Abstract
Background
Multiple sclerosis is a progressive neurological disease that results in a high incident of gait disturbance. Exploring the frequency content of the ground reaction forces generated during walking may provide additional insights to gait in patients with multiple sclerosis that could lead to specific tools for differential diagnosis. The purpose of this study was to investigate differences in the frequency content of these forces in an effort to contribute to improved clinical management of this disease.
Methods
Eighteen patients and eighteen healthy controls walked across a 10 meter long walkway. The anterior-posterior and vertical ground reaction forces generated during the stance phase of gait were evaluated in the frequency domain using fast Fourier transformation. T-tests were utilized for comparison of median frequency, the 99.5% frequency, and the frequency bandwidth between patients and healthy controls and also for comparisons between patients with mild and moderate severity.
Findings
Patients with multiple sclerosis had significantly lower 99.5% frequency (p =0.006) and median frequency (p <0.001) in the vertical ground reaction force. No differences were found in the anterior-posterior reaction force frequency content. There were no differences between patients with mild and moderate severity.
Interpretation
The lower frequency content suggests lesser vertical oscillation of the center of gravity. Lack of differences between severities may suggest presence of differences prior to currently established diagnosis timelines. Analysis of the frequency content may potentially serve to provide earlier diagnostic assessment of this debilitating disease.
Keywords: gait, Fourier transformation, power spectrum, frequency analysis, locomotion
Introduction
Multiple Sclerosis (MS) is a progressive neurological disease that results in demyelination of the axons followed by the formation of dendritic scars which preclude the repair of the damaged axons (Noseworthy et al., 2000). The result of axon demyelination is slowed or blocked nerve conduction rates (Jones, 2008). MS is characterized by an array of possible symptoms such as optic neuritis, limb weakness, neurogenic bowel and bladder problems, depression, vertigo, spasticity, and other symptoms of central nervous system dysfunction (Noseworthy et al. 2000, Snook, Motl, 2009). Additionally, MS has a large impact on mobility as approximately 50% of those diagnosed with MS will advance to a stage that will require the use of a walking aid within 15 years of initial diagnosis (Confavreux, Vukusic, 2006, Tremlett, Paty & Devonshire, 2006).
Because of the strong presence of ambulatory deficits, mobility is used extensively to characterize disease severity and progression of MS (Kurtzke, 1983, Snook, Motl, 2009). Kurtzke's detailed expanded disability status scale (EDSS) is one of the most common classifications for MS (Kurtzke, 1983). The scale ranges from 0 – 10 with 0.5 point increments and is based on impairments of 8 different functional systems, but relies heavily on ambulatory ability. Scores of 6.5 and higher are marked by constant use of bilateral walking aids while scores of 6.0 and below are consistent with independent walking or intermittent use of walking aid to cover specified distances (Kurtzke, 1983). This scale is limited, however, in that it does not discriminate in the method by which the person is able to traverse the specified distance and therefore does not tell how the person moves. For example, an individual that is able to walk 100 meters slowly and in an unsteady fashion with numerous gait compensations is classified the same as someone that is able to walk 100 meters in a steady smooth manner but then must rest due to fatigue.
Gait analysis has also been used to classify mobility of patients with MS but has been largely limited to functional assessments and use of spatial and temporal measures. Slower walking speed, shorter stride length, and longer double stance times compared to healthy controls have been reported, while differences in joint motion at the ankle and muscle firing timing in the lower extremities have also been found (Benedetti et al., 1999, Martin et al., 2006). Gait analysis for patients with MS has not been performed to the extent that allows a comprehensive overview of the mechanical differences present. Evaluation of the mechanical differences may be able to provide a more detailed register of disease progression than temporal and spatial measures or the EDSS.
