Abstract
Background
Racial/ethnic differences in the incidence and severity of heart failure (HF) are not well understood, but may be related to pre-existing variations in myocardial function.
Objective
To examine racial/ethnic differences in regional myocardial function among asymptomatic individuals free of known cardiovascular disease.
Design, setting and patients
The Multi-Ethnic Study of Atherosclerosis is a prospective, observational study of individuals without baseline cardiovascular disease, representing four major racial/ethnic groups. A total of 1099 study participants underwent cardiac MRI with tissue tagging; for each study, peak systolic strain (Ecc) and strain rate (SRs) were determined in four left ventricular (LV) regions.
Main outcome measures
Multiple linear regression was used to analyse the relationship between race/ethnicity and regional strain (Ecc and SRs) while adjusting for cardiovascular risk factors.
Results
Compared with other racial/ethnic groups, Chinese-Americans had the greatest magnitude of Ecc in a majority of LV regions (–19.60±3.78, p<0.05); Chinese-Americans also had the greatest absolute values for SRs in all regions, reflecting higher rate of systolic contraction (–2.01±0.76, p<0.05). Conversely, African-Americans had the lowest Ecc values (–17.50±4.00, p<0.05) in the majority of wall regions while Hispanics demonstrated the lowest rate of contractility in all wall regions (–1.44±0.50, p≤0.001) in comparison with the other racial/ethnic groups. These race-based differences remained significant in the majority of LV wall regions after adjusting for multiple variables, including hypertension and LV mass.
Conclusions
Important race-based differences in regional LV systolic function in a large cohort of asymptomatic individuals have been demonstrated. Further research is needed to investigate the possible mechanisms related to the race/ethnicity-based variations found in this study.
INTRODUCTION
Heart failure (HF) continues to be a common cause of major morbidity and mortality world wide. Notably, the incidence and severity of HF appear to differ across racial/ethnic groups for reasons that remain unclear.1–3 For example, hospitalisations and deaths related to HF are substantially more common among African-Americans than individuals of other racial/ethnic groups.2–5 Also, in the United States, African-Americans have a higher prevalence of HF than people of other race/ethnic groups.6 7 More recently, in a 20-year prospective study of young to middle-age adults, incident HF before 50 years of age was 20 times greater among African-Americans than among white subjects.8 These findings correlate well with similar observations from the Multi-Ethnic Study of Atherosclerosis (MESA), where incident HF was also greater among African-Americans than among Caucasians and Hispanics, with Chinese-Americans having the lowest incidence rates.9 These observed differences may be related to racial/ethnic variation in the prevalence of risk factors, subclinical diseases associated with HF, disparities in treatment and access to care and/or potential genetic heterogeneity that may influence neurohormonal sensitivity, response to HF treatments and other factors associated with clinical HF.
Studies to date of race/ethnicity-based variation in the development of HF have been limited to selected referral samples and by the availability of methods for assessing incipient myocardial dysfunction in vulnerable populations. Cardiovascular MRI offers the ability to assess regional as well as global left ventricular (LV) function in fine detail and can detect even subtle functional abnormalities in asymptomatic individuals using tissue tagging with strain analyses. In particular, circumferential LV strain and strain rate assessments by cardiac MRI have been associated with ventricular chamber performance, distribution of coronary blood flow and regional vulnerability to ischaemia and infarction. Whereas systolic strain is an absolute measure of myocardial tissue deformation in systole, strain rate reflects the timing of this tissue deformation and, hence, is a marker of the rate of contractility. The degree to which LV strain or strain rate, representing different aspects of contractile function, may vary by race/ethnicity in the population at large is unknown. Therefore, we used cardiac MRI and strain analyses to investigate possible racial/ethnic differences in subclinical regional myocardial function in a large multi-ethnic cohort of ambulatory individuals without known cardiovascular disease.
PATIENTS AND METHODS
Study population
The design and population characteristics of MESA have been described previously.10 11 Briefly, MESA is a prospective, population-based observational cohort study of 6814 men and women representing four racial/ethnic groups (Caucasian, African-American, Hispanic and Chinese-American), aged 45–84 years and free of clinical cardiovascular disease at enrolment. As part of the baseline examination, between 2001 and 2002, a total of 5004 (73%) participants received comprehensive cardiac MRI studies at the six field centres (Wake Forest University, North Caroline, USA; Columbia University, New York, USA; Johns Hopkins University, Maryland, USA; University of Minnesota, Minnesota, USA; Northwestern University, Illinois, USA and University of California at Los Angeles, California, USA). Of the 5004 individuals who underwent a cardiac MRI examination, 1522 participants with available clinical covariate data agreed to a slightly longer examination to accommodate MRI tagging analyses. Of these participants, deformation data could not be analysed owing to data acquisition failure in 289 participants (19%) and acquired data were not of sufficient quality for strain and strain rate determination in 134 participants (9%). The remaining 1099 participants with complete circumferential strain and strain rate measurements were included in this analysis. The institutional review boards of all MESA field centres approved the study protocol.
