Abstract
This paper describes recruitment challenges and lessons learned in conducting a randomized controlled exercise trial in the absence of direct access to a clinical population. One hundred thirty-five wheelchair users were enrolled in a home and community-based intervention to promote exercise adoption and maintenance. Over 44 months of recruitment, 355 individuals inquired about the study and 323 completed the screening process. Nearly half were determined ineligible (150/323, 46.4%), typically due to having restricted arm movement, cognitive impairment, or medical conditions that are contraindicated for unsupervised exercise. Respondents cited paid media advertisements and recruitment materials placed in health care providers’ offices most frequently as being how they learned about the study. RCT participant recruitment, particularly in the absence of direct access to a clinical population, required far more time and resources than anticipated to achieve sufficient enrollment. Nurturing relations with key gatekeepers, creating a visible public profile, and maintaining ongoing recruitment activities were essential to success.
Keywords: (MeSH terms) research subject recruitment, wheelchair, exercise, physically disabled
Participant recruitment is essential but increasingly difficult for randomized controlled trials (RCTs), [1–3] causing many trials to proceed without sufficient participant numbers. [4] Avoiding low participation rates, however, frequently requires more resources than initially anticipated [5]--a situation especially problematic in an era of reduced biomedical research funding. [6] Recruitment is particularly challenging for investigators lacking access to a clinical population since a patient’s personal health care provider is typically most successful at study recruitment. [7] Despite these challenges, there are limited empirical data on effective recruitment strategies to guide investigators. [8] Moreover, data are particularly limited on recruiting from understudied populations, such as those with disabilities, despite calls for increased inclusion of these populations. [9–11]
People with disabilities experience significant barriers to research participation, including lack of transportation, [2] complex health problems, [12] cognitive impairments or low literacy, and financial stress. [7] Recruiting those with disabilities can be more challenging when studies enroll participants on the basis of functional impairment, rather than by their diagnosis. Many individuals living with disabling conditions perceive their condition-related problems as unique, and seek health promotion information that addresses their specific condition. [13] Similarly, some researchers suggest that patients with disabilities, such as spinal cord injury, have unique needs. [2] Nonetheless, others have suggested that defining intervention groups by “non-disease” commonalities, such as mobility impairment, fatigue, or chronic pain, appropriately focuses on general wellness principles rather than specific disease management strategies. [13] Doing so, however, increases the difficulty of identifying and recruiting participants, even when using electronic medical record searches or computerized programs such as Research Match (https://www.researchmatch.org/) developed by the NIH Clinical and Translational Research Award Consortium.
Achieving cultural competence in working with people with disabilities as a minority population also may be critical to recruitment success. [12] Establishing rapport and a sense of collaboration [14–15] and eliminating barriers to research participation [2] can facililitate connections with the target population.
This article describes the recruitment challenges and lessons learned during a large randomized controlled exercise trial for wheelchair users when the investigators did not have direct access to the clinical population. The paper provides evidence for overcoming recruitment barriers frequently documented by researchers when enrolling participants from underserved populations.
Background
Recruitment targeting 180 participants began after receiving approval from the principal investigator’s institutional Human Subjects Committee (HSC) for a randomized controlled trial to assess a multi-component behavioral intervention for adopting and maintaining exercise among wheelchair users (NIH #R01 HD048628). Recruitment focused on seeking individuals who require use of a wheelchair as their primary method of mobility outside the home, regardless of the etiology causing mobility impairment..
Specific eligibility criteria included: permanent mobility impairment for ≥ six months that necessitated manual wheelchair use outside of the home; sufficient upper extremity mobility for upper body exercise; not regularly physically active in the past six months (assessed by questions regarding participation in moderate and vigorous physical activity over the past six months); physician consent to exercise; between 18 and 60 years old; and able to speak, read, and write English. Exclusion criteria included: body mass index of ≥50 (added after safety concerns were raised given the number of extremely obese persons who inquired); medical conditions their physician identified as contraindicated for unsupervised exercise (e.g., certain cardiac problems, chronic obstructive pulmonary disease); taking beta-blockers; cognitive impairment that precluded self-direct daily activities; and pregnant, or planning to become pregnant.
Recruitment began in May of 2006. Because none of our interdisciplinary research team is a direct service provider, recruitment required working with clinical providers and other groups in regular contact with the target population. Study information also was broadly disseminated across the metro region.
