Abstract
Since long-term immunity is a critical component of any effective vaccine, we compared over a 15 month period, the strength, durability and specificity of immunity of an attenuated smallpox vaccine Modified Vaccinia Ankara (MVA) to the New York City Board of Health (NYCBH) vaccine. The frequencies of CD8+ T cells to an immunodominant CD8 T cell epitope B8R20–27 remained remarkably stable in mice given either MVA or NYCBH. Both groups were also protected from a lethal intranasal challenge with Western Reserve strain of vaccinia virus (VACV-WR). Cytokine responses to virus-specific peptides were detectable with significant boosting upon challenge. Expression of most phenotypic markers that define antigen-specific memory CD8 T cells was similar while CD27 was differentially expressed on lung-specific T cells compared to the spleen. Our data indicate robust vaccinia-specific CD8+ T cell recall responses to lethal secondary challenge in protected mice with no apparent effect of age on T cell pools established much earlier in life.
Keywords: Vaccinia, CD8, aging
Introduction
Vaccinia virus (VACV) administered to humans by a bifurcated needle on the skin has proven to be an effective vaccine against smallpox, one of the world’s most dreaded infectious agents, but the underlying mechanisms that make this an effective vaccine are still largely unknown. Routine vaccination with the highly effective first generation smallpox vaccines such as New York City Board of Health (NYCBH), grown in the skin of calves, was discontinued in the 1970s because the risk for acquiring smallpox had decreased and the vaccine was associated with serious adverse effects [1]. As a result, a substantial portion of the world’s population has not been immunized with any strain of VACV and remains susceptible to a bioterrorist threat with smallpox. Furthermore, contraindications for use of standard vaccine would result in approximately 20–25% of the population being excluded [2]. Second generation smallpox vaccines offer a potential advantage over traditional vaccines since they use the same viruses as prior vaccines but are propagated in tissue culture rather than in animals [3, 4]. The generation of several attenuated third generation vaccine strains of VACV including a variant of the Lister strain LC16m8 [5], MVA [6, 7] and NYVAC [8] has enabled extensive testing of these vaccines in animal models as well as human clinical trials [9–16]. Fourth generation vaccines involve targeting specific genomic segments of VACV and in many cases higher doses are required to maintain immunogenicity compared to the wildtype parent strain [17].
Attenuation in MVA, one of the most extensively studied third generation vaccines was achieved by more than 500 serial passages in chicken embryo fibroblasts [13, 18]. The loss of 15% of its genome rendered MVA replication incompetent in mammalian cells. Efficacy studies indicated that MVA was immunogenic and protective in normal mice and cynomolous macaques but animals required multiple higher titer doses to achieve comparable protection to standard replicating vaccines [10, 11, 14, 15, 19]. Several MVA candidates have been tested in humans including MVA-BN [16] and MVA-TBC [20] and MVA has been recently described to efficiently elicit epitope-specific CD8 memory T cells in humans [21]. The intramuscular route of administration has proven to be more immunogenic and priming with at least two doses of MVA was required for maintaining immunogenicity and enhanced T cell as well as humoral responses [16, 20].
In humans, cellular immunity to traditional vaccines is relatively long lived and can be detected decades after immunization [22, 23]. Studies on the long term immunogenicity of MVA have been performed in a more limited fashion. Ferrier-Rembert et al assessed three non-replicating VACV vaccine candidates including MVA, NYVAC and HR using an intranasal cowpox challenge model and found that while mice were protected short term (28 days), long-term protection 150 days after immunization was incomplete [12]. Relatively little work has addressed the impact of age on pre-existing memory T cell populations to either first or third generation small pox vaccines. Studies in mice have clearly demonstrated functional CD8+ T cell memory to acute viral infections for over a year after initial generation [24–26]. The relative efficacy of the recall of poxvirus-specific T cells has not been thoroughly studied in a suitable animal model.
Using a murine model, we investigated the impact of age on memory CD8+ T cell recall responses in C57BL/6 mice immunized with either NYCBH or MVA administered by different routes based on their administration in humans. We compared the phenotype and function of antigen-specific T cells at mucosal and systemic sites prior to and following challenge with the neurovirulent strain, VACV-WR. We also examined major factors that could contribute to differences in the immune response. Our data indicate that the recall responses are similar in older mice that had been immunized with either MVA or NYCBH with robust recruitment of antigen-specific effector T cells to the site of challenge with a distinct activation profile. Overall our studies shed light on the durability of memory VACV-specific CD8 T cells in older mice to respond to a lethal challenge.
Materials and Methods
Viruses and cells
The Dryvax virus seed stock was derived from a reconstituted vial of the licensed smallpox vaccine manufactured by Wyeth. This vaccine was derived from the NYCBH strain and is referred to as NYCBH throughout the manuscript. Virus from the third passage was used in these studies. Modified vaccinia virus Ankara strain (MVA) was supplied by Bernard Moss of National Institute of Allergy and Infectious Diseases/National Institutes of Health (Bethesda, MD). Vaccinia virus WR strain (VACV-WR) was kindly provided by Girish J. Kotwal and William L. Marshall of the Department of Medicine, University of Massachusetts Medical School. NYCBH and VACV-WR were grown in CV-1 cells (ATCC CCL-70) and virus stocks were prepared from cell lysates. MVA was propagated and titrated in BHK-21 cells.
Immunization of mice and preparation of splenocytes and lung lymphocytes
Female C57BL/6 (4–8 weeks old) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were immunized once or twice (1 month apart) with 1x106 PFU MVA by the i.m. route or 1 × 106 PFU of NYCBH or MVA by tail scarification. Naïve and immunized mice were maintained for 15 months to assess immune responses. For the challenge, mice were administered 10 LD50 (LD50=5.83 × 105) PFU of VACV-WR by the i.n. route. Mice were anesthetized with isoflurane and 50 μl containing the indicated dose of virus was instilled into the nares. Splenocytes and lung lymphocytes were collected at the indicated time points post-immunization and processed as previously described [27]. All mice were maintained in the Animal Facility at the University of Massachusetts Medical School, which is regulated by AWA-1995, PHS-1986, MA140-1985, and following the AAALAC-1965 guidelines.
Tetramer and cell surface staining
Splenocytes and lung cells were washed with FACS buffer (PBS/2%FBS/0.1% sodium azide), blocked with CD16/CD32 mAb (Fc block 24G.2) (BD Biosciences, San Diego CA.) for 15 min at 4°C and performed as described [27]. The tetramer containing the immunodominant epitope of VACV-B8R20–27 was synthesized in the NIH Tetramer Facility. Splenocytes, lung cells and PBMC from immunized and unimmunized mice were initially stained with the viability marker LiveDead Aqua (Molecular Probes) for 30 min at 4C. Cell suspensions were then washed in FACS buffer, blocked with Fc block, stained with the B8R20–27 tetramer at room temperature for 30 min prior to cell surface staining. Cells were stained with mAb directed at surface phenotypic markers: CD3 (clone 145–2C11-BD), CD8 (clone 53-6.7-BD), KLRG1 (clone 2F1-eBiosciences), CD43 (clone 1B11-BioLegend), CD127 (clone A7R34-eBio) and CD27 (clone 1M7-eBio) for 30 min at 4°C. All cell preparations were fixed with Cytofix (BD) and analyzed on a FACSARIA flow cytometer. FlowJo (TreeStar Inc. Ashland, OR.) version 7.2.5 was used to analyze all the data.