MRI protocol
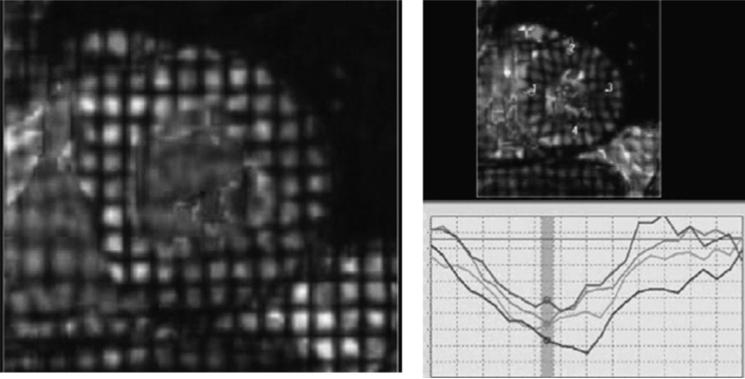
Images were obtained with 1.5 T MR scanners (SIGNA (LX and CVI), GE Medical Systems, Wankesha, Wisconsin, USA; and Siemens Medical Solutions (Vision and Sonata), Erlangen, Germany) using ECG-triggered segmented k-space fast gradient-echo (SPGR or FLASH) pulse sequence during breath holds, as previously described.12 Dedicated phase array coils were used for signal acquisition. Following the standard imaging protocol, three tagged short-axis slices were acquired at the LV base, mid-ventricle and apex. Parallel striped tags were prescribed in two orthogonal orientations (0°,90°) using identical pulse sequences with additional spatial modulation of magnetisation (SPAMM).13 The parameters for tagged MRI images were as follows: field of view 40 cm; slice thickness 8–10 mm; repetition time 3.5–7.2 ms; echo time 2.0–4.2 ms; flip angle 12°; matrix size 256×96–140; 4–9 phase encoding views per segment; temporal resolution 20–41 ms and tag spacing 7 mm (figure 1). There were no significant differences in the pulse sequences or temporal resolution of image acquisition between racial/ethnic groups.
Figure 1.
The HARP method of analysing short-axis tagged cardiac MRI images acquired from a Multi- Ethnic Study of Atherosclerosis (MESA) participant is shown. In the left panel, grid lines denote the application of non-invasive, superimposed tissue markers across a short-axis view of the left ventricular (LV) mid-wall at end-systole. Over the course of the cardiac cycle, deformation of these gridlines is analysed and these data are used to calculate the strain values in real time. In the right panel, strain curves are displayed showing systolic strain in each of the major LV segments during the end-systolic phase.
MRI data analysis
Analysis of all cardiac MRI data was performed by trained personnel at the core MRI Reading Centre. For each cardiac MRI study, LV mass, LV end-diastolic volume (LVEDV) and ejection fraction (EF) were determined using commercially available software (MASS, version 4.2 Medis, The Netherlands).12 Specialised strain analyses were performed by trained investigators (N=3, including VF and BR) who were blinded to participant clinical information and to the results of cine and other portions of the standard cardiac MRI examination. Short-axis tagged slices were analysed by the HARP method (Harmonic Phase, Diagnosoft, Palo-Alto, California, USA) to assess strain14 and directly measured strain values were used to derive strain rate.14 Strain and strain rate provide complementary information regarding segmental myocardial function.15 Regional systolic circumferential strains (Ecc) were determined in four LV segments (anterior, lateral, inferior and septal) across three LV levels (basal, mid and apical) and strain rates were derived by integrating strain measurements over time for each LV segment:
Peak systolic strain rate (SRs) was obtained from each segment. By convention, Ecc and SRs are negative and increased negativity denotes enhanced function. Inter- and intraobserver agreement for myocardial MR-tagged image analyses using the HARP technique has been shown previously.16 Since assessments of circumferential strain at the mid-wall level (compared with basal and apical levels) are considered the most representative and reproducible of short-axis strain measures, we considered mid-wall strain and SR in all primary analyses.