One tool to evaluate mechanical changes in walking is frequency domain analysis which provides the ability to examine the entire gait cycle and not just specific discrete points such as a maximum or a minimum value of a joint angle during the gait cycle. Frequency domain analysis provides details about the collection of frequencies that compose a particular signal (Giakas, 2004). In gait analysis, a resultant signal is the sum of multiple oscillations of movement that ultimately lead to the desired motion. Possible signals that could be analyzed from a walking pattern include the joint angular movement patterns of the ankle, knee, and hip or the ground reaction forces produced during the stance phase. Frequency domain analysis of the ground reaction forces (GRF) that are produced as forces applied against the ground during the stance phase of walking has previously been used to assess healthy and pathological gait and has proven to be effective in outlining differences between such populations (Stergiou et al., 2002, Giakas et al., 1996, Tsepis et al., 2004). Stergiou et al. (2002) investigated the frequency domain in an elderly population and found significantly decreased frequency content in the anterior-posterior direction compared to the healthy young controls. This was attributed to decreased forward speed which is common among elderly ambulators. Giakas et al. (1996) examined the frequency domain of GRF in scoliosis patients. Their study was important as they also examined common time-dependent measures. They found significantly higher frequency domain content in all three planes with the largest effect in the medial-lateral direction. They found no significant differences in the time-dependent measures. This is valuable considering scoliosis is a tri-planar spinal deformity that seems to affect balance and walking in all three planes and yet common time-dependent measures could not detect any differences (Giakas et al., 1996).
Thus, it is possible that frequency analysis of GRF may be of benefit in patients with MS since it may detect important differences in gait mechanics of these patients that have not previously been discussed in the related literature. In order to assess the effect that MS has on gait, GRF frequency analysis can provide quantitative information about the resultant forces produced during walking. GRFs are the resultant reaction forces broken down into the three different planar components: vertical, anterior-posterior, and medial-lateral (Winter, 1991). Any differences in these specific resultant reaction force vectors may be considered differences in the forward motion (anterior-posterior component), stabilization of side-to-side motion (medial-lateral component), or the proper maintenance of an upright position (vertical component). All of these components are regulated by precise muscle activation patterns and any differences in the frequency content would point to an alteration in the underlying neuromuscular control when comparing patients with MS and healthy controls.
The aim of this study was to investigate the frequency content of the GRF of patients with MS during over-ground walking. We compared the frequency content of the GRF signals in patients with MS to healthy age-matched controls to determine if any differences in the frequency content were present. These differences in patients with MS that are otherwise healthy would likely be due to changes in the neurological system that results from the MS disease process. It was hypothesized that the frequency content of the GRF for patients with MS would be different from healthy controls. Furthermore, as MS is a progressive disease, we expected differences between patients with mild and moderate severity levels according to EDSS classification.
Methods
Participants
Eighteen patients with MS (age mean 45.3 (SD 9.7) yrs; EDSS mean 3.9 (SD 1.6)) and eighteen age-matched healthy controls (age mean 39.2 (SD 11.0) yrs) participated in this study (Table 1). The majority of subjects were female as a reflectance of MS prevalence in the general population (Noseworthy et al., 2000). Patients provided informed consent and all procedures were approved by the University’s Medical Center Institutional Review Board. Participants were recruited through the University’s Medical Center. Inclusion criteria for the study included: 1) cognitive competency to give informed consent, and 2) age ranging from 19 years to 65 years. Additional inclusion criteria for patients with MS was an EDSS score of 1.0 – 6.0 and it was necessary that there was evidence that the MS patient’s physical and neurological examinations are “clinically acceptable”, where evidence is required that the MS patient's physical and neurological condition would not place the patient in undue risk by participating or interfere with outcome measures of the study. Exclusion criteria for the study included: 1) inability to give informed consent, 2) an EDSS score greater than 6.0 or inability to walk 25 feet with the use of a cane (patients with MS only), 3) pregnancy, breastfeeding, or within three months post partum at the initiation of the study, and 4) any other neurological or vestibular disorder. The patients with MS were also divided into a mild severity group with EDSS 0–3.0 (n = 16 limbs; EDSS mean 2.4 (SD 0.5); age mean 45.5 (SD 10.8) yrs) and a moderate severity group with EDSS 3.5–6.0 (n = 20 limbs; EDSS mean 5.2 (SD 1.1); age mean 45.2 (SD 9.3) yrs).
Table 1.