IFN-γ Enzyme-linked immunospot (ELISPOT) assay for quantitation of IFN-γ secreting cells
Peptide epitopes of VACV were based on published reports and synthesized at AnaSpec Inc (San Jose, CA) [27, 28]. ELISPOT assays were performed according to the manufacturer’s protocol (Mabtech AB, Sweden) and as previously described [29]. Freshly isolated splenocytes (2.5 × 105/ well) were incubated with the indicated peptides (4μg/ml), or concanavalin A (ConA) (5μg/ml) at 37°C for 18–20 hr in RPMI 1640 containing 10% FBS. The precursor frequency was calculated as [(number of spots in experimental well - number of spots in medium control well)/ total number of cells per well] × 106. Experiments were performed in triplicate wells.
Plaque Reduction Neutralization titers
Serial two fold dilutions of heat inactivated sera from immunized mice were incubated with VACV-WR for 60 min at 37°C. Following incubation, 0.25 mls of the virus/antibody mixture, virus alone or media was added to wells of confluent BSC-40 cells cultured in MEM. After 48 hours media was removed and crystal violet added to the plates and plaques enumerated. In wells that contained virus alone, dilutions of virus were adjusted to have 40–60 plaques per well. Titers were defined as the reciprocal serum dilution that caused a 50% reduction in viral plaques (PRNT50).
ELISA titers
Maxisorp enzyme-linked immunosorbent assay (ELISA) plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl carbonate coating buffer containing 0.5 × 106 PFU of uv inactivated VACV-WR. Plates were washed three times with PBS-0.1% Tween 20 wash buffer and blocked for 90 min with a blocking buffer (4% w/v whey protein and 0.5% tween-20 in 1X PBS). Serum samples were serially diluted in 100 μl whey buffer and added to the ELISA plate in duplicate. The plates were incubated for 3 h and washed four times. A secondary peroxidase-conjugated affinity-purified goat anti-mouse secondary antibody (Calbiochem) diluted 1:10000 in whey buffer. Plates were incubated for 1 h, washed five times, and developed with tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) for 15 min. The reaction was stopped by the addition of 1% HCl stop solution, and the plates were analyzed at 450 nm with a Multiskan EX ELISA reader (Thermo Electron, Vantaa, Finland). The endpoint titer for each serum sample represented the greatest dilution with an optical density greater than three standard deviations above the 1:100 dilution of naïve sera.
Adoptive transfer studies
Splenocytes were isolated from mice immunized with 1 × 106 PFU of NYCBH by the tail scarification route three months prior. Approximately 10% of the splenocytes were infected with VACV-WR (m.o.i.=5) for 1 hr. Infected splenocytes were washed twice and added to 90% immune splenocytes (responders) in T25 flasks in RPMI containing 0.05 mM mercaptoethanol, and 10% FBS. On day 7 of culture, CD8 T cells were isolated by MACS using the CD8α+ isolation Kit II (Miltenyi Biotec, Gladbach, Germany). Purity of CD8 T cells was greater than 90%. CD8 T cells were washed twice with PBS, and 2.5 × 106 CD8 T cells were injected i.p. into recipient naïve C57BL/6 mice. 200 μl of immune serum from mice immunized with NYCBH 3 months prior or PBS was transferred into recipient naïve C57BL/6 mice. Mice that received CD8 T cells, immune sera or PBS were challenged with 0.1 LD50 VACV-WR by the i.n. route on the same day. Four days after challenge, mice were sacrificed. Lungs were analyzed for VACV titers.
Virus titration
Lungs of infected mice were collected 4 days post challenge and frozen at −80°C for virus titration. Briefly, organs were freeze- thawed 3 times in 0.5 ml MEM/2% FBS. Organs were homogenized and dilutions of supernatants were added to confluent CV-1 cells in 6 well plates. After 48 hours, media was removed and crystal violet added to the plates and plaques enumerated. Data shown indicate the number of plaque forming units (PFU) of vaccinia virus/gram of tissue in the lungs.
Statistical analysis
Survival comparisons were performed using Mantel-Cox Log rank test. Differences between groups for all other parameters were determined using the Mann Whitney U test. p values <0.05 were considered to be significant. Graph Pad Prism software was used to perform the analysis.
Results
Stable frequencies of CD8+ B8R20–27 tetramer+ T cells in mice 15 months post immunization with replicating or non-replicating vaccinia virus vaccines
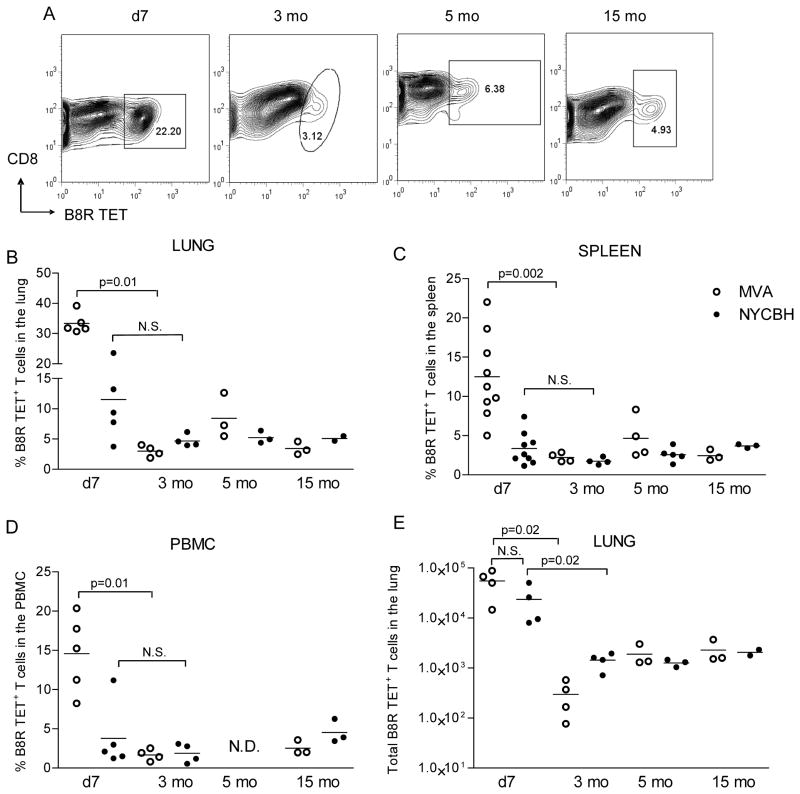
The most immunogenic and the recommended routes for administration of standard (NYCBH) and experimental (MVA) VACV vaccines in humans is using a bifurcated needle on the arm and the intramuscular route respectively; therefore, we compared the efficacy of NYCBH and MVA vaccination using similar routes in immunocompetent C57BL/6 mice. Mice were immunized with 1 dose of 106 PFU of NYCBH by scarification on the tail or with two doses of 106 PFU of MVA by the i.m. route, since multiple doses of MVA are required for sustained immunity [30]. At 7 days, 3, 5 and 15 months after immunization we compared the frequencies of CD8+ T cells to an immunodominant epitope B8R20–27 described in H-2b mice [28] using the gating strategy shown in Fig S1. The highest frequencies of CD8+ B8R20–27 tetramer+ (TET+) T cells (Fig 1A &B) as well as the total number of T cells (Fig 1E) were detected in the lungs of mice 7 days after immunization with NYCBH or 7 days after the second dose of MVA. While there was variability in individual mice the average frequency of naïve CD8 T cells that bound the B8R tetramer, was 0.62% of CD8 T cells in the spleens and 1.12% in the lungs (n=6) (data not shown). Frequencies of B8R20–27-specific memory CD8 T cells in the lungs (Fig 1B), spleens (Fig 1C) and PBMC (Fig 1D) of all groups of mice at early (3 months), intermediate (5 months) and late (15 months) time points after immunization were stable but lower compared to frequencies during the acute immune response. Frequencies of cells in the lungs of mice 7 days following administration of 1 dose of MVA by the i.m. route or 1 dose of MVA by the tail scarification route were lower compared to 2 doses of MVA by the i.m. route (data not shown). The data indicate that frequencies of antigen-specific memory CD8 T cells remain stable long term in mucosal, systemic and peripheral sites in mice administered either first or third generation smallpox vaccines.