Statistical analysis
To compare demographic and clinical characteristics across racial/ethnic groups, we used χ2 and analysis of variance with Tukey and Bonferroni tests for categorical and continuous variables, respectively. We used linear regression to evaluate the relation of racial/ethnic groups to regional Ecc and SRs across the different LV regions, while adjusting for traditional and non-traditional cardiovascular risk factors. A separate model was fit for each segment: model 1 adjusted for age, gender, body mass index, systolic and diastolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and smoking status; model 2 further adjusted for LV mass, LVEDV (a surrogate measure of preload), heart rate, glucose, C-reactive protein and drugs to control hypertension (including β blockers, ACE inhibitors and ARBs) diabetes, and dyslipidaemia; and, model 3 further adjusted for education level and income bracket.17–20 All analyses were conducted using STATA 8.0 and R software.
RESULTS
Table 1 shows the characteristics of the study sample. African-Americans had the highest LV mass compared with other racial/ethnic groups even when indexed to body surface area. Conversely, Chinese-Americans had by far the lowest LVEDV, as well as the highest EF. For regional systolic function, African-Americans presented the lowest mean Ecc (p<0.05, –17.50±4.00, mean±SD across all LV regions) when compared with Caucasian, African-Americans and Hispanics. However, when considering the rate of contraction indexed by systolic strain rate, Hispanics had the lowest values (p<0.01, –1.44±0.50, mean±SD) when compared with participants of other radical/ethnic groups.
Table 1.
Demographic and clinical characteristics of the 1099 study participants
| Characteristics | Caucasians (n = 364) | Chinese-Americans (n = 101) | African-Americans (n = 308) | Hispanics (n = 326) |
|---|---|---|---|---|
| Age (years)** | 67.0±9.4 | 70.1±7.6 | 64.6±10.1 | 65.8±9.8 |
| Male (%) | 53.3 | 55.4 | 51.6 | 56.1 |
| Education level (%)** | ||||
| Grade 8 | 1.38 | 19.80 | 4.29 | 30.37 |
| Grades 9–11 | 3.03 | 12.87 | 8.91 | 10.43 |
| Completed high school/GED | 18.73 | 8.91 | 19.14 | 24.54 |
| Some college | 25.34 | 21.78 | 34.98 | 23.93 |
| Bachelor's degree | 20.94 | 24.75 | 14.19 | 4.91 |
| Graduate or professional school | 30.58 | 11.88 | 18.48 | 5.83 |
| Income bracket (%)** | ||||
| <$12 000 | 4.80 | 29.00 | 11.30 | 21.67 |
| $12 000–24 999 | 14.97 | 39.00 | 19.18 | 26.32 |
| $25 000–34 999 | 9.60 | 9.00 | 13.70 | 17.65 |
| $35 000–49 999 | 16.67 | 6.00 | 20.21 | 16.10 |
| $50 000–99 999 | 29.94 | 11.00 | 28.08 | 15.17 |
| ≥$100 000 | 24.01 | 6.00 | 7.53 | 3.10 |
| Body mass index (kg/m2)** | 27.5±4.5 | 23.3±3.29 | 28.8±4.9 | 28.6±4.1 |
| Body surface area (m2)** | 1.89±0.21 | 1.64±0.16 | 1.91±0.21 | 1.80±0.18 |
| Systolic blood pressure (mm Hg)** | 124.2±19.7 | 129.4±21.5 | 130.1±21.0 | 131±20.8 |
| Diastolic blood pressure (mm Hg)** | 69.8±9.9 | 71.4±10.8 | 74.3±10.4 | 72.4±10.0 |
| Hypertension (n, %)** | 136 (37.4) | 46 (45.5) | 165 (53.6) | 132 (40.5) |
| Current cigarette smoking (n, %)** | 32 (8.8) | 6 (5.9) | 54 (17.8) | 38 (11.7) |
| Diabetes (n, %)* | 40 (11.0) | 23 (22.8) | 64 (20.8) | 65 (19.9) |
| Total cholesterol (mg/dl)* | 197.6±35.1 | 189.1±32.4 | 189.8±36.2 | 195.4±33.6 |