Subject demographics.
| Clinical Characteristic |
MS | Control | ||
|---|---|---|---|---|
| Mild Severity Mean(SD) (n=16 limbs) |
Moderate Severity Mean(SD) (n=20 limbs) |
Combined Mean(SD) (n=36 limbs) |
Mean(SD) (n=36 limbs) |
|
| Gender | 14 f, 2 m | 18 f, 2 m | 32 f, 4 m | 15 f, 3 m |
| Age (years) | 45.5 (10.8) | 45.2 (9.3) | 45.3 (9.7) | 39.2 (11.0) |
| Body Mass (kg) | 97.9 (14.0)* | 67.3 (12.6)* | 80.9 (20.2)† | 70.8 (11.7)† |
| Height (cm) | 167.3 (6.0) | 163.9 (9.8) | 165.4 (8.4)† | 170.9 (7.4)† |
| Walk Velocity (m/s) | 1.1 (0.2)* | 0.9 (0.1)* | 1.0 (0.2)† | 1.2 (0.3)† |
| EDSS | 2.4 (0.5)* | 5.2 (1.1)* | 3.9 (1.6) | |
Significant difference between mild and moderate severity groups.
Significant difference between combined patients with MS and controls. (p<0.05)
Gait Analysis Procedures
Subjects walked across a 10 meter long walkway with an embedded 60 cm × 40 cm force platform (Kistler 9281B, Kistler Instrumentation Corporation, Amhurst, NY). The force platform is colored to match the walkway to prevent targeting. The starting point was determined by having the subject perform two practice walkovers. For these practice trials, the subject was told that we were checking the focus of the cameras. For the collection trials, subjects walked at a comfortable, self-selected pace while GRFs were captured at a sampling rate of 600 Hz. We collected one limb at a time, as only one force platform was available in the laboratory, so each limb was analyzed separately. The limb collected first was randomly selected. Upon completion of each trial and prior to the subsequent, subjects were instructed to rest for 1 minute to ensure that fatigue did not confound the results. Data was collected from heel contact to toe off on the force platform, representing an entire stance phase. This process was repeated until five successful walkovers were obtained. A walkover was defined as successful if the foot of interest, and only the foot of interest, landed entirely on the force platform. This typically meant a total of 5–7 walkovers per leg. Next, the contralateral limb was collected using the same process. For a few subjects, the starting point was adjusted after the first contralateral trial. The five trials from each limb were individually analyzed for frequency content. The frequency content outcome variables of all trials for a single limb on a subject were averaged to create one value per limb for each outcome variable. This analysis procedure yielded 36 limbs for the MS group and 36 limbs for the healthy controls.
Frequency Analysis
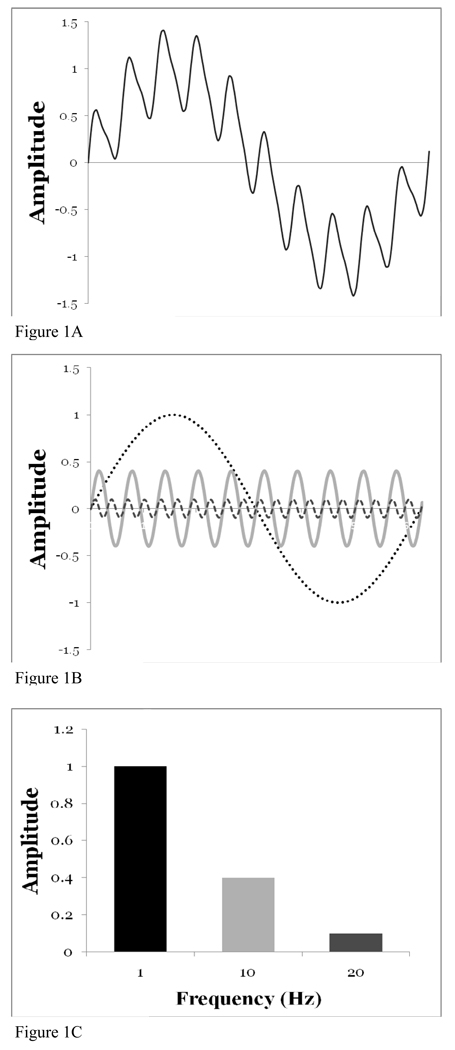
Frequency analysis was performed by converting the anterior-posterior and vertical GRF curve into the frequency domain using fast Fourier transformation. Because walking is dominated by forward motion and maintaining vertical position, only the anterior-posterior and vertical signals were evaluated. The fast Fourier transform function within the analysis package of MATLAB (Matlab 7.0, Mathworks, Inc., Concord, MA) was utilized. The fast Fourier transformation (FFT) is a method of calculating the content frequencies within a signal and has been used previously on GRF signals (Giakas, 2004, Stergiou et al., 2002). Through Fourier analysis, a signal can be thought of as the resultant of many different signals summed together. For example, in Figure 1A, a simple signal is shown. Upon inspection, it is clear that Figure 1A is a sum of 3 simple sine waves with amplitudes of 1, 1/10, and 1/20 and frequencies of 1, 10, and 20 (Figure 1B). A biological signal resulting from the ground reaction forces can be similarly thought of as the summation of an infinite number of sine and cosine waves with varying frequencies and amplitudes created from the movement of different anatomical components (i.e. joints, muscles, bones, nerves, etc.). Through the breakdown of the signal, FFT will calculate the amount of movement (amplitude) at each frequency. The collection of all the frequencies is termed the power spectrum and may be plotted as frequency versus amplitude (Figure 1C). A biological signal would be expected to have more frequency components than an artificially produced signal.