Initial efforts followed a recruitment plan that anticipated enrolling 60 participants in three cohorts. Experience recruiting for the first cohort, however, led to altering and expanding the original recruitment plans. The revised plans, vetted and approved by the HSC, included increasing the number of cohorts and altering eligibility criteria. Making these changes early was important for consistent sampling and recruitment over the entire project. The initial small cohort that prompted these changes was designated pilot data to preserve overall project integrity. While beyond the scope of this paper, adding cohorts with differing start dates may have an effect on study outcomes and should be considered when analyzing findings.
Eligibility criteria were revisited to assess what could be changed to achieve a greater sample size without compromising scientific rigor. Issues that resulted in exclusions from our initial efforts were reviewed and four specific criteria were altered. The upper age limit was increased to 65 years, using a powered wheelchair was acceptable if there was sufficient upper arm mobility for exercise, those on beta blockers with pharmacologically controlled heart rates and physician consent were no longer excluded, and those randomly assigned to the treatment group workshop but unable to attend the one-day workshop were provided a DVD of the content so they would receive the intervention content of the workshop and could remain in the study. To mitigate against creating a selection effect for educational content delivery method, everyone assigned to the intervention arm was strongly encouraged to attend the workshop. Individuals were informed of the DVD only in the event they reported a conflict would prevent their workshop attendance. We then focused on strategies to reduce participation burden and enhance retention. Specific recruitment and retention strategies are described below.
Recruitment and Retention
A catchy study name (Project Workout on Wheels or Project WOW) and a logo (Figure 1) were developed for all study materials (flyers, brochures, posters, pens, magnets, and data collection sheets) to establish project identity. Study brochures and advertisements provided a phone number and address for interested individuals to contact study staff. The study brochure included a pre-printed, addressed and postage-paid tear off that could be completed and returned. Upon initial telephone contact, the study was described and those interested in enrolling were formally screened for initial eligibility.
Figure 1.

Study logo and example of ad
Recruitment strategies
Recruitment efforts included working with entities that have direct contact with the target population. Strategies ranged from meeting with hospitals, healthcare facilities, healthcare providers, and durable medical equipment suppliers to place posters and brochures in waiting rooms; working with disability service agencies such as independent living centers and social and rehabilitation services to mail study brochures directly to individuals; placing paid advertisements in newspapers, newsletters, fliers, direct mail coupon packets (ValPak), movie theaters, and on metro buses; using media outlets such as television, radio, and the internet; and having project staff attend community events such as health fairs and informational fairs for disability groups. Additionally, the research team built upon ongoing collaborative relationships with local disability leaders developed through previous studies For example, several members agreed to speak on health promotion for consumer groups. We also employed a snowball technique. Study enrollees were mailed brochures and invited to share study information with wheelchair using friends or acquaintances. They were offered a $10 store certificate as a small incentive if someone enrolled based on their referral. A detailed list of recruitment sites and activities is presented in Table 1. Notably, a wheelchair using member of the study team actively participated in study recruitment, including direct contact activities with the target population.
Table 1.
Recruitment locations and activities
| Recruitment Locations and Activities | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Displayed materials |
Mailed info to patients/clients |
Mailed info to med providers |
Hosted an exhibit table |
Posted info on website |
Posted info in newsletter |
Inserted an ad (paid or free) in newsletter |
Broadcasted/ published feature article |
Hosted speaker |
||
| Health Orgs | Health care offices and providers | x | ||||||||
| Rehabilitation facilities | x | |||||||||
| Hospitals | x | x | x | |||||||
| Physical therapy clinics | x | |||||||||
| Durable Medical Equipment vendors | x | x | x | |||||||
| Professional orgs (e.g., NASW) | x | x | x | x | ||||||
| Disability Orgs | Independent Living Centers | x | x | x | x | |||||
| State Social/Rehabiltation Services | x | x | x | x | ||||||
| Specific dx groups (e.g., MS Society) | x | |||||||||
| Transportation providers | x | |||||||||
| Colleges and University Disability Resource Offices | x | |||||||||
| Community events | Health Fairs | x | ||||||||
| Disabled Sports Expos | x | |||||||||
| Specialty health clinics (seating, SCI) | x | x | ||||||||
| Miscellaneous (C of C, YMCAs, public library, public housing) | x | x | ||||||||
| Media | ||||||||||
| Major city newspapers | x | x | ||||||||
| Small town daily papers | x | |||||||||
| Ethnic publications (spanish, etc.) | x | |||||||||
| Aging magazines | x | |||||||||
| County recreation guides | x | |||||||||
| Business advertising sheets | x | |||||||||
| Direct mail coupon packet (Val Pak) | x | |||||||||
| Public venue | ||||||||||
| News segment on ABC-affiliate | x | |||||||||
| Public buses | x | |||||||||
| Movie Theaters | x | |||||||||
| Radio (commercial & public stations) | x | |||||||||
| Radio (aired during KU basketball) | x | |||||||||
| Internet | ||||||||||
| Project website | x | |||||||||
| Social networking sites | x | |||||||||
We maintained records of all recruitment efforts and regularly reviewed the effectiveness of each to assess return on investment and to inform subsequent cohort recruitment activities. Activities yielding high contacts were continued; those resulting in low or no contacts were not.