| LDL cholesterol (mg/dl) | 118.9±29.1 | 112.2±29.8 | 116.4±31.6 | 118.7±30.3 |
| HDL cholesterol (mg/dl) | 52.1±14.8 | 50.7±11.2 | 53.3±16.3 | 47.5±13.8 |
| Triglycerides (mg/dl)** | 132.7±85.9 | 135.57±76.1 | 100.8±53.9 | 147.3±77.0 |
| C-reactive protein (mg/dl)* | 2.9±3.6 | 1.5±2.0 | 3.6 ± 4.3 | 3.7±6.0 |
| Taking drugs for hypertension (n, %)** | 117 (32.1) | 36 (35.6) | 149 (48.4) | 104 (31.9) |
| Taking drugs for dyslipidaemia (n, %)* | 83 (22.8) | 29 (28.7) | 52 (16.9) | 52 (16) |
| Taking drugs for diabetes (n, %)* | 24 (6.6) | 17 (16.8) | 39 (12.7) | 43 (13.2) |
| LV mass (g)** | 145.8±40.8 | 122.8±33.7 | 156.0±42.2 | 146.1±35.7 |
| LV mass indexed to BSA (g/m2) | 76.8±16.9 | 74.3±16.4 | 81.2±18.6 | 80.7±15.0 |
| LVEDV (ml) | 125.0±33.5 | 107.1±24.4 | 127.2±33.5 | 124.3±29.4 |
| LVEDV indexed to BSA (ml/m2) | 66.3±14.9 | 65.0±12.5 | 66.5±15.0 | 68.6±13.0 |
| Stroke volume (ml)** | 85.3±22.3 | 76.7±17.2 | 85.4±20.5 | 84.2±17.9 |
| Stroke volume indexed to BSA (ml/m2)* | 45.2±10.4 | 46.8±9.7 | 44.5±9.0 | 46.6±8.3 |
| Ejection fraction (%)** | 68.5±6.9 | 71.9±6.7 | 67.7±8.4 | 68.7±7.5 |
| Peak systolic strain (%)** | –18.49±4.12 | –19.60±3.78 | –17.50±4.00 | –18.53±3.98 |
| Systolic strain rate (s–1)** | –1.63±0.63 | –2.01±0.76 | –1.67±0.75 | –1.44±0.50 |
Values are presented as mean±SD or frequencies (n, %).
p<0.05
p<0.01 for race/ethnic differences.
BSA, body surface index; GED, General Education Diploma; HDL, high-density cholesterol; LDL, low-density cholesterol; LV, left ventricular; LVEDV, left ventricular end-diastolic volume.
Racial/ethnic variation in circumferential strain
The peak systolic strain, represented by Ecc (%) measured at the LV mid-level, for the total sample was determined by region (mean±SD): anterior –18.3±4.1; lateral –20.5±3.9; inferior –13.8±4.5 and septal –16.1±3.9. Chinese-Americans demonstrated the greatest circumferential shortening (ie, most negative absolute values of Ecc relative to other groups) in all myocardial regions except in the septum. Race-based differences in systolic myocardial function were most marked in the lateral wall, where mean Ecc for Chinese-Americans (–21.8±3.6) was significantly greater (absolute strain values) than seen in Caucasians (–20.4±4.0, p<0.05), African-Americans (–20.0±4.2, p=0.0001) and Hispanics (–20.54±3.6, p=0.009). Peak systolic strain was also most favourable in Chinese-Americans for the anterior and inferior walls, but only significantly so in comparison with African-Americans (p<0.05).
Accordingly, African-Americans demonstrated the least favourable peak systolic strain, with the lowest systolic shortening values in all myocardial regions except the septum (table 2). These race-based differences in mechanical behaviour were most evident in the anterior and inferior walls, where mean absolute values for Ecc in African-Americans were lowest (anterior –17.50±4.0; inferior –12.40±4.7) in comparison with those in Caucasians (anterior –18.50±4.1, p=0.01; inferior –14.1±4.7, p<0.0001), Chinese-Americans (anterior –19.60±3.8, p=0.0001; inferior –15.02±3.7, p<0.0001) and Hispanics (anterior –18.52±4.0, p=0.009; inferior –14.3±3.8, p<0.0001). Most of these differences remained significant even after adjustment for multiple variables including blood pressure, hypertension status and LV mass.
Table 2.