Figure 1. Long term TET+ frequencies in target organs of immunized mice.
Using the gating strategy shown in Fig S1 to identify live CD3+CD8+ T cells, (A) representative frequencies of B8R20 27 TET+ T cells in the lung lymphocytes of mice immunized with NYCBH 7 days, 3, 5 and 15 months earlier are shown. Frequencies of TET+ cells in the (B) lung, (C) spleen (D) PBMC and (E) the absolute numbers of TET+ T cells in the lungs of MVA and NYCBH immunized animals are depicted at the indicated time points post immunization. Each symbol represents the frequency of TET+ T cells obtained in target organs of individual mice; mean values are denoted by horizontal lines. p values <0.05 were considered to be significant.
MVA and NYCBH immunized mice survive a lethal VACV-WR challenge
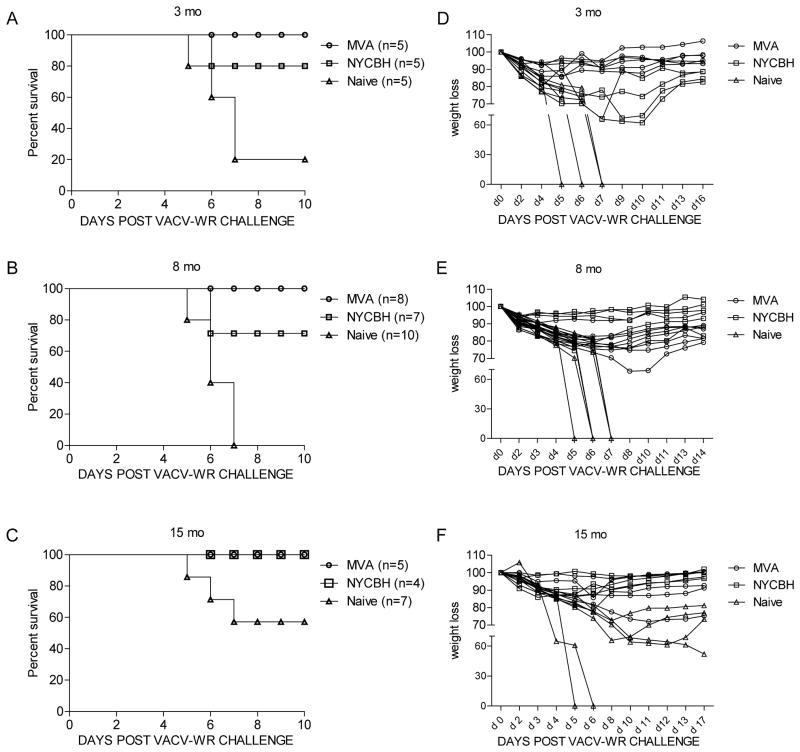
We next determined the ability of MVA and NYCBH immunized mice to protect against a lethal challenge dose of a neurovirulent strain of VACV-WR. The LD50 for VACV-WR in 3 month and 1 year old C57BL/6 mice was established at 5.83 × 105 PFU. Mice were next monitored for weight loss and survival after challenge with 10 LD50 of VACV-WR by the i.n. route. Three and eight months after immunization with 2 doses of MVA, 100% of mice were protected and >75% of mice immunized with a single recommended dose of NYCBH survived a lethal VACV-WR challenge. Less than 20% of non-immunized mice survived (Fig 2A, B). In contrast, mice immunized 3 months earlier with 1 dose of 106 PFU of MVA by the tail scarification route had 20% survival and mice immunized with 1 dose of 106 PFU of MVA by the i.m. route had 80% survival (Fig S2A). In mice that survived the challenge, significant weight loss, which is another measure of disease severity was detected in 4/4 NYCBH immunized mice with milder weight loss detected in mice immunized with two doses of MVA (Fig 2D) or 1 dose of MVA by the i.m. route (Fig S2B). At the 15 mo time point, although fewer naïve (40%) mice succumbed to the same challenge dose compared to either the 3 or 8 mo time point, severe weight loss (≥ 25% loss in body weight) was detected in all naïve mice that survived. Mice immunized with either MVA or NYCBH survived the lethal challenge with mild weight loss (≤ 15% loss in body weight) detected in 3/5 mice immunized with 2 doses of MVA and 3/4 mice immunized with NYCBH (Fig 2F). One mouse immunized with two doses of MVA had weight loss that was equivalent to naïve mice.
Figure 2. Survival and weight loss curves of MVA or NYCBH immunized mice following lethal i.n. challenge with VACV-WR.
Groups of female C57BL/6 mice (n = 5–10) were immunized with 2 doses of MVA, 1 dose of NYCBH or left unimmunized. Survival and weight loss curves following challenge with 10 LD50 of VACV-WR by the i.n. route in mice immunized (A, D) 3 months, (B, E) 8 months and (C, F) 15 months earlier. The percentage of weight relative to the initial body weight (100%) was plotted and the data are presented as percent change in body weight following challenge. Experiments were performed twice at the 3 mo time point with comparable results and once at the 8 and 15 mo time point. For survival comparisons, p=0.09 NYCBH vs naïve; p=0.02 MVA vs naïve at the 3 mo time point. p=0.01 NYCBH vs Naïve; p=0.0003 MVA vs naïve at the 8 mo time point.
We also assessed the ability of the two vaccine candidates to induce neutralizing antibodies by evaluating PRNT50 titers in the sera of mice 3 and 6 months after immunization. We found that 50% and 20% of mice immunized with 2 doses of MVA seroconverted at early (3 months) and late (6 months) time points after immunization with 50% PRNT titers of 20 (Table 1). In contrast, 75–80% of mice immunized with NYCBH had PRNT50 titers that ranged from 20–40 at both time points. Significantly higher ELISA titers compared to PRNT50 titers were detected in the sera of both MVA and NYCBH immunized mice.
Table 1.
Seroconversion in immunized mice.
| Vaccine | Number of mice/group | Time point Post- vaccination | Reciprocal Neutralizing antibody titer | Sero conversion rate | Reciprocal ELISA titer |
|---|---|---|---|---|---|
| MVA | 4 | 3 month | 20 | 25,600 | |
| ≤ 10 | 50% | 6400 | |||
| ≤ 10 | 12800 | ||||
| 20 | 1600 | ||||
| 5 | 6 month | 20 | 25600 | ||
| ≤ 10 | 25600 | ||||
| ≤ 10 | 20% | 25600 | |||
| ≤ 10 | 51200 | ||||
| ≤ 10 | 51200 | ||||
| NYCBH | 4 | 3 month | 20 | 12,800 | |
| ≤ 10 | 75% | 12,800 | |||
| 40 | 12,800 | ||||
| 20 | 25,600 | ||||
| 5 | 6 month | 40 | 6400 | ||
| 40 | 3200 | ||||
| 20 | 80% | 3200 | |||
| 20 | 3200 | ||||
| ≤ 10 | n.t.* |
Groups of mice were immunized with 2 doses of MVA i.m. or 1 dose of NYCBH by the t.s. route. PRNT50 antibody titers and ELISA titers against VACV-WR were assessed at the indicated time points in sera of immunized mice. PRNT50 antibody titers higher than 20 were considered evidence of seroconversion.
n.t.=not tested.