Strategies to reduce participant burden
We also incorporated strategies to reduce participant burden and increase the likelihood that eligible individuals would choose to enroll. Transportation needs, a major participation barrier for individuals with disabilities [2], were addressed directly because our project required travel for fitness testing at four points during the year-long study enrollment (all participants) and to attend the one-time workshop (experimental group only). Participants with their own transportation were reimbursed per mile. Others were provided taxi service, with a wheelchair-lift if needed. All participants received a $25 monetary incentive for their time and effort each time they reported for fitness testing and received an additional $25 at the fourth testing if they attended the previous three testing appointments.
Obtaining signed consents from personal physicians can also burden potential participants, particularly given mobility and transportation issues. To reduce this burden we obtained participants’ written permission for us to contact their providers by FAX. Doing so also allowed us to identify when providers were the source of delays in returning consents and to communicate directly with provider offices to resolve issues.
Strategies to enhance retention during recruitment
Everyone was contacted within 24 hours of their initial inquiry. We focused on establishing a good rapport at this initial contact and maintained contact as each person moved through the screening and enrollment process. On-going contact from initial inquiry was particularly important because project recruitment activities for each cohort began three months before study initiation. Consequently, the study protocol stipulated that eligible individuals receive up to four contacts by phone and mail prior to their study start date. These contacts included receipt of a Project WOW study magnet to place on their refrigerator as a visible reminder of their upcoming participation.
A final strategy for retaining participants involved the randomization and consenting processes. All participants received an exercise intervention; the difference between the two arms involved the intensity of the intervention. To protect against the threats to study validity of resentful demoralization or compensatory rivalry [16] and to avoid this dual pitfall of control participants thinking that they did not receive the better approach, the consent process was divided into two phases. The first focused on general study parameters (e.g., trial length, testing protocol details, testing intervals, monetary incentives, risks, etc) and included information about being randomly assigned to one of two groups--only discussing commonalities between groups. The second phase occurred after randomization and detailed information specific to their assigned intervention group.
Data Analysis
SPSS version 17 [17] was used for data entry and analysis of our recruitment and retention efforts. Means and frequencies were used for participant demographic data, recruitment methods, and eligibility status. We examined whether there were significant differences between those who inquired and became study enrollees and those who declined to participate on race, age, or time waited between screening and initiation, and between those determined to be eligible versus those determined ineligible on race. These analyses used independent t-tests for continuous variables (age and days between screening and initiating) and chi square analyses for categorical variables (race).
Findings
Given the extensive recruitment activities, we wanted to know what each yielded in terms of initial inquiries. Over the 44 months of recruitment, we received 355 inquiries. All contacts were asked how or where they learned about the study. Over 92% could report how they heard about Project WOW. Figure 2 displays the relative yield of each recruitment method. The best recruitment strategy was through media sources (33.5%), although health care providers and disability service agencies also were successful sources (21.6% and 16.2%, respectively). Figure 3 depicts the number of contacts generated by the different media and health care provider sources. ValPak, a direct mail advertising company, generated the greatest response (with 57 callers citing this method), while the second best method was placing posters with brochures in waiting rooms of clinics and in local hospitals (49 calls). All recruitment activities combined resulted in a steady stream of inquiries throughout the entire enrollment period, likely due to our ongoing efforts and the posters that remained in health providers’ and durable medical equipment suppliers’ waiting rooms. The greatest influx of calls, however, occurred within 10 days following direct mailings through ValPak or airing of radio announcements. Although all healthcare providers were asked to share study brochures with patients, only 11 inquiries reported being informed directly by a healthcare provider; others reported simply picking up the brochure in healthcare settings.