Relation of racial/ethnic group to systolic strain and strain rate across LV wall regions
| Caucasians | African-Americans | Hispanics | |
|---|---|---|---|
| Systolic strain, Ecc (%) | |||
| Model 1 | |||
| Anterior | 0.82±0.48 | 1.40±0.50* | 0.62±0.48 |
| Lateral | 1.53±0.47* | 1.85±0.49** | 1.43±0.47* |
| Inferior | 0.62±0.52 | 2.04±0.55** | 0.20±0.53 |
| Septal | –0.40±0.45 | –0.27±0.47 | –0.35±0.46 |
| Model 2 | |||
| Anterior | 0.97±0.50 | 1.63±0.53* | 0.43±0.50 |
| Lateral | 1.66±0.50** | 1.88±0.53** | 1.50±0.50* |
| Inferior | 0.65±0.56 | 2.02±0.60** | 0.20±0.57 |
| Septal | –0.44±0.47 | –0.39±0.50 | –0.42±0.47 |
| Model 3 | |||
| Anterior | 1.14±0.53* | 1.67±0.55* | 0.55±0.52 |
| Lateral | 1.50±0.53* | 1.84±0.54** | 1.54±0.52* |
| Inferior | 0.58±0.59 | 2.11±0.61** | 0.36±0.58 |
| Septal | –0.35±0.49 | –0.34±0.50 | –0.24±0.48 |
| Systolic strain rates, SRs (s–1) | |||
| Model 1 | |||
| Anterior | 0.43±0.07** | 0.44±0.08** | 0.63±0.07** |
| Lateral | 0.47±0.08** | 0.52±0.09** | 0.63±0.08** |
| Inferior | 0.33±0.08** | 0.37±0.09** | 0.56±0.09** |
| Septal | 0.21±0.08* | 0.30±0.08** | 0.44±0.08** |
| Model 2 | |||
| Anterior | 0.33±0.08** | 0.28±0.08** | 0.53±0.08** |
| Lateral | 0.36±0.09** | 0.39±0.09** | 0.54±0.09** |
| Inferior | 0.25±0.09* | 0.27±0.10* | 0.47±0.09** |
| Septal | 0.09±0.08 | 0.16±0.09 | 0.35±0.08** |
| Model 3 | |||
| Anterior | 0.33±0.08** | 0.27±0.08** | 0.53±0.08** |
| Lateral | 0.31±0.09** | 0.36±0.09** | 0.54±0.09** |
| Inferior | 0.20±0.10* | 0.24±0.10* | 0.46±0.10** |
| Septal | 0.07±0.09 | 0.13±0.09 | 0.30±0.08** |
p<0.05
p≤0.001.
Regression coefficients (±SE) for strain and strain rate are in relation to the Chinese-Americans as the referent group.
Model 1 is adjusted for sex, age, body mass index, systolic and diastolic BP, LDL cholesterol, HDL cholesterol and smoking status.
Model 2 is further adjusted for LV mass, LVEDV, heart rate, glucose, C-reactive protein, diabetes and drugs to control dyslipidaemia, diabetes and hypertension.
Model 3 is further adjusted for education level and income bracket.
BP, blood pressure; HDL, high-density cholesterol; LDL, low-density cholesterol; LV, left ventricular; LVEDV, left ventricular end-diastolic volume.
When analysing other slices, similar patterns of regional function were found, with Chinese-Americans presenting greater absolute values in all regions, except in the septum (basal level), where the differences remained significant in most of the adjusted models when compared with African-Americans (p<0.05). At the apical level, Chinese-Americans presented greater Ecc, which was significantly different in the inferior and septal walls (p<0.05, unadjusted model) in comparison with African-Americans. These differences remained significant in most of the adjusted models for the inferior region (p<0.05).
Racial/ethnic variation in systolic strain rate
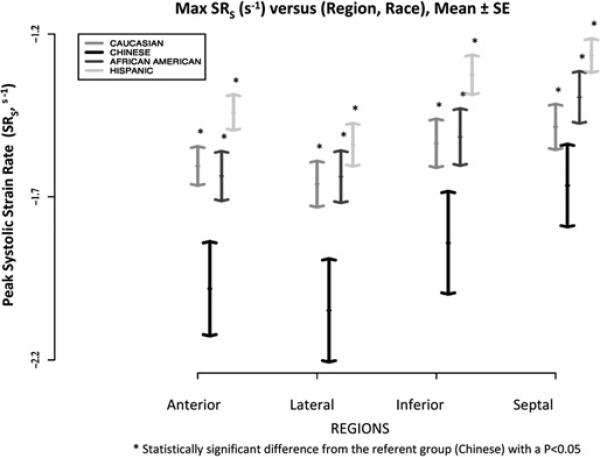
Peak systolic strain rate, represented as SRs (s–1), for the total population was estimated at the level of the mid-wall in each LV wall region: anterior –1.6±0.6; lateral –1.7±0.7; inferior –1.5±0.7; and, septal –1.4±0.6. Compared with all other racial/ethnic groups, Chinese-Americans had the greatest rate of contractile function in all myocardial regions; these absolute differences were highly significant (p≤0.001) in the anterior, lateral and posterior walls, with a non-significant trend only in the septum when Chinese-Americans were compared with Caucasians (p=0.09). Regional systolic function remained significantly greater in Chinese-Americans than in all other racial/ethnic groups and particularly in the anterior and lateral walls before and after adjustment for multiple variables (table 2, figure 2).
