 10.1152/jn.1993.70.6.2620. [DOI] [PubMed] [Google Scholar]
- Nicholls JG, Purves D. Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J. Physiol. 1970;209:647–667. doi: 10.1113/jphysiol.1970.sp009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, ten Donkelaar H, Nicholson C. The Central Nervous System of Vertebrates. Vol. 1. Springer-Verlag; Berlin: 1998. [Google Scholar]
- Nusbaum MP, B. KW., Jr Swim initiation in the leech by serotonin-containing interneurones, cells 21 and 61. J. Exp. Biol. 1986;122:277–302. doi: 10.1242/jeb.122.1.277. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Friesen WO, B. KW, Jr, Pearce RA. Neural mechanisms generating the leech swimming rhythm: swim-initiator neurons excite the network of swim oscillator neurons. J. Comp. Physiol. A. 1987;161:355–66. doi: 10.1007/BF00603961. [DOI] [PubMed] [Google Scholar]
- O'Gara BA, Chae H, Latham LB, Friesen WO. Modification of leech behavior patterns by reserpine-induced amine depletion. J. Neurosci. 1991;11:96–110. doi: 10.1523/JNEUROSCI.11-01-00096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gara BA, Friesen WO. Termination of leech swimming activity by a previously identified swim trigger neuron. J. Comp. Physiol. A. 1995;177:627–36. doi: 10.1007/BF00207191. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Dubuc R, Grillner S. A new population of neurons with crossed axons in the lamprey spinal cord. Brain Res. 1991;564:143–148. doi: 10.1016/0006-8993(91)91364-7. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Grillner S. Monosynaptic excitatory amino acid transmission from the posterior rhombencephalic reticular nucleus to spinal neurons involved in the control of locomotion in lamprey. J. Neurophysiol. 1989;62:1079–1089. doi: 10.1152/jn.1989.62.5.1079. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion: From Mollusc to Man. Oxford University Press; New York: 1999. [Google Scholar]
- Orlovsky GN, Deliagina TG, Wallén P. Vestibular control of swimming in lamprey. I. Responses of reticulospinal neurons to roll and pitch. Exp. Brain Res. 1992;90:479–488. doi: 10.1007/BF00230930. [DOI] [PubMed] [Google Scholar]
- Ort CA, Kristan WB, Jr., Stent GS. Neuronal control of swimming in the medicinal leech. II. Identification and connection of motor neurons. J. Comp. Physiol. 1974;94:121–154. 121. [Google Scholar]
- Paggett KC, Gupta V, McClellan AD. Adaptive variations of undulatory behaviors in larval lamprey: comparison of swimming and burrowing. Exp. Brain. Res. 1998;119:213–223. doi: 10.1007/s002210050335. [DOI] [PubMed] [Google Scholar]
- Paggett KC, Jackson AW, McClellan AD. Organization of higher-order brain areas that initiate locomotor activity in larval lamprey. Neuroscience. 2004;125:25–33. doi: 10.1016/j.neuroscience.2004.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Grillner S. The activity-dependent plasticity of segmental and intersegmental synaptic connections in the lamprey spinal cord. Eur. J. Neurosci. 2000;12:2135–2146. doi: 10.1046/j.1460-9568.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- Payton B. Structure of the leech nervous system. In: Muller KJ, Nicholls JG, Stent GS, editors. Neurobiology of the Leech. Cold Spring Harbor Laboratory; New York: 1981. pp. 35–50. [Google Scholar]