Figure 2.
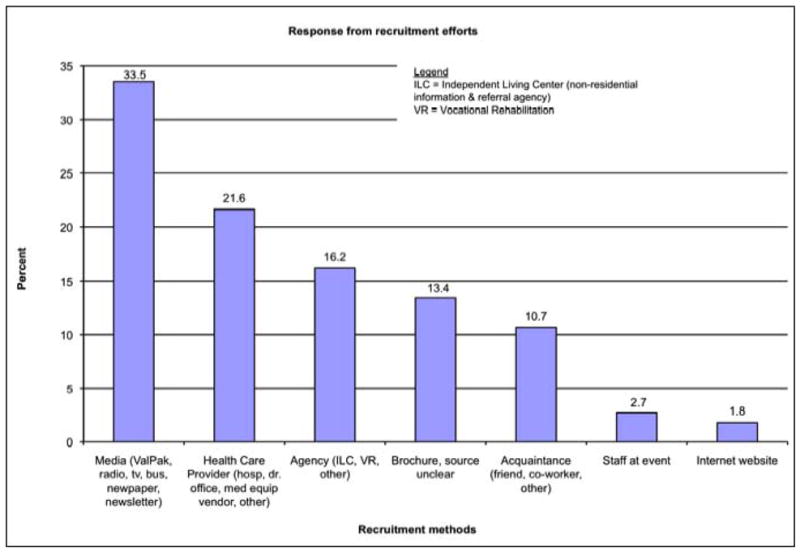
Percent of inquiries received by recruitment method cited
Figure 3.
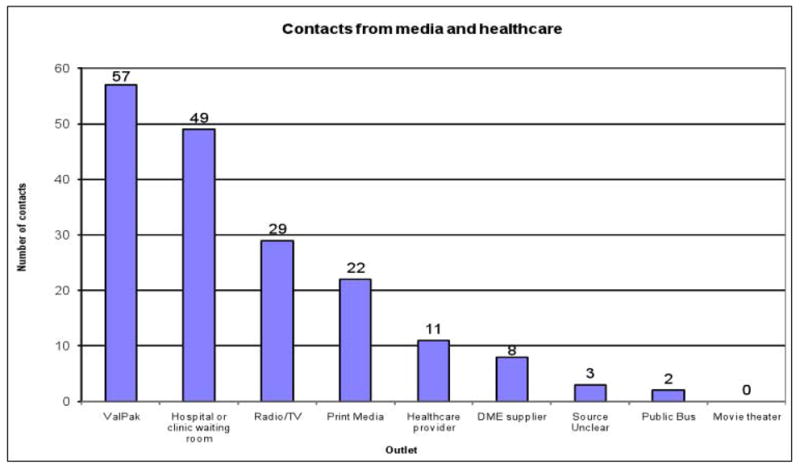
Number of inquiries received from media outlets and health care sources
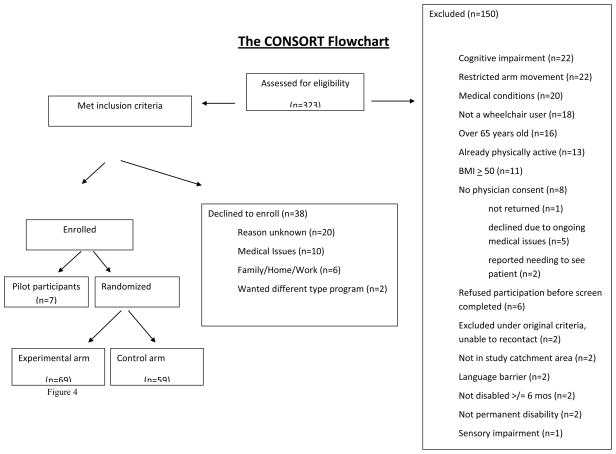
Reaching potential participants is a necessary first step; enrolling and retaining eligible participants are the ultimate goals. Of the 355 inquiries, 32 could not be reached for screening, which resulted in our screening 323 individuals (refer to Figure 2 for CONSORT diagram). While this was encouraging, nearly half were determined ineligible (150/323, 46.4%). The most common reasons were cognitive impairment that precluded compliance with study protocols, arm limitations that prevented sufficient movement for aerobic exercise, medical conditions that contraindicated unsupervised exercise, and not being a wheelchair user (Figure 2).