Figure 2.
Peak systolic strain rate (SR) values across racial/ethnic groups and myocardial regions (mid-level) are shown. Each bar represents mean (± standard error).
Conversely, Hispanics had the lowest rate of contractile function of all racial/ethnic groups and particularly in the anterior and inferior walls. In fact, Hispanics had a significantly lower rate of contractility than Chinese-Americans in all myocardial wall regions after full multivariable adjustment (table 2, figure 2). Even when compared with other racial/ethnic groups, regional myocardial rate of contractility was the most diminished among Hispanics as denoted by the lowest SRs in all wall regions (figure 2).
In analyses stratified by sex or age group (<65 vs ≥65 years), results remained similar in all the main multivariable adjusted models (data not shown). In the total sample, results also remained similar when the main analyses were repeated with additional adjustment for stroke volume indexed to body surface area (data not shown). When the analyses were performed at the basal LV level, similar results were again found, with Chinese-Americans presenting significantly greater absolute values of strain rate, principally when compared with Hispanics, in most of the regions and statistical models analysed (p<0.01). At the apical level, Chinese-Americans also presented greater values, especially when compared with Hispanics, in all regions and for all adjusted statistical models (p<0.01).
DISCUSSION
Compared with conventional measures of gross systolic LV function, such as LVEF, strain-based analyses provide region-specific as well as more sensitive information about myocardial performance.21 22 Using strain-based analyses, this study is the first to provide evidence of significant race/ethnicity-based variations in regional LV performance in a large and asymptomatic multi-ethnic population. Compared with other racial/ethnic groups, Chinese-Americans demonstrated the fastest rate of myocardial contraction and the largest magnitude of regional LV function as measured by cardiac MRI tagging and strain analysis across most myocardial wall regions. Conversely, African-Americans showed the least magnitude of contraction in almost all regions. With respect to myocardial rate of contractile function, Hispanics exhibited the lowest systolic strain rate in all LV regions. These results suggest that subtle differences in myocardial function exist across racial/ethnic groups, even after accounting for variation in the burden of traditional and non-traditional risk factors.
Racial/ethnic variation in early asymptomatic myocardial dysfunction
Previous studies have described race-based variation in LV geometry, with African-Americans having markedly greater LV mass and wall thickness than white subjects,23 24 even after adjusting for age and hypertension.25 26 In our asymptomatic study sample, we observed similar variations in LV mass across four racial/ethnic groups that additionally included Hispanic and Chinese-Americans. Hypertrophic remodeling has been associated with poorer systolic and diastolic myocardial function in previous MESA studies.19 27 In this study, important race-based subclinical differences were found in both regional and global systolic function even after controlling for LV mass and hypertension. Chinese-Americans had the most favourable, and African-Americans and Hispanics the least favourable, patterns of myocardial function. Notably, racial/ethnic differences were more prominent with respect to the LV rate of contractile function versus absolute magnitude of LV tissue deformation, suggesting that subtle alterations in strain rate versus strain may be particularly relevant for characterising subclinical racial/ethnic variations in LV function. Such subtle alterations in LV function may be among the earliest signs of myocardial pathology that both precede and accompany changes in LV structure along the pathway of progression towards overt HF.19 21
Racial/ethnic myocardial predisposition and clinical implications
Multiple studies have shown that among patients at risk for clinical HF, African-Americans and Hispanics have greater morbidity and mortality than other racial-ethnic groups, particularly Asian-Americans.2 4 5 28 Such racial/ethnic variations have not been fully explained by differences in the prevalence of risk factors for HF or even disparities in treatment and access to care,2 29 30 prompting speculation about the role of possible inherent and/or other biological mechanisms. Our study provides evidence that early differences in subclinical regional myocardial function do exist among certain racial/ethnic groups. The degree to which these early functional differences contribute to variation in clinical outcomes is unclear. However, although regional circumferential shortening has not been related to incident HF, more global measures of circumferential strain have been shown to predict future development of HF.31 In addition, the pattern of our findings, showing African-Americans and Hispanics having less magnitude and rate of regional myocardial contractility than Chinese-Americans and Caucasians, is consistent with race/ethnicity-based variation in incident HF reported in the same MESA population9 and in other prospective cohort studies.8
Observed race/ethnicity-based differences in regional myocardial function may be a marker of exposure to a variety of genetic and environmental factors that have yet to be identified. It has been suggested that more rapid progression towards advanced disease in African-Americans and Hispanics may be driven by a higher prevalence of risk factors for HF, particularly hypertension.1 8 32 The racial/ethnic differences in myocardial dysfunction in this study were significant even after adjusting for multiple traditional and non-traditional cardiovascular risk factors, including factors such as hypertension and LV mass as well as socioeconomic variables. However, such factors may predispose African-American and Hispanic individuals to subtle subclinical differences in myocardial dysfunction that may combine with other risk-related and socioeconomic mechanisms to account for the stark differences in HF-related morbidity and mortality seen among different racial/ethnic groups.
