Abstract
The aim of this study was to investigate the effects and mechanisms of gastric electrical stimulation (GES) on proximal stomach distention-induced visceral sensitivity. Isobaric gastric distention was performed using a barostat system in 8 normal and 6 vagotomized dogs and animal behaviors were noted and graded. The normal dogs were studied in 4 sessions: control (no GES), short pulse GES, long pulse GES, and dual-pulse GES, and the vagotomized dogs were studied in three sessions: control (no GES), long pulse GES and guanethidine. It was found that: 1) proximal stomach distention-induced behavioral changes were mediated by vagal and sympathetic pathways. The total behavior score (TBS) was 40.6±7.4 in the controls, 15.3±8.9 in vagotomized dogs (P=0.006 vs. control) and 8.8±0.9 in the vagotomized dogs with guanethidine (P=0.04 vs. vagotomy). The behavioral changes were mediated via the vagal pathway at distention pressures below 20mmHg, but mediated via both the vagal and sympathetic pathways at distention pressures equal to and above 20mmHg. 2) GES with long pulses or dual pulses but not short pulses reduced the distention-induced behavioral score (P=0.003, P=0.006 and P=0.7, respectively) and the effects of GES of long pulses might be mediated via the vagal and sympathetic pathways. In conclusion, gastric distention-induced visceral sensitivity is mediated via the vagal pathway at low distention pressures but via both vagal and sympathetic pathways at high distention pressures. GES with long but not short pulses reduces distention-induced visceral sensitivity.
Keywords: visceral pain/discomfort, visceral hypersensitivity, gastric electrical stimulation, vagal pathway, sympathetic pathway
1. Introduction
A subset of functional dyspepsia (FD) patients presents hypersensitivity to proximal stomach distention which is regarded as a result of abnormal central nervous system processing. The visceral hypersensitivity is clinically associated with a higher prevalence of postprandial discomfort, belching, and weight loss (Tack et al., 2001). In hypersensitive FD, proximal stomach distention activates components of the lateral discomfort system and bilateral frontal inferior gyri, putatively involved in regulation of hunger and satiety at significantly lower distention pressures in comparison with that of healthy volunteers (Vandenberghe et al., 2007). However, it is unknown whether peripheral vagal or sympathetic pathways are involved in this process.
Treatment options for FD are limited, and there are no medications that are specifically developed for the treatment of FD; some therapies are directed toward certain underlying pathophysiologies, such as delayed gastric emptying, impaired gastric accommodation and visceral hypersensitivity (Hiyama et al., 2007; Kupcinskas et al., 2008; Madisch et al., 2006; Oshima et al., 2006; Smith, 2005; Weinland et al., 2008). Gastric electrical stimulation (GES) was proposed for the treatment of gastrointestinal motor dysfunctions as early as in 1963 (Bilgutay et al., 1963). A great progress has been made recently on the effects, mechanisms and clinical applications of GES. GES has been under investigation for its therapeutic potentials for treating various gastrointestinal disorders, such as gastroparesis, intestinal pseudo-obstruction and constipation, as well as obesity (Abell et al., 2003; Anand et al., 2007; Andersson et al., 2006; Cigaina, 2002; Gourcerol et al., 2007a; Gourcerol et al., 2007b; Lin et al., 2007; Maranki et al., 2007; Nie et al., 2006; Sanmiguel et al., 2006; Tack, 2007; Zhang et al., 2006b). However, none of previous studies have investigated possible effects and mechanisms of different methods of GES on visceral sensitivity.
Altered mechano-sensory functions or hypersensitivity to luminal distention plays an important role in the pathophysiology of functional gastrointestinal disorders, including FD and irritable bowel syndrome (IBS). Mechanical stimulation of the sigmoid colon lowers sensory thresholds in patients with IBS but not in healthy controls (Mertz et al., 1995). Repeated mechanical stimulation increases visceral sensory thresholds in asymptomatic subjects but not in FD patients (Holtmann et al., 2000).