IFN-γ secretion by virus-specific CD8 T cells
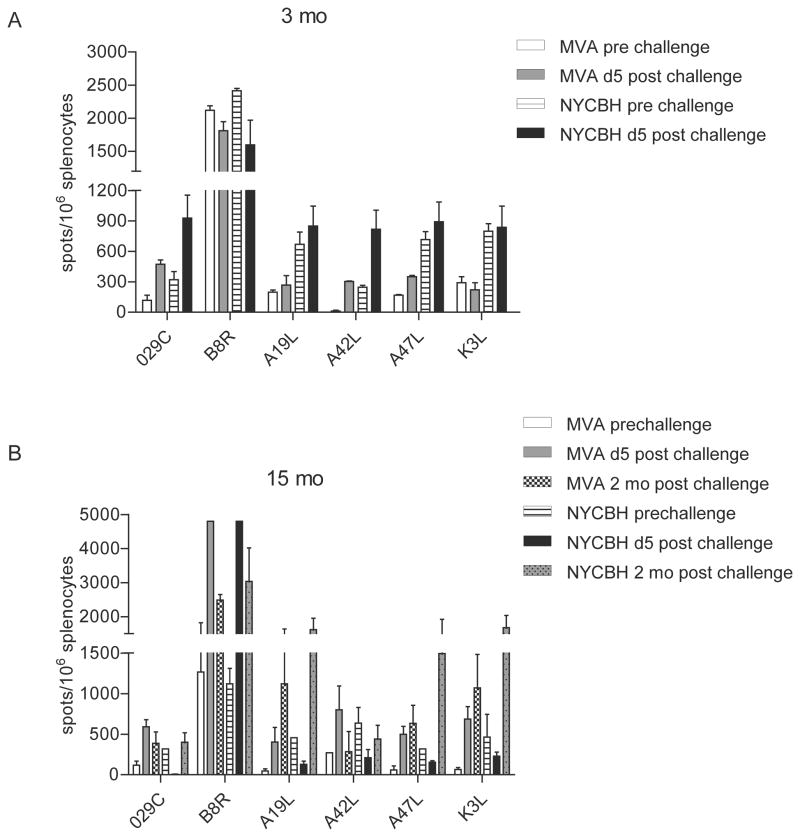
To assess the immunogenicity of first and third generation smallpox vaccines we compared IFN-γ responses in the splenocytes of immunized mice after stimulation with 6 previously identified VACV-specific T cell peptides. At 3 and 15 months post immunization, responses to all six epitopes were detected in mice immunized with either MVA or NYCBH. However, pre-challenge responses were lower in splenocytes obtained from mice immunized with MVA compared to mice immunized with NYCBH (Fig 3A&B). In order to determine whether responses to these epitopes were boosted following challenge with VACV-WR, we quantitated the number of IFN-γ producing cells in splenocytes of mice obtained after challenge. Responses to the immunodominant B8R epitope were significantly boosted 5 days post challenge in both groups of mice at the 15 month time point with lower responses to the other 5 subdominant epitopes. Two months post virus challenge, the number of epitope-specific CD8 T cells that were able to secrete IFN-γ compared to prechallenge levels were higher indicating that these responses were boosted upon virus challenge. While responses to the 6 peptides varied, our results indicate the presence of functional T cells at early and late time points post immunization in both groups of mice. In addition, these cells were able to respond to a lethal challenge by secreting IFN-γ in response to virus-specific peptides.
Figure 3. Short and long term ability of splenocytes to respond to VACV-specific peptides.
IFN-γ responses in splenocytes obtained prior to and 5 days post i.n. challenge to 6 VACV-specific CD8 T cell peptides (4 μg/ml). Responses in mice immunized with MVA or NYCBH (A) 3 and (B) 15 months earlier are shown. Elispot Assays were performed using triplicate wells for each condition and individual mice/group. Data shown are mean values ± S.E.M. for 2–5 mice/group.
Robust recruitment of TET+ T cells at mucosal and systemic sites following lethal challenge with VACV-WR
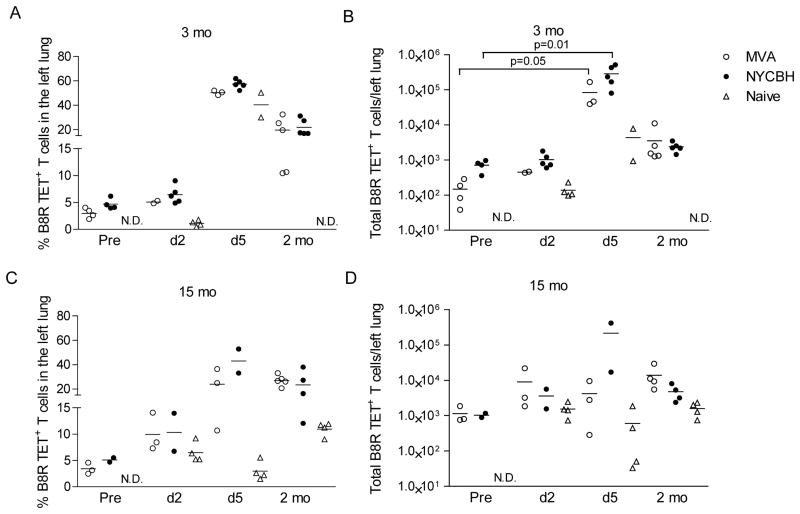
We next examined the frequencies of B8R20–27 TET+ T cells in the spleen, PBMC and lung and evaluated their contributions to the recall immune response. We administered 10 LD50 of VACV-WR to mice that had been immunized 3 or 15 months earlier with MVA or NYCBH. Mice were sacrificed at early (2 and 5 days) and late time points (2 months) after challenge. The frequency as well as total number of antigen-specific T cells was significantly boosted in the lungs of mice 5 days after challenge in both groups immunized 3 months earlier (Fig 4A&B) with similar trends at the 15 month time point (Fig 4C&D). In mice immunized with NYCBH, fewer mice per group were sacrificed at the fifteen month time point. A similar pattern of responses was seen in the spleen and PBMC (data not shown). In mice that survived the secondary challenge, the residual frequencies of cells (2 months after challenge) were higher in the lungs of mice compared to the pre-challenge levels. Our data indicate that antigen-specific CD8 T cells were efficiently recruited to the site of challenge even 15 months after immunization with either NYCBH or MVA.
Figure 4. Recall efficacy of B8R20 27 TET+ cells in the lungs of mice after lethal challenge with VACV-WR.
(A and C) Frequencies and (B and D) the absolute numbers of TET+ T cells in the lungs of mice immunized 3 or 15 months earlier. Values prior to (pre) and post challenge (d2, d5 and 2 mo) with VACV-WR are shown.