Figure 1.
A) Artificial signal resulting from summation of three different sine waves occurring with different frequencies and amplitudes. B) Signal from 1a broken down into its three component sine waves. In this manner it is much easier to see the dominant large amplitude sine wave as well as the higher frequency waves contributing to the summed signal. C) Power spectrum of artificial signal (Fig 1A) shows plot of frequency vs. amplitude for 3 sine waves in decomposed signal (Fig 1B).
From the power spectrum curve, it is possible to calculate different dependent variables of interest. Based on previous studies (Giakas, 2004, Stergiou et al., 2002) and our pilot work, we chose the dependent variables to be investigated in this study as: 99.5% frequency, median frequency, and frequency bandwidth. When considering frequency in relation to gait, it is important to consider walking is a relatively slow movement; consider a person walking on average between 60–120 steps per minute, or 1–2 steps per second (steps*s−1). This means the cadence frequency is only 1–2 Hz. For this reason, we would expect that the majority of the signal would be contained at a lower frequency. The 99.5% frequency (f99.5) is that which contains 99.5% of our signal. It is calculated as:
| (1.1) |
where P is the power calculated as the integral of the frequency versus amplitude signal plot and f max is the maximum frequency of the signal. The median frequency (fmed) occurs at the point at which half the signal power is above and half of it is below. It is calculated as follows:
| (1.2) |
where P is the signal power and fmax is the maximum frequency of the signal. The frequency bandwidth (fband) is a difference between maximum and minimum frequencies when the power is at greater than half the maximum power (Giakas, 2004, Stergiou et al., 2002). It is calculated as follows:
| (1.3) |
Statistical Analysis
Independent t-tests were performed to examine differences between patients with MS and healthy controls (patients with MS n=36; healthy controls n=36) for each of the dependent variables. With only one embedded force platform, the right and left limbs were collected separately and thus considered statistically independent for the analysis. In order to test for differences between patients with mild and moderate severity levels, patients with MS were grouped according to EDSS as either mild severity (score 0–3.0; n = 16 limbs) or moderate severity (score 3.5–6.0; n = 20 limbs). Mann-Whitney t-tests were used for comparisons between the two groups. The alpha-level for significance was set at 0.05. Furthermore, to verify any significant measures were not affected by velocity, we adjusted for velocity by employing an ordinary least squares regression. Visual inspection of P-P plots suggested that there were no violations of model assumptions. The ordinary least squares regression model assumes that residuals are independently and identically distributed normally. Ordinary least squares regression treat two variables as independent and is then able to assess significance while accounting for the effect from both variables.