- Pearce RA, Friesen WO. Intersegmental coordination of leech swimming: comparison of in situ and isolated nerve cord activity with body wall movement. Brain Res. 1984;299:363–366. doi: 10.1016/0006-8993(84)90720-0. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Friesen WO. Intersegmental coordination of the leech swimming rhythm. I. Roles of cycle period gradient and coupling strength. J. Neurophysiol. 1985a;54:1444–1459. doi: 10.1152/jn.1985.54.6.1444. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Friesen WO. Intersegmental coordination of the leech swimming rhythm. II. Comparison of long and short chains of ganglia. J. Neurophysiol. 1985b;54:1460–1472. doi: 10.1152/jn.1985.54.6.1460. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Friesen WO. A model for intersegmental coordination in the leech nerve cord. Biol. Cybern. 1988;58:301–11. doi: 10.1007/BF00363939. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Neural adaptation in the generation of rhythmic behavior. Annu. Rev. Physiol. 2000;62:723–753. doi: 10.1146/annurev.physiol.62.1.723. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog. Brain Res. 2004;143:123–9. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Poon M, Friesen WO, Stent GS. Neuronal control of swimming in the medicinal leech. V. Connexions between the oscillatory interneurones and the motor neurones. J. Exp. Biol. 1978;75:45–63. doi: 10.1242/jeb.75.1.45. [DOI] [PubMed] [Google Scholar]
- Poon MLT. Induction of swimming in lamprey by L-DOPA and Amino Acids. J Comp. Physiol. 1980;136:337–344. 337. [Google Scholar]
- Puhl JG, Mesce KA. Keeping it together: mechanisms of intersegmental coordination for a flexible locomotor behavior. J. Neurosci. 2010;30:2373–2383. doi: 10.1523/JNEUROSCI.5765-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan KA, Buchanan JT. Cellular and synaptic actions of acetylcholine in the lamprey spinal cord. J. Neurophysiol. 2008;100:1020–31. doi: 10.1152/jn.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan KA, Placas PG, Buchanan JT. Cholinergic modulation of the locomotor network in the lamprey spinal cord. J. Neurophysiol. 2004;92:1536–1548. doi: 10.1152/jn.01053.2003. [DOI] [PubMed] [Google Scholar]
- Rouse DT, Jr., McClellan AD. Descending propriospinal neurons in normal and spinal cord-transected lamprey. Exp. Neurol. 1997;146:113–124. doi: 10.1006/exnr.1997.6511. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells. J. Neurophysiol. 1967a;30:1000–1023. doi: 10.1152/jn.1967.30.5.1000. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). II. Dorsal cells and giant interneurons. J. Neurophysiol. 1967b;30:1024–1042. doi: 10.1152/jn.1967.30.5.1024. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Synaptic interactions of identified nerve cells in the spinal cord of the sea lamprey. J. Comp. Neurol. 1974a;154:189–206. doi: 10.1002/cne.901540206. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J. Comp. Neurol. 1974b;154:207–23. doi: 10.1002/cne.901540207. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Neurobiology of lampreys. Physiol. Rev. 1979;59:1007–1077. doi: 10.1152/physrev.1979.59.4.1007. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Effects of groups of propriospinal interneurons on fictive swimming in the isolated spinal cord of the lamprey. J. Neurophysiol. 1985;54:959–977. doi: 10.1152/jn.1985.54.4.959. [DOI] [PubMed] [Google Scholar]
- Schmitt DE, Hill RH, Grillner S. The spinal GABAergic system is a strong modulator of burst frequency in the lamprey locomotor network. J. Neurophysiol. 2004;92:2357–2367. doi: 10.1152/jn.00233.2004. [DOI] [PubMed] [Google Scholar]
- Selverston AI, Moulins M. Oscillatory neural networks. Annu. Rev. Physiol. 1985;47:29–48. doi: 10.1146/annurev.ph.47.030185.000333. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall ME, Trontelj P, Utevsky SY, Nkamany M, Macdonald KS. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc. Biol. Sci. 2007;274:1481–1487. doi: 10.1098/rspb.2007.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigvardt KA, Grillner S, Wallén P, Van Dongen PA. Activation of NMDA receptors elicits fictive locomotion and bistable membrane properties in the lamprey spinal cord. Brain Res. 1985;336:390–395. doi: 10.1016/0006-8993(85)90676-6. [DOI] [PubMed] [Google Scholar]