Of the 173 eligible participants, 135 (78.0%) initiated the program and 38 ultimately opted not to participate, 47.4% of whom reported why (Figure 2). There were no statistically significant differences between eligible individuals who enrolled versus those who declined to participate on race, age, or number of days between screening and study initiation. However, there were significant age and racial differences between those determined ineligible versus eligible. Individuals deemed ineligible were significantly older (mean age 48.9 + 13.5 years) than eligible individuals (43.8 + 12.6 years) t = −3.453, p=.001. Further, 63.9% of African Americans screened were ineligible compared to 38.1% of whites, while the ‘other’ and the ‘multiracial’ groups were equally split between ineligible (55.6% and 50.0%, respectively) and eligible (44.4% and 50.0%, respectively).
Discussion and Lessons Learned
Sustained efforts and a variety of recruitment methods and sources were used to enroll 135 participants in a randomized controlled trial of a home-based behavioral intervention for exercise adoption and maintenance among community-dwelling wheelchair users. Despite extensive efforts and extension of the initial recruiting time frame, we did not reach our original goal of 180 participants. This is not unusual and validates other investigators’ reports that recruitment is more time intensive and expensive than anticipated. [3] A recent review of RCT participant recruitment noted that 21% of trials that reported a targeted sample size failed to enroll the desired numbers. [18] It is assumed this is likely higher for studies that do not report a target sample size.
Our target population had many co-morbid conditions which made them ineligible to participate in unsupervised exercise and this contributed to our recruitment difficulties. Ineligibility due to co-morbidity also is not unique to our study. Others, targeting different populations, have found co-morbidity often leads to exclusion from RCTs. [19 20–21]
Although the significant racial difference in eligibility determination are disconcerting, further investigation does not reveal systematic bias. Of the 46 African Americans determined ineligible, most were ineligible because they were not wheelchair users (n=10), had cognitive impairment (n=8), restricted arm movement (n=7), or a BMI above 50 (n=7). Four had doctors unwilling to provide consent due to disability exacerbation (n=1), having uncontrolled high blood pressure (n=1), or the doctor reported needing to see the patient before providing medical permission (n=2); two did not have their disability at least six months; and two did not meet the original eligibility criteria, yet were unable to be contacted subsequently. Other exclusion reasons included being already physically active (n=1), having sensory impairment (n=1), having medical conditions (i.e., congestive heart failure and chronic obstructive pulmonary disease) that preclude unsupervised exercise (n=1), not having a permanent disability (n=1), refusing participation before eligibility was established (n=1), and being over 65 (n=1). Recruitment efforts for this study yielded substantial interest by African Americans, but ultimately the study did not maximize enrolling more African Americans primarily due to the eligibility criteria. Future studies should investigate strategies that promote higher participation rates in studies by African Americans.
Revisiting eligibility criteria, as we did, also is increasingly common. Others report that more flexible inclusion criteria help investigators achieve a sufficient sample size. [18–19] This flexibility, however, must be balanced with scientific rigor and patient safety, and some factors are beyond the control of investigators. For example, physician determination that someone is not medically cleared to participate in a study, physicians declining to sign medical clearance forms (as opposed to indicating the person is not medically cleared), or health problems that arise between consenting and baseline testing are all uncontrollable events. Lesson learned #1: Expect recruiting populations with disabilities, or with many co-morbidities, to be more complex than recruiting non-disabled populations.
The necessity and decision to enroll more cohorts than originally anticipated resulted in expending substantially more staff hours and advertising funds. Developing and maintaining relationships with groups and people supporting recruitment efforts takes time. Study staff met with administrators of healthcare agencies and hospitals; attended healthcare providers’ and disability service agencies’ staff meetings; gave talks to interested professional and consumer groups; and attended community events. Time and effort also were devoted to interviews with print media sources and for television and radio segments about the study. And, advertising costs were significantly higher than initially budgeted as the increased number of cohorts required over three times the number of advertising rounds than originally planned—also resulting in greater staff time to identify new advertising venues, determine pricing structures, follow each entity’s advertising material requirements, and sign contracts. Advertising was expensive across all venues (direct mail, radio, newspapers, buses, movies) and although ValPak was the most expensive, it was also the most cost effective. ValPak ads cost the study $205 per inquiry, radio ads cost $316 per inquiry, and print media ads cost $398 per inquiry.