A number of previous studies have shown that GES is capable of influencing extrinsic innervation. Short pulse GES used in the treatment of nausea and vomiting in patients with gastroparesis was reported to influence the central nervous system (CNS) control mechanisms of nausea and vomiting via the activation of vagal afferent (McCallum et al. 2010). GES with parameters known to enhance antral contractions was found to produce a mean increase vagal afferent firing, similar to the increase in firing of vagal mechanosensitive afferent fibers stimulated by gastric distension in rats (Andrews et al., 1980; Gonzale et al., 1981; Peles et al., 2003). GES with different parameters was reported to activate gastric distention-sensitive vagal afferent neurons in the nucleus tractus salitarii (Qin et al. 2005) in rats. GES with long pulses was found to inhibit antral contractions via the sympathetic mechanism (Ouyang et al. 2005). Since visceral signaling to the CNS is mediated through vagal and sympathetic afferent pathways, and GES is possibly capable of altering extrinsic nerves, we hypothesized that GES may influence visceral sensitivity.
The aims of this study were therefore 1) to study possible vagal and sympathetic mechanisms involved in the visceral sensitivity to gastric distention; and 2) to investigate the effects and possible autonomic mechanisms of GES on gastric distention-induced visceral sensitivity in dogs.
2. Material and methods
2.1. Animals
Fourteen healthy female dogs (20–26 kg) were used in this study. After an overnight fast, the dogs were anesthetized with an initial intravenous infusion of sodium thiopental (5 mg/kg; Abbott Laboratories, North Chicago, IL, USA) and maintained on IsoFlo (1.5% isoflurane, inhalation anesthesia; Abbott Laboratories, North Chicago, IL, USA) in oxygen–nitrous oxide (1:1) carrier gases delivered from a ventilator following endotracheal intubation. Laparotomy was performed. One pair of cardiac pacing wires were implanted on the gastric serosa of the great curvature 14 cm (G1) above the pylorus. The two electrodes in the pair were arranged circumferentially with a distance of 2cm. The electrodes were allowed to penetrate into the subserosal layer and were affixed to the serosa by non-absorbable sutures. The connecting wires of the electrodes were tunneled through the anterior abdominal wall subcutaneously along the right side of the trunk and placed outside the skin around the right hypochondrium for attachment to the recorder (World Precision Instruments, Sarasota, FL, USA). A gastric cannula was placed in the anterior wall of the stomach, 10 cm proximal the pylorus.
In 6 of the 14 dogs, bilateral truncal vagotomy was performed at the level of hernia of the diaphragm. A segment (1 cm) of the ventral/dorsal trunk of the vagus innervating the stomach was excised to prevent the regeneration of these nerves. The ramifications to the stomach were also severed to ensure that all these vagal nerves innervating the stomach were denervated (Ouyang et al., 2004).
The dogs were transferred to recovery cages after receiving medications for postoperative pain control. The study was initialed after a two-week recovery from surgery. The surgical procedures and experimental protocol were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston, Texas.
2.2. Experimental protocol
All experiments were conducted in the morning after an overnight fast in alert and conscious dogs. Before the initiation of experiments, the dogs were trained to stand on a table for a period of up to two weeks until no distress signs were observed. During the experiments, the animal was placed in a sling with a minimal restrain: the dog was freely standing (not supported by the sling) in the sling with the leash loosely attached to one of the poles of the sling. The experiment in the normal dogs was composed of four randomized sessions on separate days: control (no GES), pulse train GES, long pulse GES and dual-pulse GES. The experiment in the vagotomized dogs was composed of three randomized sessions on separate days: vagotomy control session (marked as Vag.), long pulse GES session (Vag.+LP) and guanethidine session (Vag.+Gua.). In each session, isotonic proximal stomach distention with different pressures (5mmHg, 10 mmHg, 15 mmHg, 20 mmHg, 25 mmHg, 30 mmHg) was applied via a noncompliant balloon placed in the fundus via the gastric cannula using a barostat system (G & J Electronics Inc., Toronto, ON, Canada). These pressures were chosen because they were in the physiological range and could be tolerated by the animals. Animal behavioral changes were noted and graded at each distention level. The protocol of each session included a) 15 min at baseline, b) 5 min with gastric distention at a specific pressure, and c) 5-min recovery period without distention. Steps b) and c) are repeated with the distention pressure increased from 5mmHg to 30mmHg with a step of 5mmHg.
In the sessions with GES, GES was applied during the entire session. In the sessions with guanethidine, guanethidine (3mg/kg, i.v. Sigma, St. Louis.) was given after the baseline recording but 5 min before the initiation of gastric distention. The selection of the dose was based on a previous canine study in which guanethidine at this dose was reported to block the effect of intestinal electrical stimulation on intestinal motility (Liu et al., 2006b).