Results
MS showed significantly lower 99.5% frequency in the vertical direction (p = 0.006) and significantly lower median frequency in the vertical direction (p < 0.001) compared to healthy controls. Frequency bandwidth in the vertical direction was not different between patients with MS and healthy controls (p = 0.191). In the anterior-posterior direction, there were no differences between MS and healthy controls for 99.5% frequency (p = 0.930), median frequency (p = 0.072), or frequency bandwidth (p = 0.193) (Table 2). The average walking velocity was significantly lower (p < 0.001) for patients with MS (mean 1.02 (SD 0.19) m/s ) compared to healthy controls (mean 1.23 (SD 0.25) m/s).
Table 2.
GRF frequency content values (Hz) for anteroposterior and vertical directions for MS subjects and healthy controls.
| MS Subjects Mean (SD) (n=36) |
Controls Mean (SD) (n=36) |
p-value | ||
|---|---|---|---|---|
| 99.5% | A–P | 15.20 (8.73) | 15.36 (5.87) | 0.930 |
| Vertical | 4.07 (0.77) | 4.54 (0.62) | 0.006* | |
| Median | A–P | 0.96 (0.79) | 1.30 (0.79) | 0.072 |
| Vertical | 0.31 (0.06) | 0.39 (0.07) | 0.001* | |
| Bandwidth | A–P | 1.13 (0.75) | 1.37 (0.81) | 0.193 |
| Vertical | 0.53 (0.09) | 0.56 (0.08) | 0.191 | |
Sig, p < 0.05
To adjust for velocity, we employed an ordinary least square regression for those outcomes with significant differences. The 99.5% frequency in the vertical direction was significantly different between patients with MS and healthy controls (p=0.047) while adjusting for any affects from velocity (p=0.225). Median frequency in the vertical direction was significantly different between patients with MS and healthy controls (p<0.001) while adjusting for any affects from velocity (p=0.453). Results showed velocity did not significantly affect the outcomes and our multivariate analysis results were consistent with our bivariate results.
When MS subjects were divided into groups based on EDSS scores, there was no differences between the mild and moderate groups in the vertical direction for 99.5% frequency (p = 0.086), median frequency (p = 0.941), or frequency bandwidth (p = 0.493) (Table 3). Similarly, there were no differences between the mild and moderate severity groups in the anterior-posterior direction for 99.5% frequency (p = 0.348), median frequency (p = 0.534), or frequency bandwidth (p = 0.493) (Table 3).
Table 3.
GRF frequency content values (Hz) for anteroposterior and vertical directions for MS subjects divided into two groups according to severity (EDSS 0–3.0 and EDSS 3.5–6.0). No significant differences were found.
| MS EDSS 0–3.0 Mean (SD) (n=16) |
MS EDSS 3.5–6.0 Mean (SD) (n=16) |
p-value | ||
|---|---|---|---|---|
| 99.5% | A–P | 12.82 (4.36) | 18.16 (11.11) | 0.348 |
| Vertical | 4.21 (0.51) | 3.96 (0.92) | 0.086 | |
| Median | A–P | 0.80 (0.53) | 1.13 (0.94) | 0.534 |
| Vertical | 0.32 (0.05) | 0.31 (0.06) | 0.941 | |
| Bandwidth | A–P | 0.96 (0.49) | 1.31 (0.88) | 0.493 |
| Vertical | 0.54 (0.06) | 0.52 (0.11) | 0.510 | |
Sig, p < 0.05
Discussion
The purpose of this study was to compare and measure any differences in the frequency domain of GRFs in patients with MS and healthy controls during over-ground walking. The use of frequency domain analysis in gait measures allows for analysis of the entire gait cycle instead of discrete points or events in the cycle (Stergiou et al., 2002, Giakas et al., 1996). We hypothesized that patients with MS would exhibit differences in frequency domain during walking in the vertical and anterior-posterior directions. The only differences found were in the vertical direction where the patients with MS had 99.5% frequency and median frequency that were significantly decreased compared to healthy controls. Decreases in the frequency components in patients with MS indicates a loss of higher frequency phenomena in the GRFs during walking that are otherwise present in healthy controls. Furthermore, it was found that these differences persisted when the outcomes were adjusted for velocity, signifying that the differences found were in fact due to the effect of MS and not simply a result of the two groups walking at different speeds.