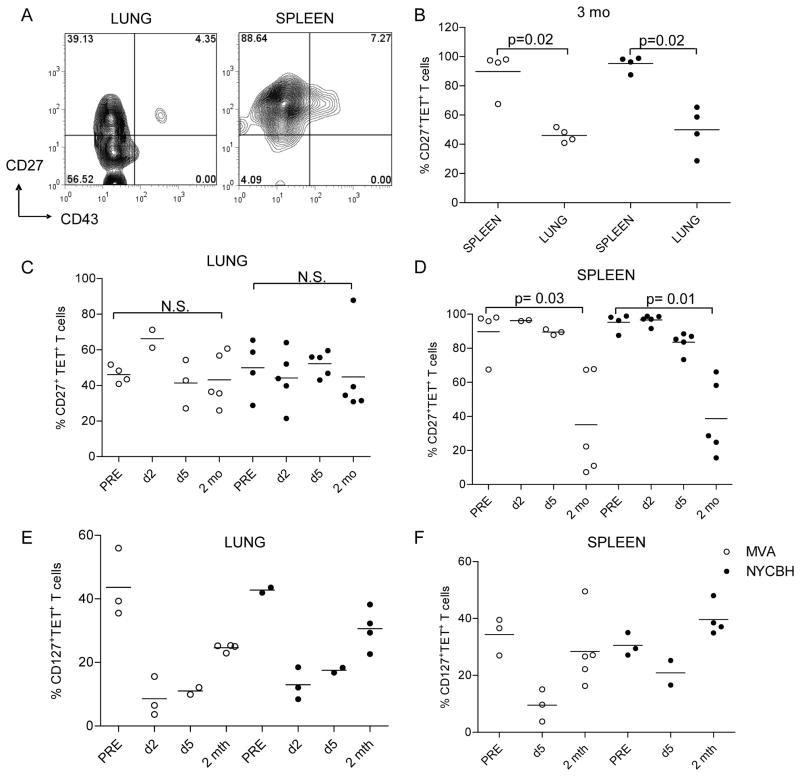
Differential expression of CD27 on TET+ T cells isolated in mucosal versus systemic sites
We further examined markers on CD8+ T cells associated with activation, antigen exposure and immune memory prior to, immediately after and 2 months following lethal VACV-WR challenge. Hikono et al found approximately 90% of antigen-specific T cells in the spleen expressed CD27, a marker associated with memory T cells 1–2 yr after i.n. infection with Sendai virus [26]. We too found that 90% TET+ T cells in the splenocytes of mice immunized with MVA or NYCBH expressed CD27 at 3 (Fig 5A, B&D) and 15 months (data not shown) after infection. Interestingly, significantly lower frequencies of the memory B8R20–27 TET+ T cells in the lung were found to express CD27 compared to the spleen (p ≤ 0.02, Mann Whitney U) (Fig 5B&C). Lungs of mice immunized with 1 dose of MVA also had a similar phenotype with a significant portion of B8R20–27 TET+ T cells not expressing CD27 (data not shown). To determine whether the phenotype of antigen-specific T cells changed at mucosal and systemic sites upon activation, we assessed CD27 expression on T cells following challenge. The frequency of CD27+ TET+ T cells in the lung remained stable at 2 days, 5 days and 2 mo following challenge. In contrast, while 90% TET+ T cells in the spleen expressed CD27 prior to and at early time points post challenge, these frequencies dropped significantly to ~50% 2 months post secondary challenge. Our results indicate that while most antigen-specific CD8 T cells in the spleen express CD27 prior to and early after challenge, a significant proportion of antigen-specific memory T cells in the lung do not express CD27. The IL-7R (CD127) is expressed on a subset of virus-specific memory CD8 T cells and is dynamically regulated following antigen activation [31]. In our studies, 30–50% TET+ T cells expressed CD127 fifteen months after immunization with either MVA or NYCBH (Fig 5E and F). As has been shown by other groups, we found that 2 and 5 days following challenge when cells were activated, CD127 expression was low on TET+ T cells in the lungs and spleens of mice immunized with either vaccine. Two months post secondary challenge, frequencies of CD127+TET+ cells returned to levels comparable to pre-challenge frequencies.
Figure 5. Distinct patterns of CD27 expression and downregulation of CD127 on VACV-specific memory CD8 T cells.
Splenocytes and lung lymphocytes were isolated from mice 3 months after immunization with MVA and cells were stained with the B8R20 27 tetramer and antibodies to CD27, CD127 and CD43. (A) A representative profile of CD27 and CD43 expression on TET+/CD8+ cells in the lungs of a mouse immunized with MVA. Numbers indicate the percentage of TET+ cells in each quadrant. (B) Frequencies of TET+/CD8+ cells that express CD27 in the lungs and spleens of mice 3 months after immunization with MVA or NYCBH. Frequencies of cells that express CD27 in the (C) lungs and (D) spleens following challenge with VACV-WR (d2, d5 and 2 mo). Data show the frequencies of CD127+ TET+ T cells isolated from mice immunized 15 months prior in the (E) lung and (F) spleen prior to and at the indicated time points post challenge. Each symbol represents the frequency of TET+ T cells obtained in target organs of individual mice. Mean values are denoted by horizontal lines. p values <0.05 were considered to be significant.
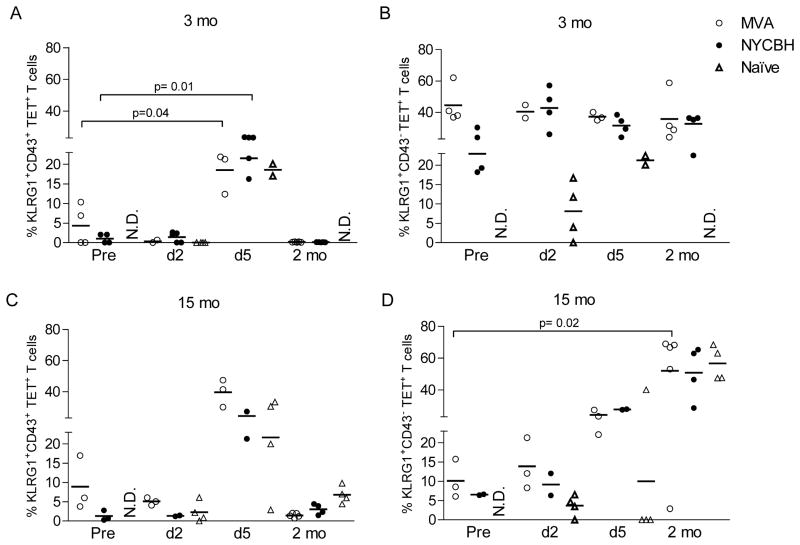
Lower frequency of KLRG1+ TET+ CD8 T cells 15 months post immunization
KLRG1 is an inhibitory C-type lectin expressed on NK cells and activated CD8 T cells [32] and has been implicated previously as a senescence marker [33, 34]. In humans and mice, most acute infections result in mainly KLRG1lo memory CD8+ T cells [33, 35]. We were interested in determining what proportion of VACV-specific T cells that survived long term expressed the senescence marker KLRG1. In addition, we assessed the level of activation of TET+ KLRG1+ cells during and after lethal challenge using an antibody directed against an activated isoform of CD43. The vast majority of memory T cells did not express CD43 prior to challenge as expected, while peak expression of CD43 was detected 5 days post challenge in all groups of mice (Fig 6A&C). In addition, 20% of TET+ T cells that did not express KLRG1 expressed CD43 on day 5 (data not shown). Two months after challenge CD8 T cells remained quiescent and did not express CD43.
Figure 6. KLRG1 expression on TET+ memory CD8 T cells in the lung.