2.3. Placement of the barostat balloon and gastric distention
Gastric distention was achieved using an intragastric balloon and a barostat system. Before the baseline recording in each experimental session, the gastric cannula was opened and a double-lumen catheter with a finely folded adherent plastic bag (1200ml capacity; maximal diameter: 17 cm, MUI Scientific, Mississauga, ON, Canada) attached to its distal end was inserted into the proximal stomach through the gastric cannula. The balloon was placed to the direction of the fundus. The catheter was secured once the desired position of the balloon was reached. When inflated, the balloon covered the fundus and some area of gastric corpus. The catheter was connected to an electronic barostat system. The folded balloon was fully opened by briefly inflating the balloon which was then deflated. Gastric distention was performed at a pressure from 5mmHg to 30mmHg with a stepwise increase of 5mmHg. There was a 5-min recovery period without distention between two consecutive distention levels. The method of a stepwise increase in distention pressure has been commonly used in clinical research for the assessment of visceral sensation in health and patients with functional dyspepsia (Tack et al., 2001).
2.4. Gastric electrical stimulation
GES was applied by a universal stimulator (WPI, Sarasota, FL, USA). Three types of GES were chosen for this study: 1) short pulse GES: the stimulus was composed of repetitive trains of 10 short pulses: frequency of 100Hz, amplitude of 5mA, width of 0.33ms with train on time of 0.1s and off time of 0.4s; This set of parameters was found to reduce visceral discomfort in a rodent model of visceral hypersensitivity in our lab (Sun et al. 2010). 2) long pulse GES: the electrical stimulus was composed of repetitive single pulses with a pulse width of 100ms, amplitude of 5mA and frequency of 5 pulses per minute which is in the vicinity of the physiological frequency of the canine gastric slow wave; GES with similar parameters was found to improve gastric dysrhythmia (Lin et al., 1998); 3) dual pulse GES: it was composed of ten short pulses with a width of 0.33ms followed by a long pulse of 100ms, repeated at a frequency of 5/min. This type of GES was found to improve drug-induced emesis and gastric dysrhythmia in dogs (Chen et al., 2003; Song et al., 2006). Since none of previous studies have investigated the role of GES in ameliorating visceral discomfort in dogs, we chose these parameters based on their effects on other measurements.
2.5. Assessment of visceral sensitivity to gastric distention
The dog is not a good model for studying visceral pain, instead the rat is a well-established model for assessing pain via the measurement of abdominal EMG and abdominal withdraw score. In this study, however, we focused on visceral sensitivity that was defined as any behavioral changes in response to visceral stimulation (gastric distention). Animal behavioral changes to gastric distention in dogs were assessed using a previously established method (Chen et al., 2003; Xu et al., 2005; Xu et al., 2004), including movement due to discomfort, salivation, licking tongue, murmuring, yawning, closing eyes, abnormal breathing and vomiting (belching, liquid or solid vomiting). Some behaviors included pain, discomfort, nausea and vomiting. The degree of severity for each of these behavioral changes was graded as, none = 0, mild = 1, moderate = 2 and severe = 3. Vomiting was scored as 3 (Chen et al. 2003). Accordingly, the highest behavioral score was 3 (the highest score per sign) × 8 (the maximum number of the signs) = 24 for each distention level. The total behavioral score (TBS) was defined as the sum of the scores during all 6 distention periods, with the highest score of 144. To make the evaluation objective, the person who evaluated the signs was blinded from the study design and objectives.
2.6. Assessment of gastric tone
Gastric tone was measured by a barostat system (Distender Series II, G & J Electronics, Inc., Toronto, Canada). A barostat balloon was inserted into the stomach via the gastric cannula and connected to the barostat device. Gastric volume was measured at an operating pressure that was the minimum distention pressure plus 2 mmHg. Gastric tone was reflected by the intragastric balloon volume: a higher gastric volume was indicative of reduced gastric tone and vice versa. The recording of gastric volume with and without GES lasted 20 min or more to ensure that the recording was stable.
2.7. Statistical analysis
All data are presented as mean ± SE. Analysis of variance (ANOVA) was applied to assess the difference in visceral sensitivity among different sessions. Paired t-test and unpaired t-test were used to investigate the difference in visceral sensitivity between two sessions in the same group of the animals, and between two different groups of animals, respectively. P < 0.05 was considered significant.