Lesson learned #2: Anticipate devoting more time and resources to recruitment than initially believed necessary.
The name of our study, Project Workout on Wheels, or Project WOW, our logo featuring a wheelchair user in motion, and our use of a wide variety of recruitment methods and venues, garnered recognition of our study and enhanced recruitment. Many participants reported hearing about the study several times before actually calling and many who called reported receiving study brochures from acquaintances who obtained them in physician offices or other places, further validating our practice of widely and repeatedly disseminating materials. Lesson learned #3: Achieving visibility and recognition takes time but pays off.
Initial recruitment strategies relied heavily on working with rehabilitation specialists within the healthcare system. These recruitment avenues are not always open to investigators wishing to identify study participants. Nine medical centers in the metro area agreed to assist with recruitment activities, but four others declined. One large health insurer agreed to provide study information to employees, but not to their enrollees. It is not clear why these groups refused to allow recruitment efforts, as most did not give a reason or responded very generally when asked. Institutional refusals may have related to concern that the study might identify potentially unflattering information about these institutions. Whatever the reasons, the refusal of some organizations to allow study information to be disseminated to their patients and clients made study recruitment even more difficult. Lesson learned #4: Don’t assume health care agencies will value research activities and welcome and facilitate study recruitment.
Connecting directly with target populations is essential for recruitment efforts. Self-directed wheelchair users without contraindications for unsupervised exercise generally are well integrated into the community, but reaching these individuals in venues outside traditional health care facilities requires considerable effort to identify and approach numerous community-based organizations and businesses. While this resulted in increased study visibility, it did not yield high responses. Many groups declined to participate (e.g., a national retail pharmacy chain). We also used social networking sites. Although this method required little time and minimal cost, it yielded few inquiries. This may be a more promising method in the future as more individuals with disabilities adopt this technology.
We also posted study information free of charge on the local chamber of commerce website to reach local employers. However, reaching out to the business community was unproductive. Efforts to directly promote the study to several large employers generated no interest and no one cited the chamber of commerce as an information source. For our study, this may be due to low numbers of persons using wheelchairs being employed at a single business. Nonetheless, identifying internal “gatekeepers” within organizations who have the authority to act on a study recruitment request and gaining their support may facilitate recruitment. Lesson learned #5: Nurturing relations with key gatekeepers is essential throughout recruitment.
Summary/Conclusions
Recruiting participants into trials, especially from populations with a low prevalence, poses many challenges. In particular, we found recruiting wheelchair users into an exercise trial to be more time intensive and expensive than originally expected. The two most effective methods—placing paid advertisements and disseminating study materials in health care provider facilities and offices—still generated fewer participants than expected. Creative thinking and trying less typical approaches, such as advertising, through direct mail coupons were more productive than traditional approaches to raise visibility and increase contacts. Revising eligibility criteria without reducing scientific rigor and maintaining ongoing contact with participants between screening and enrollment, as well as throughout the study, also were important.
In sum, awareness of study population-specific situations, such as a high incidence of co-morbidities, creating study identity and visibility, nurturing relations with gatekeepers of institutions from which one seeks study participants, thinking creatively about media and advertising options, and planning for the necessary time and expense of study recruitment, are all essential lessons for successful recruitment.
Figure 4.
CONSORT diagram
Acknowledgments
Data from project funded by NIH grant #R01 HD048628-01
Footnotes
The authors declare no conflicts of interest.
Clinical trial #NCT00866112
Previous presentation of all or partial material:
Nary, D.E., Nesbitt-Daly, J., & Grobe, K.F. (2008, October). Recruiting wheelchair users for an exercise intervention. Poster presentation at the American Public Health Association Annual Meeting, San Diego, CA.