Interestingly, frequency analysis of surface electromyographic (EMG) signals has been used for purposes of quantifying fatigue (Judkins et al., 2006). Increased muscle fatigue has been shown to correlate with decreased median frequency; while increased frequency bandwidth corresponds to recruitment of additional motor units (Judkins et al., 2006). Although GRF and EMG are entirely different entities, it is interesting to consider that we have shown that patients with MS have decreased median frequency of their vertical GRF. This seems to correspond well with the fact that fatigue is one of the most common symptoms of MS (Noseworthy et al. 2000). This may also explain why we did not find any significant differences in the frequency bandwidth; patients with MS have motor control problems resulting from the altered nerve conduction which does not necessarily mean increased motor unit recruitment.
We also investigated whether any differences would be seen in frequency components between patients with MS at a mild severity level (EDSS 0–3.0) compared to a moderate severity level (EDSS 3.5–6.0). Results showed no differences between the mild and the moderate groups. The lack of any differences between groups leads us to believe that the decay of higher frequency phenomena is happening prior to any clinical diagnosis. Practically, the EDSS score is given to individuals after a diagnosis has been made at which time it appears that the loss of higher frequency phenomena has already occurred. Interestingly, when the two groups were compared separately to healthy controls, the differences in the vertical direction median frequency persisted for both groups but the 99.5% frequency was not significant for the mild severity group (p=0.088). This would seem to imply a stronger effect for median frequency in the vertical direction.
Frequency domain analysis has the advantage of exploring movement as a result of oscillations. Many discrete time-dependent measures compare values or timing of minima and maxima of movement or force. Frequency domain analysis is able to consider multiple component frequencies. The frequency of a movement is independent of the amount of time to perform a task as the frequency of movement will only increase/decrease based on the number of oscillations that occur per second (i.e. if a 1 Hz movement is done in one second then only one oscillation has occurred, but if it is done over 0.5 seconds then only half an oscillation has occurred but the frequency measure has not been affected). Normal walking has certain characteristic movement frequencies resulting from the interaction of joints, bones, muscles, nerves, and other anatomical features and that divergence from these frequencies results from differences in the interactions of the nervous system and the muscular system required for healthy gait. Frequency analysis can determine differences in the frequencies present from these different interactions, but with the limitation of not specifically noting which interaction is problematic. In a patient with MS that has no other underlying health conditions, it is feasible to suggest that the differences in frequencies are a result of problems within the neuromuscular system.
The frequency domain content in the patients with MS had significantly lower median frequency and 99.5% frequency in the vertical GRF. The marked lower frequency is consistent with slower oscillations in movement. This would be expected in a slower, conservative movement, most likely explained by the altered nerve signal conduction associated with MS. As a result of demyelination, nerve fiber function is compromised by slowing axonal conduction velocity (White, Dressendorfer, 2004). With slowed axonal conduction velocity and signal leakage, patients with MS experience slowed muscle activation of the lower extremities. This would be reflected by the decreased median frequency and 99.5% frequency in the vertical direction. Frequency analysis of GRF has previously shown its potential to discriminate changes associated with aging most likely due to changes in the neuromuscular system. Stergiou et al.(2002) were able to find distinct frequency content differences in ground reaction forces from walking in healthy young individuals and healthy elderly individuals. Differences in the anterior-posterior direction were present but not in the vertical direction. The authors attributed the differences in frequency characteristics to decreased walking speed associated with the elderly group. However, we feel in light of the current findings, it is more plausible that they actually measured differences in the fore-aft oscillation of the center of gravity and not necessarily the resultant forward velocity. This would seem to explain our results; although our patients with MS walked slower, they did not have differences in the frequency of the fore-aft oscillation of their center of gravity. The findings of Stergiou et al. (2002) and the present study suggest that the changes in frequency component organization may be affected differently by aging (anterior-posterior differences) and by certain pathologies such as MS (vertical differences). This is important as problems with vertical GRF, which reflects the support forces, may lead to increased need for cane and/or wheelchair. Pittock et al. (2004) reported that in a 10 year prospective study with patients with MS that were ambulatory without the use of a cane or wheelchair (EDSS 3–5), half of these patients required a cane or wheelchair to maintain mobility at the end of the follow-up period.