Lung lymphocytes were isolated 3 or 15 months after immunization with MVA or NYCBH and stained with the B8R20 27 tetramer and antibodies against KLRG1 and CD43 as described. Data show the frequencies of (A and C) cells expressing KLRG1 and CD43 (KLRG1+CD43+) or (B and D) cells expressing KLRG1 but not CD43 (KLRG1+CD43−) among TET+/CD8+ cells in lung lymphocytes prior to and following challenge (d2, d5, 2 mo) with VACV-WR. The data in each subpanel are gated on B8R20 27 TET+CD8+ cells. Each symbol represents the frequency of TET+ T cells obtained in lungs of individual mice; mean values are denoted by horizontal lines. p values <0.05 were considered to be significant.
Three months post immunization, approximately 40–50% of TET+ T cells in the lungs expressed KLRG1 with little to no expression of CD43 (Fig 6A&B). A lower frequency of TET+ T cells (~20%) expressed KLRG1 15 months post immunization (Fig 6C&D). The frequencies of KLRG1+ cells increased in the lungs 5 days post challenge in mice immunized 3 or 15 months earlier. The frequency of TET+ T cells that expressed KLRG1 two months post secondary challenge (in mice immunized 15 months earlier) were higher compared to pre-challenge frequencies of KLRG1+ cells.
Collectively our findings indicate similar phenotypic profiles on antigen-specific T cells from mice immunized with either MVA or NYCBH in mucosal as well as systemic sites.
Virus-specific CD8 T cells reduce viral loads
We used adoptive transfer experiments to investigate the protective capacity of individual components of the adaptive immune response. In pilot experiments, mice were challenged with 10 LD50 of VACV-WR following transfer of immune splenocytes, immune CD8 T cells, immune sera (from mice immunized with NYCBH by the t.s. route) or PBS. Mice in all groups had significant weight loss with no survivors beyond day 8 (data not shown). We speculated that this very high challenge dose overwhelmed the modest number of CD8 T cells transferred and that the dilution of immune serum in recipient mice may have been insufficient to protect against the high challenge dose used.
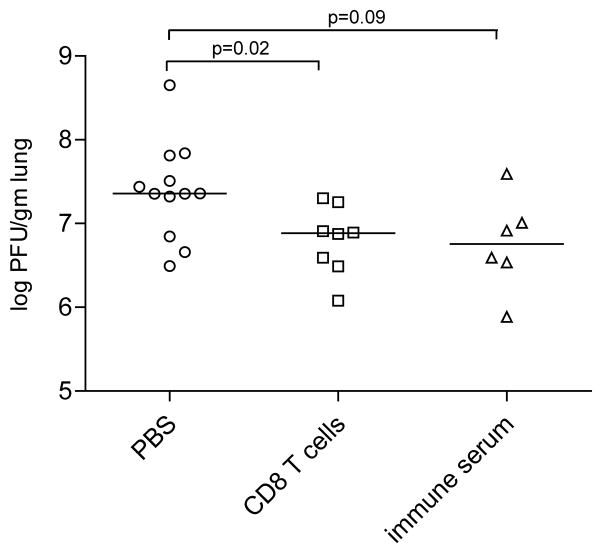
Splenocytes obtained from mice immunized 3 months prior with NYCBH by the t.s. route were stimulated with live virus to increase the frequency and activation of VACV-specific CD8 T cells as described in Materials and Methods. 51Cr release assays on bulk culture cells indicated that CD8 T cells were able to lyse VACV-infected targets (data not shown). Naïve syngeneic mice received VACV-specific CD8 T cells or sera from mice immunized with NYCBH 3 months prior. The reciprocal PRNT50 titer of serum that was transferred into mice was 40 consistent with our findings (data not shown). Mice that received virus-specific CD8 T cells, immune serum or PBS were challenged with 0.1 LD50 of VACV-WR by the i.n. route. Four days following this challenge, viral titers were significantly lower in the lungs of mice that received immune CD8 T cells compared to naïve mice that received PBS (p=0.02) (Figure 7). While viral titers were not significantly different in the lungs of mice that received immune sera compared to naïve mice, there was a trend towards significance. Overall our data indicate that memory CD8 T cells limit viral replication in challenged mice. Furthermore, our findings indicate that antibodies and CD8 T cells may act in concert to provide protection during secondary VACV challenge.
Figure 7. CD8 T cells reduce viral loads in the lung.
Naïve mice were challenged with 0.1 LD50 of VACV-WR by the i.n. route following adoptive transfer of PBS, CD8 T cells or immune sera from NYCBH mice as described in Materials and Methods. Data shown are PFU/gm of lung tissue four days following challenge in mice that received PBS (open circles, n=12), CD8 T cells (open squares, n=8) or immune sera (open triangles, n=6) prior to challenge. Each symbol represents viral titers obtained in lungs of individual mice; mean values are denoted by horizontal lines. p values <0.05 were considered to be significant.
Discussion
Long term immunity is one of the cornerstones of a successful vaccine. Pathogen-specific memory T cells undergo changes in phenotype, function and location over time which would influence their ability to respond to a secondary challenge [36]. In this study we assessed the impact of increasing age on the frequency, phenotype and recall potential of VACV-specific CD8 T cell memory pools in mice that received a traditional first generation replicating or a third generation non-replicating smallpox vaccine. We demonstrated long term stable CD8 T cell responses in mice immunized with either MVA or NYCBH. Both groups of mice were able to mount robust secondary CD8 T cell responses when challenged with a lethal dose of VACV-WR. We also assessed patterns of activation and memory markers on antigen-specific T cells and found distinct differences in cells that respond at mucosal versus systemic sites. Our studies provide important insights into the impact of aging on VACV-specific CD8 memory T cells established earlier in life and their ability to respond to a lethal poxvirus challenge. Overall, our results indicate that both first and third generation smallpox vaccines were able to induce long-term protection against a lethal VACV-WR challenge.
We compared immune responses in mice that were administered two doses of MVA by the i.m. route to mice that were administered 1 dose of NYCBH by tail scarification specifically because these routes and the number of immunizations have been shown to induce optimal responses for these vaccines in both humans and mice [3]. We too have noted that mice administered 1 dose of MVA by the tail scarification route were more susceptible to lethal challenge with VACV-WR at early and late time points and had less robust CD8 T cell responses. We administered 10–100 fold less MVA (106 PFU) compared to other immunization protocols in order to be consistent with the dose administered to mice immunized with NYCBH in this study.
To characterize the antibody responses in immunized mice, we assessed both neutralizing antibody titers as well as ELISA titers directed against the challenge virus VACV-WR. We found low levels of neutralizing antibodies in the sera of MVA immune mice at all time points. Administration of 106 PFU of MVA by the i.m. route has previously been shown not to induce VACV-specific neutralizing antibodies [12]. Surprisingly, while a greater proportion of mice immunized with NYCBH had neutralizing antibodies, the overall titers were also low. Other studies have also reported low neutralizing antibody levels in immune mice that have been scarified with standard vaccines [37, 38]. As expected ELISA titers were higher than neutralizing antibody titers in both groups with higher levels in the sera of mice immunized with MVA compared to NYCBH at the 6 month time point. We do not think that these ELISA titers however are associated with protection.