Nary, D.E., Froehlich-Grobe, K., & Aaronson, L. (2010). Recruitment issues in a randomized controlled exercise trial targeting wheelchair users. Poster to be presented at the Scholars Summer Workshop for trainees receiving support through the NIH/NICHD Supplements to Promote Diversity in Health-Related Research, August 11–12, 2010, Washington, DC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aitken L, Gallagher R, Madronio C. Principles of recruitment and retention in clinical trials. International Journal of Nursing Practice. 2003;9(6):338–46. doi: 10.1046/j.1440-172x.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas DD, Yilmaz B. Recruitment of spinal cord injury patients to clinical trials: Challenges and solutions. Top Spinal Cord Injury Rehabilitation. 2006;11(3):12–23. [Google Scholar]
- 3.Blanton S, Morris DM, Prettyman MG, McCulloch K, Redmond S, Light KE, et al. Lessons learned in participant recruitment and retention: The EXCITE Trial. Physical Therapy. 2006;86(11):1520–33. doi: 10.2522/ptj.20060091. [DOI] [PubMed] [Google Scholar]
- 4.Treweek S, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, Sullivan F, Wilson S, Jackson C, Jones R, Mitchell E. Strategies to improve recruitment to randomised controlled trials. Cochrane Database of Systematic Reviews. 2010;(4) doi: 10.1002/14651858.MR000013.pub5. Art. No.: MR000013. [DOI] [PubMed] [Google Scholar]
- 5.Morton LM. Encouraging participation in medical research: What strategies work? Journal of Clinical Epidemiology. 2008;61:969–70. doi: 10.1016/j.jclinepi.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorsey ER, de Roulet J, Thompson JP, Reminick JI, Thai A, White-Stellato Z, et al. Funding of US Biomedical Research, 2003–2008. Journal of the American Medical Association. 2010;303(2):137–43. doi: 10.1001/jama.2009.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell KR, Hammond F, Bickett AK, Temkin AK, Dikmen S. Participant recruitment and retention in rehabilitation research. American Journal of Physical Medicine & Rehabilitation. 2008;87:330–8. doi: 10.1097/PHM.0b013e318168d092. [DOI] [PubMed] [Google Scholar]
- 8.Auster J, Janda M. Recruiting older adults to health research studies: A systematic review. Australasian Journal on Ageing. 2009;28(3):149–51. doi: 10.1111/j.1741-6612.2009.00362.x. [DOI] [PubMed] [Google Scholar]
- 9.USDHHS. Healthy People 2010: Understanding and Improving Health. 2. Washington DC: USDHHS. US Government Printing Office; 2000. Chapter 6: Disability and secondary conditions. [Google Scholar]
- 10.USDHHS. Physical activity and health: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 11.USDHHS. The Surgeon General’s call to action to improve the health and wellness of persons with disabilities. USDHHS, Office of the Surgeon General; 2005. [PubMed] [Google Scholar]
- 12.Kosma M, Cardinal BJ, McCubbin JA. Recruitment techniques among understudied populations and their implications for physical activity promotion. QUEST. 2004;56:413–20. [Google Scholar]
- 13.Stuifbergen AK. Building health promotion interventions for persons with chronic disabling conditions. Family & Community Health. 2006;29S:28S–34S. doi: 10.1097/00003727-200601001-00006. [DOI] [PubMed] [Google Scholar]
- 14.Calamaro CJ. Culture competence in research: Research design and subject recruitment. Journal of Pediatric Health Care. 2008;22:329–332. doi: 10.1016/j.pedhc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Kitchin R. The researched opinions on research: disabled people and disability research. Disability & Society. 2000;15:25–47. [Google Scholar]
- 16.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston, MA: Houghton Mifflin Company; 2002. [Google Scholar]
- 17.SPSS Inc. SPSS Base 16.0.2 for Windows User’s Guide. Chicago: SPSS Inc; 2008. [Google Scholar]
- 18.Toerien M, Brookes ST, Metcalfe C, de Salis I, Tomllin Z, Peters TJ, et al. A review of reporting of participant recruitment and retention in RCTs in six major journals. Trials. 2009;10(52) doi: 10.1186/1745-6215-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott MM, Domanchuk K, Dyer A, Ades P, Kibbe M, Criqui M. Recruiting participants with peripheral arterial disease for clinical trials: Experience from the Study to Improve Leg Circulation (SILC) Journal of Vascular Surgery. 2009;49:653–9. doi: 10.1016/j.jvs.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guihan M, Garber SL, Bombardier CH, Durazo-Arizu R, Goldstein B, Holmes SA. Lessons learned while conducting research on prevention of pressure ulcers in veterans with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2007;88(7):858–61. doi: 10.1016/j.apmr.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Spilker B, Cramer JA. Patient recruitment in clinical trials. NY: Raven; 1992. [Google Scholar]