- Sirota MG, Di Prisco GV, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur. J. Neurosci. 2000;12:4081–92. doi: 10.1046/j.1460-9568.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Skinner FK, Mulloney B. Intersegmental coordination in invertebrates and vertebrates. Curr. Opin. Neurobiol. 1998;8:725–732. doi: 10.1016/s0959-4388(98)80114-1. [DOI] [PubMed] [Google Scholar]
- Stent GS, Kristan WB, Jr., Torrence SA, French KA, Weisblat DA. Development of the leech nervous system. Int. Rev. Neurobiol. 1992;33:109–193. doi: 10.1016/s0074-7742(08)60692-3. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Cottrell GW, Kleinfeld D, B. KW., Jr Imaging reveals synaptic targets of a swim-terminating neuron in the leech CNS. J. Neurosci. 2003;23:11402–10. doi: 10.1523/JNEUROSCI.23-36-11402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegner J, Matsushima T, El Manira A, Grillner S. The spinal GABA system modulates burst frequency and intersegmental coordination in the lamprey: differential effects of GABAA and GABAB receptors. J. Neurophysiol. 1993;69:647–657. doi: 10.1152/jn.1993.69.3.647. [DOI] [PubMed] [Google Scholar]
- Teräväinen H, Rovainen CM. Fast and slow motoneurons to body muscle of the sea lamprey. J. Neurophysiol. 1971;34:990–998. doi: 10.1152/jn.1971.34.6.990. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Ménard A, Pombal M, Grillner S. Forebrain dopamine depletion impairs motor behavior in lamprey. Eur. J. Neurosci. 2008;27:1452–1460. doi: 10.1111/j.1460-9568.2008.06125.x. [DOI] [PubMed] [Google Scholar]
- Ullén F, Orlovsky GN, Deliagina TG, Grillner S. Role of dermal photoreceptors and lateral eyes in initiation and orientation of locomotion in lamprey. Behav. Brain Res. 1993;54:107–10. doi: 10.1016/0166-4328(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Boutin T, Petropoulos D, Brocard F, Dubuc R. The trigeminal sensory relay to reticulospinal neurones in lampreys. Neurosci. 2005;131:535–546. doi: 10.1016/j.neuroscience.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Ohta Y, Bongianni F, Grillner S, Dubuc R. Trigeminal inputs to reticulospinal neurones in lampreys are mediated by excitatory and inhibitory amino acids. Brain Res. 1995;695:76–80. doi: 10.1016/0006-8993(95)00936-k. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Pearlstein E, Le Ray D, Robitaille R, Dubuc R. A cellular mechanism for the transformation of a sensory input into a motor command. J. Neurosci. 2000;20:8169–76. doi: 10.1523/JNEUROSCI.20-21-08169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana Di Prisco G, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–5. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Wallén P, Grillner S. Synaptic effects of intraspinal stretch receptor neurons mediating movement-related feedback during locomotion. Brain Res. 1990;530:161–6. doi: 10.1016/0006-8993(90)90675-2. [DOI] [PubMed] [Google Scholar]
- Vinay L, Bongianni F, Ohta Y, Grillner S, Dubuc R. Spinal inputs from lateral columns to reticulospinal neurons in lampreys. Brain Res. 1998a;808:279–293. doi: 10.1016/s0006-8993(98)00835-x. [DOI] [PubMed] [Google Scholar]
- Vinay L, Bussieres N, Shupliakov O, Dubuc R, Grillner S. Anatomical study of spinobulbar neurons in lampreys. J. Comp. Neur. 1998b;397:475–492. [PubMed] [Google Scholar]
- Wallén P, Lansner A. Do the motoneurones constitute a part of the spinal network generating the swimming rhythm in the lamprey? J. Exp. Biol. 1984;113:493–497. 493. [Google Scholar]