Limitations
Several limitations of this study merit consideration. The selection and enrolment of our study sample was not completely random and, consequently, the prevalence of risk factors approximates to, but does not exactly reflect, that of the larger MESA study cohort. In addition, selection and enrolment of the larger MESA study may not exactly reflect that of the general population. Although our analyses included socioeconomic factors such as income and education, these variables may not absolutely reflect access and adherence to appropriate care for treatment of HF risk factors. Observed absolute differences between racial/ethnic groups with respect to strain and strain rate were small compared with relative differences, which were of the order of >10%. Strain and strain rate are novel markers of subclinical myocardial function, and clinically important differences in these measures have yet to be defined. Therefore, even small absolute differences may represent biologically important racial/ethnic variation among individuals without known cardiovascular disease. Since our analyses are cross-sectional, future studies are needed to investigate the longitudinal relation of regional strain-related abnormalities with the incidence of clinical HF.
Notably, we did not find racial/ethnic differences in regional function as represented by peak systolic strain (Ecc) in the septal wall. Possibly, the interventricular septum wall contributes less to absolute circumferential strain than the LV free wall because it may be subject to a complicated set of stresses, or tractions, both from the right and left ventricles. Although the septum may alter its position in response to interventricular pressure differences,33 segment-length strain in the septum has been shown to depend on stress distribution across the right and left ventricular free walls.34 Interestingly, these limitations may not apply in the same way to the rate of myocardial contraction in the septum. Indeed, this study did detect significant racial/ethnic differences in systolic strain rate (SRs) in the septal wall that were more congruent with SRs differences in the LV free wall.
Importantly, myocardial strain is influenced by variations in loading conditions, which can manifest as alterations in wall stress. Although our analyses included adjustment for LVEDV, a surrogate for preload, we were unable to adjust specifically for wall stress since blood pressure measurements at the time of cardiac MRI were unavailable. However, since the vast majority of participants in the community-based MESA cohort are haemodynamically stable at the time of their examinations, variation in wall stress in our study sample is likely to be small. Several complementary strain and tagging-related measures of LV function, such as radial and longitudinal strain and apical-to-basal torsion, could add valuable information to our analysis of circumferential strain. Unfortunately, these additional parameters of myocardial function were unavailable in our study sample, primarily due to logistical factors limiting their measurement in a large population-based cohort such as MESA.
CONCLUSION
Our findings demonstrate race/ethnicity-based differences in regional myocardial function in the largest asymptomatic population studied to date with MRI tagging. We observed that Chinese-Americans have greater systolic strain and strain rate than Caucasians, Hispanics and African-Americans, across the majority of LV wall regions, even after adjusting for multiple cardiovascular risk factors and socioeconomic variables. Further research is needed to investigate the possible mechanisms related to the race/ethnicity-based variations found in this study.
Acknowledgements
The authors thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesanhlbi.org.
Funding This study was supported by NHLBI grant RO1-HL66075-01 and MESA study contracts NO1-HC-9808, NO1-HC-95168 and NO1-HC-95169. JACL is also supported by the Reynolds Foundation, SC is supported by the Ellison Foundation, and VRSF was a recipient of a research grant from CAPES, Ministry of Education, Brazilian government.