Based on the above results, we believe that frequency analysis of the GRFs could potentially serve as an additional screening tool. We have successfully shown that differences are present between patients with MS and healthy controls. It is not known, however, at what point in the disease progression the differences became significant. As previously mentioned, MS is a progressive neurological disorder, thus as the disease progresses, demyelination of axon sheaths would continue and action potentials would continue to have decreased conduction velocity and increased signal leakage resulting in slower, lower frequency movements (Noseworthy et al., 2000). In this manner, the loss of high frequency phenomena in MS gait may be a simple on/off type of mechanism that becomes apparent prior to current clinical diagnosis. It is also possible that the loss of high frequency phenomena is progressive in a stepwise manner such that those MS patients that are able to ambulate without assistive devices, such as our subjects, are in a specific window of the frequency content and progression to the use of assistive devices and eventually non-ambulatory states may have further decreases. Unfortunately, a limitation of GRF frequency analysis is that it may be difficult to get successful GRF signals from individuals that require assistive devices. Future work will attempt to address the point in disease progression where differences in GRF frequency become notable and whether there is further high frequency loss in individuals with EDSS greater than 6. It is important to note that in many MS cases, the patients' symptoms persist for years before any imaging or lumbar puncture evidence leads to an MS diagnosis which delays pharmacological or therapeutic interventions which may successfully slow disease progression (Anlar, 2009, Veugelers et al., 2009). Our study showed that frequency components were altered in all patients with MS regardless of severity since there were no differences between EDSS groups. Thus alterations in frequency components are present even in mildly affected patients and may be evident before clinical diagnosis. It is possible that the use of GRF frequency analysis could help with differential diagnosis leading to earlier MS screening and ultimately diagnosis and intervention.
It is of note that our study did not control for walking velocity but rather subjects walked at self-selected comfortable walking pace. This was done to preserve the dynamics of each individual's gait. Altering walking speed affects muscle activation patterns (Sinkjaer et al., 1996), muscle fiber length behavior (Cronin et al., 2009), and certain stride parameters (Dingwell et al., 2010). It is possible that forcing our subjects to walk at a controlled velocity would have changed our results. However, based on our ordinary least squares regression analysis, we are confident that despite that patients with MS walked with slower velocity than our healthy controls, differences in the vertical direction frequency content are in fact a direct result of the underlying changes from MS. Another limitation was that our subjects' body masses were significantly different which may affect GRF. However, the weight of our subjects would most likely affect amplitudes of forces but not necessarily the frequency content. Further analysis with weight matched subjects should be considered.
Conclusion
Our results show that the frequency components of GRFs in patients with MS are significantly lower than healthy controls and are altered regardless of EDSS classification. The lower frequency content indicates that patients with MS are adapting lower oscillation frequencies in their gait compared to healthy controls as a result of an altered neuromuscular system. This movement pattern is most likely a result of the slowed nerve conduction symptomatic of MS, a result of demyelination of the axon sheath.
This study has shown that frequency analysis of GRFs can be used as an effective tool for identifying pathological gait in MS. It is possible that a successful intervention, either exercise or pharmacological, could result in improved frequency content of the GRF signal. More studies are necessary to assess use of GRF frequency analysis in patients with MS as an outcome measure for treatment and its potential use as an early screening tool. In addition, future work should incorporate electromyography as this may help provide additional insights to correlations between muscle activity and GRF frequency components.