3. Results
3.1. Effects and mechanisms of gastric distention on animal behaviors
Gastric distention induced animal behavioral changes suggestive of visceral sensation, and the changes were mediated by both vagal and sympathetic pathways. The total behavioral score during 6 distention periods was 40.6±7.4 in the control dogs (P<0.001 vs. baseline without distention), 15.3±8.9 in vagotomized dogs (P=0.006 vs. the control dogs) and 8.8±0.9 in the vagotomized dogs with guanethidine (P=0.04 vs. the vagotomized dogs without guanethidine).
Further more, the gastric distention-induced behavioral changes were found to be mediated exclusively via the vagal pathway at distention pressures below 20mmHg, but mediated via both the vagal and sympathetic pathway at distention pressures equal to and above 20mmHg. As shown in Fig. 1, at pressures below 20mmHg: 1) there was a significant and pressure-dependent increase in the behavioral score in the control dogs; this gastric distention-induced increase in the behavioral score was almost completely disappeared in the vagotomized dogs, suggesting the involvement of the vagal pathway; 2) application of guanethidine to the vagotomized dogs did not reduce the behavioral score any further, suggesting the exclusion of the sympathetic pathway.
Fig. 1.
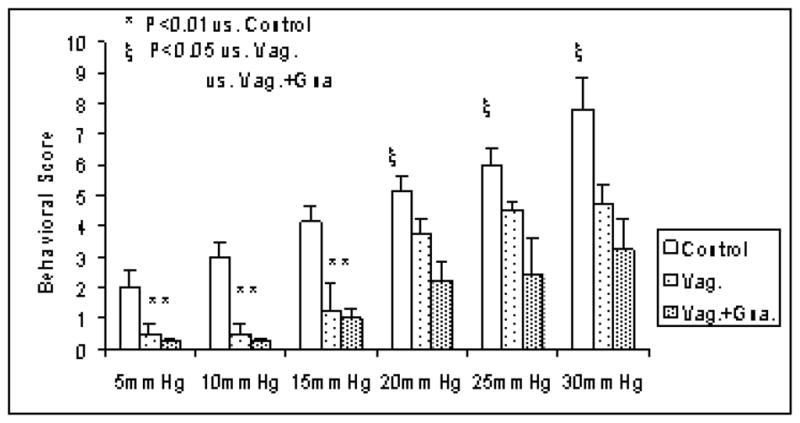
Involvement of vagal and sympathetic pathways at different distention pressures in normal and vagotomized dogs. In nomal dogs, a pressure-dependent increase in the behavioral score was noted; In vagotomized dogs, this gastric distention-induced increase in the behavioral score was almost completely disappeared at pressures below 20mmHg, and was decreased at pressures of 20mmHg and above.
At pressures of 20mmHg and above: 1) there was a similar pressure-dependent increase in the behavioral score in the control dogs; however, vagotomy reduced but not completely blocked the effects of gastric distention on the behavioral score (P<0.05, vs. control, at 20, 25 and 30mmHg), suggesting the partial involvement of vagal pathway; 2) application of guanethidine to the vagotomized dogs further reduced the behavioral score (P<0.05, vs. vagotomized dogs without guanethidine at 20, 25 and 30mmHg), suggesting the involvement of the sympathetic pathway.
3.2. Effects and mechanisms of GES on gastric distention-induced behavioral changes
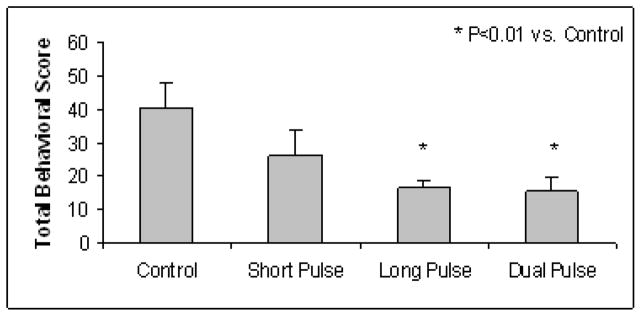
GES of long pulses or dual pulses but not short pulse reduced the gastric distention-induced behavioral scores and the effect might be mediated through both vagal and sympathetic pathways. As shown in Fig. 2, the TBS during distention in the normal control dogs was 40.6±7.4 in the control session, 26.2±8.1 with GES of short pulse (P=0.7 vs. control), 16.4±2.1 with long pulse GES (P<0.003 vs. control), and 15.3±4.3 with dual pulse GES (P<0.006 vs. control). Since both long pulse GES and dual pulse GES had the same component of long pulses, these findings suggested that the ameliorating of GES on gastric distention-induced visceral discomfort was attributed to the long pulses.