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of the Johns Hopkins University.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Thomas KL, East MA, Velazquez EJ, et al. Outcomes by race and etiology of patients with left ventricular systolic dysfunction. Am J Cardiol. 2005;96:956–63. doi: 10.1016/j.amjcard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Dries DL, Exner DV, Gersh BJ, et al. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340:609–16. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 3.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care and outcomes of elderly patients hospitalized with heart failure. Jama. 2003;289:2517–24. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 4.Alexander M, Grumbach K, Remy L, et al. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. Am Heart J. 1999;137:919–27. doi: 10.1016/s0002-8703(99)70417-5. [DOI] [PubMed] [Google Scholar]
- 5.Bourassa MG, Gurne O, Bangdiwala SI, et al. Natural history and patterns of current practice in heart failure. The Studies of Left Ventricular Dysfunction (SOLVD) Investigators. J Am Coll Cardiol. 1993;22:14A–9. doi: 10.1016/0735-1097(93)90456-b. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 12.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 13.Axel L, Dougherty L. Heart wall motion: improved method of spatial modulation of magnetization for MR imaging. Radiology. 1989;172:349–50. doi: 10.1148/radiology.172.2.2748813. [DOI] [PubMed] [Google Scholar]
- 14.Osman NF, Prince JL. Visualizing myocardial function using HARP MRI. Phys Med Biol. 2000;45:1665–82. doi: 10.1088/0031-9155/45/6/318. [DOI] [PubMed] [Google Scholar]
- 15.Urheim S, Edvardsen T, Torp H, et al. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–64. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 16.Castillo E, Osman NF, Rosen BD, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–91. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Fernandes VR, Bluemke DA, et al. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–8. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen BD, Cushman M, Nasir K, et al. Relationship between C-reactive protein levels and regional left ventricular function in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2007;49:594–600. doi: 10.1016/j.jacc.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 19.Rosen BD, Edvardsen T, Lai S, et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 20.Rosen BD, Saad MF, Shea S, et al. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47:1150–8. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 21.Borg AN, Ray SG. A unifying framework for understanding heart failure? Response to “Left Ventricular Torsion By Two-Dimensional Speckle Tracking Echocardiography in Patients With Diastolic Dysfunction and Normal Ejection Fraction” by Park SJ, et al. J Am Soc Echocardiogr. 2009;22:318–20. doi: 10.1016/j.echo.2008.11.026. author reply 21–2. [DOI] [PubMed] [Google Scholar]
- 22.Cottrell C, Kirkpatrick JN. Echocardiographic strain imaging and its use in the clinical setting. Expert Rev Cardiovasc Ther. 2010;8:93–102. doi: 10.1586/erc.09.165. [DOI] [PubMed] [Google Scholar]
- 23.Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43:1182–8. doi: 10.1161/01.HYP.0000128738.94190.9f. [DOI] [PubMed] [Google Scholar]
- 24.Olutade BO, Gbadebo TD, Porter VD, et al. Racial differences in ambulatory blood pressure and echocardiographic left ventricular geometry. Am J Med Sci. 1998;315:101–9. doi: 10.1097/00000441-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Arnett DK, Rautaharju P, Crow R, et al. Black-white differences in electrocardiographic left ventricular mass and its association with blood pressure (the ARIC study). Atherosclerosis Risk in Communities. Am J Cardiol. 1994;74:247–52. doi: 10.1016/0002-9149(94)90365-4. [DOI] [PubMed] [Google Scholar]
- 26.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–7. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 27.Edvardsen T, Rosen BD, Pan L, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imagingethe Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2006;151:109–14. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Rathore SS, Krumholz HM. Race, ethnic group and clinical research. BMJ. 2003;327:763–4. doi: 10.1136/bmj.327.7418.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafata JE, Pladevall M, Divine G, et al. Are there race/ethnicity differences in outpatient congestive heart failure management, hospital use and mortality among an insured population? Med Care. 2004;42:680–9. doi: 10.1097/01.mlr.0000129903.12843.fc. [DOI] [PubMed] [Google Scholar]
- 30.Deswal A, Petersen NJ, Urbauer DL, et al. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J. 2006;152:348–54. doi: 10.1016/j.ahj.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–6. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 32.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 33.Lima JA, Guzman PA, Yin FC, et al. Septal geometry in the unloaded living human heart. Circulation. 1986;74:463–8. doi: 10.1161/01.cir.74.3.463. [DOI] [PubMed] [Google Scholar]
- 34.Kent RS, Carew TE, LeWinter MM, et al. Comparison of left ventricular free wall and septal diastolic compliance in the dog. Am J Physiol. 1978;234:H392–8. doi: 10.1152/ajpheart.1978.234.4.H392. [DOI] [PubMed] [Google Scholar]