Acknowledgments
We kindly acknowledge Mr. Jeffrey Kaipust and Mr. Ryan Hasenkamp for their help in data collection and processing. This work was funded by the MARS Foundation, a University of Nebraska Medical Center College of Public Health Graduate Fellowship, and the Nebraska Research Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anlar O. Treatment of multiple sclerosis. CNS & neurological disorders drug targets. 2009;vol. 8(no. 3):167–174. doi: 10.2174/187152709788680670. [DOI] [PubMed] [Google Scholar]
- Benedetti MG, Piperno R, Simoncini L, Bonato P, Tonini A, Giannini S. Multiple Sclerosis. no. 5. vol. 5. Houndmills, Basingstoke, England: 1999. Gait abnormalities in minimally impaired multiple sclerosis patients; pp. 363–368. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain : a journal of neurology. 2006;vol. 129(no. 3):606–616. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- Cronin NJ, Ishikawa M, Grey MJ, af Klint R, Komi PV, Avela J, Sinkjaer T, Voigt M. Mechanical and neural stretch responses of the human soleus muscle at different walking speeds. Journal of Physiology. 2009;vol. 587(no. 13):3375–3382. doi: 10.1113/jphysiol.2008.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell JB, John J, Cusumano JP. Do humans optimally exploit redundancy to control step variability in walking? PLoS Comput. Biology. 2010;vol. 6(no. 7):e1000856. doi: 10.1371/journal.pcbi.1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakas G. Power Spectrum Analysis and Filtering. In: Stergiou N, editor. Innovative Analyses of Human Movement. Champaign, IL: Human Kinetics; 2004. pp. 223–258. [Google Scholar]
- Giakas G, Baltzopoulos V, Dangerfield PH, Dorgan JC, Dalmira S. Comparison of gait patterns between healthy and scoliotic patients using time and frequency domain analysis of ground reaction forces. Spine. 1996;vol. 21(no. 19):2235–2242. doi: 10.1097/00007632-199610010-00011. [DOI] [PubMed] [Google Scholar]
- Jones P. 1/31/2008-last update. Multiple Sclerosis Encyclopaedia. 2008 Available: http://www.mult-sclerosis.org/myelin.html [2010, 04/25]
- Judkins TN, Oleynikov D, Narazaki K, Stergiou N. Robotic surgery and training: electromyographic correlates of robotic laparoscopic training. Surgical endoscopy. 2006;vol. 20(no. 5):824–829. doi: 10.1007/s00464-005-0334-z. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;vol. 33(no. 11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Martin CL, Phillips BA, Kilpatrick TJ, Butzkueven H, Tubridy N, McDonald E, et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Multiple Sclerosis. 2006;vol. 12(no. 5):620–628. doi: 10.1177/1352458506070658. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. The New England journal of medicine. 2000;vol. 343(no. 13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Mayr WT, McClelland RL, Jorgensen NW, Weigand SD, Noseworthy JH, Weinshenker BG, Rodriguez M. Change in MS-related disability in a population-based cohort: a 10-year follow-up study. Neurology. 2004;vol. 62(no. 1):51–59. doi: 10.1212/01.wnl.0000101724.93433.00. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. Journal of Neurophysiology. 1996;vol. 76(no. 2):1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabilitation and neural repair. 2009;vol. 23(no. 2):108–116. doi: 10.1177/1545968308320641. [DOI] [PubMed] [Google Scholar]
- Stergiou N, Giakas G, Byrne JB, Pomeroy V. Clinical biomechanics. no. 8. vol. 17. Bristol, Avon: 2002. Frequency domain characteristics of ground reaction forces during walking of young and elderly females; pp. 615–617. [DOI] [PubMed] [Google Scholar]
- Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006;vol. 66(no. 2):172–177. doi: 10.1212/01.wnl.0000194259.90286.fe. [DOI] [PubMed] [Google Scholar]
- Tsepis E, Giakas G, Vagenas G, Georgoulis A. Frequency content asymmetry of the isokinetic curve between ACL deficient and healthy knee. Journal of Biomechanics. 2004;vol. 37(no. 6):857–864. doi: 10.1016/j.jbiomech.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Veugelers PJ, Fisk JD, Brown MG, Stadnyk K, Sketris IS, Murray TJ, Bhan V. Multiple sclerosis. no. 11. vol. 15. Houndmills, Basingstoke, England: 2009. Disease progression among multiple sclerosis patients before and during a disease-modifying drug program: a longitudinal population-based evaluation; pp. 1286–1294. [DOI] [PubMed] [Google Scholar]
- White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Med. 2004;vol. 34(no. 15):1077–1100. doi: 10.2165/00007256-200434150-00005. [DOI] [PubMed] [Google Scholar]
- Winter DA. The Biomechanics and motor control of human gait : normal, elderly and pathological. 2nd edn. Waterloo, Ont: University of Waterloo Press; 1991. [Google Scholar]