In our hands, MVA and NYCBH were comparable in inducing long term protection against a lethal VACV-WR challenge. We used a well accepted intranasal challenge model with VACV-WR, a neurovirulent VACV strain [14, 39, 40]. Ferrier–Rembert et al found that MVA and NYVAC were markedly less effective than VACV-Lister in protection from a lethal cowpox challenge and Coulibaly et al found that higher doses of MVA were required for protection against ectromelia challenge [9, 12]. These results may be explained by the pathogenic orthopoxviruses that were used to evaluate cross-protection in VACV-immunized animals. Interestingly, despite the weight loss and low antibody titers in mice immunized with either MVA or NYCBH, both groups were protected from a lethal challenge with VACV-WR. The low levels of neutralizing antibodies induced in these mice may have been sufficient for protection. Alternatively the immunization may have elicited the generation of antibody secreting cells that were triggered to secrete neutralizing antibodies upon VACV challenge in our immune mice. There was a trend towards significant differences in viral titers in naïve mice that received VACV immune sera (from mice immunized 3 months prior with NYCBH) compared to naïve mice that received PBS prior to VACV-WR challenge. We believe that adoptive transfer of only 200 μl of serum diluted the low levels of neutralizing antibodies which led to this borderline significance and that these antibodies do play a role in protection. Transfer of increased amounts of immune serum may have led to significant differences.
Moutafsti et al recently demonstrated that peptide immunization of naïve T cells were able to induce robust B8R-specific T cells that were sufficient for protection from a lethal intranasal challenge [41]. Cornberg et al demonstrated that E7R-specific memory CD8 T cells were able to reduce viral titers in naïve syngeneic recipient mice administered 1 × 106 PFU of VACV [42]. Our data using adoptive transfer of virus-specific CD8 T cells showed significant decreases in viral titers compared to titers in the lungs of naïve mice that were challenged. Our data support findings that CD8 T cells may contribute to limit viral replication during VACV-WR challenge by the intranasal route.
The efficacy of viral vaccines depends in part on the generation of a pool of potent, long-lived memory T cells ready to expand rapidly upon re-exposure to antigen. We were interested in knowing whether effective T cell memory was maintained long term in mice immunized with either vaccine and if there were distinct differences in the phenotype of cells generated by immunization with MVA or NYCBH. We used the expression of KLRG1 as an indicator of short lived terminal effector CD8 T cells (SLECs) as well as a marker of immune senescence since long lived protective memory T cells express low levels of KLRG1 [33, 34, 43]. We too found that a significant portion of TET+ T cells express KLRG1 during acute vaccinia infection in the spleen and the lung (data not shown). However a higher frequency of TET+ T cells remain KLRG1+ (approximately 40–50% ) 3 months after infection with MVA or NYCBH compared to T cells in mice with LCMV or Sendai virus infections [25, 33, 34]. By 15 months however, a lower frequency of TET+ T cells (15–20%) expressed KLRG1indicating that attrition of KLRG1+TET+ T cells occurred between 3 and 15 months. Interestingly, two months post secondary challenge in 15 mo immune mice, 50% of the TET+ T cells were KLRG1+ suggesting that the recent challenge had increased the frequencies of KLRG1+ TET+ T cells. The data suggest that the decrease in number of KLRG1+ memory T cells may be related to the long time from exposure to a primary or secondary VACV infection and that the further away one is from immunization, the fewer KLRG1+ cells survive.
Members of the TNF-receptor family which include CD27 are involved in orchestrating activation, differentiation and death of immune cells [44]. Studies have shown that the interaction between CD27 and its cellular ligand CD70 regulates formation of effector and memory T cells after antigenic challenge in vivo [44, 45] and a recent study demonstrated that CD27 signaling directed IL-2 production that is essential for the survival of T cells in nonlymphoid tissue [46]. Influenza-specific and murine CMV-specific CD8+CD27- T cells are thought to represent a specialized memory T cell subset, which preferentially localize outside the secondary lymphoid organs and may represent memory T cells with high cytolytic potential [45]. On the other hand, cells with a CD27hi CD43low phenotype have been shown to mediate the strongest recall response in Sendai virus infection while cells with a CD27low CD43low phenotype mediated the weakest response [26]. Our data indicate that CD27 expression is down regulated on a significant portion of memory T cells in peripheral sites such as the lung while virtually all antigen-specific cells express CD27 in the spleen. The frequency of CD27+ cells did not change in the lung in response to a recent challenge and at two months following secondary challenge when memory T cells were quiescent again. In contrast the frequency of CD27+ T cells decreased in the spleen post secondary challenge. Our data support evidence that repeated exposure to antigen or localization at sites of antigenic challenge may result in the loss of CD27 expression in lung memory T cells. Alternatively, our data may suggest that the quality of virus-specific T cells in the lung which is the first line of defense against virus challenge may be compromised since a smaller proportion of cells express CD27.
The exact mechanisms that contribute to protection from a lethal poxvirus challenge are complex and likely involve multiple components of the immune system. Several factors including the innate immune response, cellular and humoral arms of the immune system, initial viral dose and the kinetics of virus replication in mucosal and systemic sites are likely to contribute to protection. The findings from this study do shed light on the impact of age on established memory CD8 T cell pools, the long term recall efficacy and immunobiology of aging VACV-specific CD8 T cells and will provide insight into future vaccine development and use of third generation smallpox vaccines.
Supplementary Material
Initial gating strategy to identify cells in the lymphocyte gate was based on forward and side scatter profiles. Viable CD3+ cells were gated on by exclusion of the viability marker LiveDead Aqua. CD8 T cells were next delineated using antibodies directed against CD8. Antigen-specific B8R20-27 tetramer +CD8+ cells were identified using the B8R20-27 tetramer. Tetramer positive cells were then assessed for expression of KLRG1, CD27, CD127 and CD43.
(A) Survival and (B) weight loss curves in mice (n=5) immunized 3 months earlier with 106 PFU of MVA by t.s. or the i.m. route. The percentage of weight relative to the initial body weight (100%) was plotted and the data are presented as percent change in body weight following infection.
Acknowledgments
This project has been funded in whole or part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract N01-AI-25490 and grant U19 AI057319.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health.