- Wallén P, Robertson B, Cangiano L, Low P, Bhattacharjee A, Kaczmarek LK, Grillner S. Sodium-dependent potassium channels of a Slack-like subtype contribute to the slow afterhyperpolarization in lamprey spinal neurons. J. Physiol. 2007;585:75–90. doi: 10.1113/jphysiol.2007.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P, Williams TL. Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J. Physiol. 1984;347:225–239. doi: 10.1113/jphysiol.1984.sp015063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JC. Neuronal basis of leech swimming: separation of swim initiation, pattern generation, and intersegmental coordination by selective lesions. J. Neurophysiol. 1981;45:698–723. doi: 10.1152/jn.1981.45.4.698. [DOI] [PubMed] [Google Scholar]
- Weeks JC. Segmental specialization of a leech swim-initiating interneuron, cell 205. J. Neurosci. 1982;2:972–985. doi: 10.1523/JNEUROSCI.02-07-00972.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JC. Synaptic basis of swim-initiation in the leech. II. A pattern-generating neuron (cell 208) which mediates motor effects of swim-initiating neurons. J. Comp. Physiol. A. 1982;148:265–279. 265. [Google Scholar]
- Weeks JC, Kristan WB., Jr. Initiation, maintenance and modulation of swimming in the medicinal leech by the activity of a single neurone. J. Exp. Biol. 1978;77:71–88. 71. [Google Scholar]
- Weisblat DA. Development of the nervous system. In: Muller KJ, Nicholls JG, Stent GS, editors. Neurobiology of the Leech. Cold Spring Harbor Laboratory; New York: 1981. pp. 173–196. [Google Scholar]
- Wilkinson JM, Coggeshall RE. Axonal numbers and sizes in the connectives and peripheral nerves of the leech. J. Comp. Neurol. 1975;162:387–96. doi: 10.1002/cne.901620307. [DOI] [PubMed] [Google Scholar]
- Willard AL. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J. Neurosci. 1981;1:936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TL, Grillner S, Smoljaninov VV, Wallén P, Kashin S, Rossignol S. Locomotion in lamprey and trout: The relative timing of activation and movement. J Exp Biol. 1989;143:559–566. 559. [Google Scholar]
- Wilson DM. The central nervous control of flight in a locust. J. Exp. Biol. 1961;38:471–490. [Google Scholar]
- Yu X, Friesen WO. Entrainment of leech swimming activity by the ventral stretch receptor. J. Comp. Physiol. A. Neuroethol. Sens. Neural Behav. Physiol. 2004;190:939–949. doi: 10.1007/s00359-004-0549-9. [DOI] [PubMed] [Google Scholar]
- Yu X, Nguyen B, Friesen WO. Sensory feedback can coordinate the swimming activity of the leech. J. Neurosci. 1999;19:4634–4643. doi: 10.1523/JNEUROSCI.19-11-04634.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV. Activity of individual reticulospinal neurons during different forms of locomotion in the lamprey. Eur. J. Neurosci. 2005;22:2271–2282. doi: 10.1111/j.1460-9568.2005.04395.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Palmer R, McClellan AD. Increase in descending brain-spinal cord projections with age in larval lamprey: implications for spinal cord injury. J. Comp. Neurol. 2002;447:128–137. doi: 10.1002/cne.10208. [DOI] [PubMed] [Google Scholar]
- Zhang W, Grillner S. The spinal 5-HT system contributes to the generation of fictive locomotion in lamprey. Brain Res. 2000;879:188–192. doi: 10.1016/s0006-8993(00)02747-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, Pombal MA, El Manira A, Grillner S. Rostrocaudal distribution of 5-HT innervation in the lamprey spinal cord and differential effects of 5-HT on fictive locomotion. J. Comp. Neurol. 1996;374:278–90. doi: 10.1002/(SICI)1096-9861(19961014)374:2<278::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zhang W, Pombal MA, El Manira A, Grillner S. Rostrocaudal distribution of 5-HT innervation in the lamprey spinal cord and differential effects of 5-HT on fictive locomotion. J. Comp. Neurol. 1996;374:278–290. doi: 10.1002/(SICI)1096-9861(19961014)374:2<278::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Zheng M, Friesen WO, Iwasaki T. Systems-level modeling of neuronal circuits for leech swimming. J. Comput. Neurosci. 2007;22:21–38. doi: 10.1007/s10827-006-9648-7. [DOI] [PubMed] [Google Scholar]