We thank Jane Nowicki, John Cruz, Smita Jaiswal and Anita Leporati for technical assistance. We thank Pamela Pazoles and Marcia Woda for help with flow cytometry analysis. We thank the NIH tetramer facility for providing us with the vaccinia virus-specific B8R20-27 Kb restricted MHC tetramer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fenner F. Smallpox: emergence, global spread, and eradication. History and philosophy of the life sciences. 1993;15(3):397–420. [PubMed] [Google Scholar]
- 2.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005 Mar 18;23(17–18):2078–81. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Artenstein AW. New generation smallpox vaccines: a review of preclinical and clinical data. Reviews in medical virology. 2008 Jul–Aug;18(4):217–31. doi: 10.1002/rmv.571. [DOI] [PubMed] [Google Scholar]
- 4.Artenstein AW, Grabenstein JD. Smallpox vaccines for biodefense: need and feasibility. Expert review of vaccines. 2008 Oct;7(8):1225–37. doi: 10.1586/14760584.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugimoto M, Yasuda A, Miki K, Morita M, Suzuki K, Uchida N, et al. Gene structures of low-neurovirulent vaccinia virus LC16m0, LC16m8, and their Lister original (LO) strains. Microbiology and immunology. 1985;29(5):421–8. doi: 10.1111/j.1348-0421.1985.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 6.Mayr A, Munz E. Changes in the vaccinia virus through continuing passages in chick embryo fibroblast cultures. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene 1 Abt Medizinisch-hygienische Bakteriologie, Virusforschung und Parasitologie. 1964 Dec;195(1):24–35. [PubMed] [Google Scholar]
- 7.Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author's transl) Deutsche medizinische Wochenschrift (1946) 1974 Nov 22;99(47):2386–92. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 8.Tartaglia J, Perkus ME, Taylor J, Norton EK, Audonnet JC, Cox WI, et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992 May;188(1):217–32. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- 9.Coulibaly S, Bruhl P, Mayrhofer J, Schmid K, Gerencer M, Falkner FG. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology. 2005 Oct 10;341(1):91–101. doi: 10.1016/j.virol.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004 Mar 11;428(6979):182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 11.Earl PL, Americo JL, Wyatt LS, Espenshade O, Bassler J, Gong K, et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proceedings of the National Academy of Sciences of the United States of America. 2008 Aug 5;105(31):10889–94. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrier-Rembert A, Drillien R, Tournier JN, Garin D, Crance JM. Short- and long-term immunogenicity and protection induced by non-replicating smallpox vaccine candidates in mice and comparison with the traditional 1st generation vaccine. Vaccine. 2008 Mar 25;26(14):1794–804. doi: 10.1016/j.vaccine.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 13.McCurdy LH, Larkin BD, Martin JE, Graham BS. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin Infect Dis. 2004 Jun 15;38(12):1749–53. doi: 10.1086/421266. [DOI] [PubMed] [Google Scholar]
- 14.Meseda CA, Garcia AD, Kumar A, Mayer AE, Manischewitz J, King LR, et al. Enhanced immunogenicity and protective effect conferred by vaccination with combinations of modified vaccinia virus Ankara and licensed smallpox vaccine Dryvax in a mouse model. Virology. 2005 Sep 1;339(2):164–75. doi: 10.1016/j.virol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, Pistoor FH, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. Journal of virology. 2005 Jun;79(12):7845–51. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006 Mar 15;24(12):2065–70. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs BL, Langland JO, Kibler KV, Denzler KL, White SD, Holechek SA, et al. Vaccinia virus vaccines: past, present and future. Antiviral research. 2009 Oct;84(1):1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. The Journal of general virology. 1991 May;72( Pt 5):1031–8. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 19.Abdalrhman I, Gurt I, Katz E. Protection induced in mice against a lethal orthopox virus by the Lister strain of vaccinia virus and modified vaccinia virus Ankara (MVA) Vaccine. 2006 May 8;24(19):4152–60. doi: 10.1016/j.vaccine.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Parrino J, McCurdy LH, Larkin BD, Gordon IJ, Rucker SE, Enama ME, et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine. 2007 Feb 9;25(8):1513–25. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer VS, Kastenmuller W, Gasteiger G, Franz-Wachtel M, Lamkemeyer T, Rammensee HG, et al. Long-term immunity against actual poxviral HLA ligands as identified by differential stable isotope labeling. J Immunol. 2008 Nov 1;181(9):6371–83. doi: 10.4049/jimmunol.181.9.6371. [DOI] [PubMed] [Google Scholar]
- 22.Demkowicz WE, Jr, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long–lived responses to vaccinia virus. Journal of virology. 1996 Apr;70(4):2627–31. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nature medicine. 2003 Sep;9(9):1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 24.Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. European journal of immunology. 2002 Jun;32(6):1567–73. doi: 10.1002/1521-4141(200206)32:6<1567::AID-IMMU1567>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Hikono H, Kohlmeier JE, Ely KH, Scott I, Roberts AD, Blackman MA, et al. T-cell memory and recall responses to respiratory virus infections. Immunological reviews. 2006 Jun;211:119–32. doi: 10.1111/j.0105-2896.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 26.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. The Journal of experimental medicine. 2007 Jul 9;204(7):1625–36. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathew A, O'Bryan J, Marshall W, Kotwal GJ, Terajima M, Green S, et al. Robust intrapulmonary CD8 T cell responses and protection with an attenuated N1L deleted vaccinia virus. PloS one. 2008;3(10):e3323. doi: 10.1371/journal.pone.0003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. The Journal of experimental medicine. 2005 Jan 3;201(1):95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathew A, Terajima M, West K, Green S, Rothman AL, Ennis FA, et al. Identification of murine poxvirus-specific CD8+ CTL epitopes with distinct functional profiles. J Immunol. 2005 Feb 15;174(4):2212–9. doi: 10.4049/jimmunol.174.4.2212. [DOI] [PubMed] [Google Scholar]
- 30.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proceedings of the National Academy of Sciences of the United States of America. 2004 Mar 30;101(13):4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008 Apr 15;180(8):5309–19. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaser C, Kaufmann M, Pircher H. Virus-activated CD8 T cells and lymphokine-activated NK cells express the mast cell function-associated antigen, an inhibitory C-type lectin. J Immunol. 1998 Dec 15;161(12):6451–4. [PubMed] [Google Scholar]
- 33.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. The Journal of experimental medicine. 2008 Mar 17;205(3):625–40. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007 Aug;27(2):281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, et al. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Experimental gerontology. 2003 Aug;38(8):911–20. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 36.Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annual review of immunology. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- 37.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007 Feb 26;25(10):1814–23. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jentarra GM, Heck MC, Youn JW, Kibler K, Langland JO, Baskin CR, et al. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: scarification vaccination. Vaccine. 2008 Jun 2;26(23):2860–72. doi: 10.1016/j.vaccine.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smee DF, Bailey KW, Sidwell RW. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antivir Chem Chemother. 2001 Jan;12(1):71–6. doi: 10.1177/095632020101200105. [DOI] [PubMed] [Google Scholar]
- 40.Snyder JT, Belyakov IM, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against Lethal Vaccinia Virus Challenge in HLA-A2 Transgenic Mice by Immunization with a Single CD8+ T-Cell Peptide Epitope of Vaccinia and Variola Viruses. Journal of virology. 2004 Jul;78(13):7052–60. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moutaftsi M, Salek-Ardakani S, Croft M, Peters B, Sidney J, Grey H, et al. Correlates of protection efficacy induced by vaccinia virus-specific CD8+ T-cell epitopes in the murine intranasal challenge model. European journal of immunology. 2009 Mar;39(3):717–22. doi: 10.1002/eji.200838815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornberg M, Sheridan BS, Saccoccio FM, Brehm MA, Selin LK. Protection against vaccinia virus challenge by CD8 memory T cells resolved by molecular mimicry. Journal of virology. 2007 Jan;81(2):934–44. doi: 10.1128/JVI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008 Feb 1;180(3):1309–15. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 44.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005 Aug 1;175(3):1665–76. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 45.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, et al. Properties of murine (CD8+)CD27– T cells. European journal of immunology. 2005 Nov;35(11):3131–41. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- 46.Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. The Journal of clinical investigation. 2009 Dec 1; doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Initial gating strategy to identify cells in the lymphocyte gate was based on forward and side scatter profiles. Viable CD3+ cells were gated on by exclusion of the viability marker LiveDead Aqua. CD8 T cells were next delineated using antibodies directed against CD8. Antigen-specific B8R20-27 tetramer +CD8+ cells were identified using the B8R20-27 tetramer. Tetramer positive cells were then assessed for expression of KLRG1, CD27, CD127 and CD43.
(A) Survival and (B) weight loss curves in mice (n=5) immunized 3 months earlier with 106 PFU of MVA by t.s. or the i.m. route. The percentage of weight relative to the initial body weight (100%) was plotted and the data are presented as percent change in body weight following infection.