Fig. 2.
Effects of GES on gastric distention-induced behavioral changes. GES of long pulses or dual pulses but not short pulses reduced the gastric distention-induced behavioral scores.
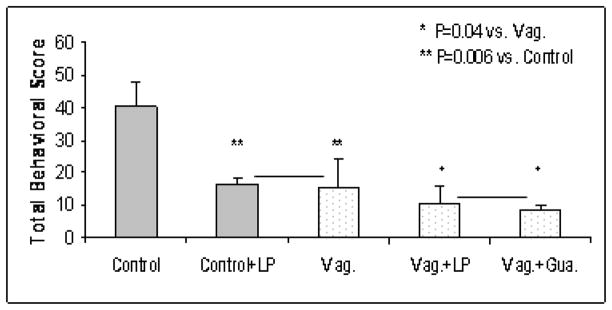
Further more, from Fig. 3, we can see that the reduction in the TBS with long pulse GES in the normal control dogs (panel of “Control+LP”, 16.4±2.1, P=0.006 vs. control) was the same as that in the vagotomized dogs (panel of “Vag”, 15.3±8.9, P=0.006 vs. control). This meant that the effect of long pulse GES was the equivalent to that of vagotomy. By comparing the TBS shown in panels “Vag.+LP” and “Vag.+Gua.” in Fig. 3, we can see that the effects of long pulse GES on the vagotomized dogs (10.8±4.9, P=0.04, vs. Vag.) was equivalent to that of the application of guanethidine (8.8±1.1, P=0.04, vs. Vag.).
Fig. 3.
Possible mechanisms of GES on gastric distention-induced behavioral changes. The TBS during gastric distention was similarly reduced in normal dogs with GES of long pulse and vagotomized dogs; In vagotomized dogs, the TBS during gastric distention was also similarly reduced by GES of long pulses and guanethidine.
3.3. Effects of GES on gastric tone
Long pulse GES with the same parameters that were effective in reducing the behavioral score did not alter gastric tone. The gastric balloon volume (reflecting gastric tone) during all pressure levels of gastric distention was 3,374±241ml in the control session without GES, 3,334±152.9ml with GES of long pulses and 3,729±269.3ml with GES of short pulses (P>0.05, ANOVA).
4. Discussion
In this study we found that gastric distention induced behavioral changes suggestive of visceral sensation in a pressure-dependent manner in normal dogs. The induction of the behavioral changes with gastric distention at low pressures (below 20 mmHg) was almost completely blocked by vagotomy alone, whereas at high pressures, the effect of gastric distention was blocked by both vagotomy and the administration of guanethidine. GES of long pulses or dual pulses but not short pulse trains reduced the gastric distention-induced increase in visceral sensitivity (or the TBS) and the ameliorating effect of GES in the normal dogs was found to be comparable with vagotomy and the ameliorating effect of GES in the vagotomized dogs was similar to the application of guanethidine.
Distension of the gut is one of the main origins of primary abdominal discomfort of the gut (Wood, 2007). Sensory information of the gut reaching the central nervous system gives rise to both painful and non-painful sensation, and influences feeding and illness behaviors (Grundy et al., 2006). In this study, visceral sensitivity to gastric distention was observed and the effects of GES on distention-induced visceral sensitivity were assessed using animal behavioral scores. This behavioral score grading method was used in a number of previous studies (Chen et al., 2003; Xu et al., 2005; Xu et al., 2004) and included all major behavioral changes suggestive visceral sensation in dogs. In rats, abdominal electromyogram is an established method for assessing visceral pain. However, such a method has not been validated in dogs. Therefore, in this study, we used general animal behaviors to assess visceral sensitivity.
We found that gastric distention induced a pressure-dependent increase in the animal behavioral score in the normal dogs. However, vagotomy alone almost completely blocked gastric distention-induced behavioral changes at lower distention pressures and this suggested the involvement of the vagal pathway with gastric distention. Interestingly, both vagal and sympathetic pathways were found to be involved with gastric distention at distention pressures of 20mmHg or above. Basic research has shown that vagal and spinal afferent nerve fibers transmit gastrointestinal sensory information to the central nervous system. Vagal mechanoreceptors generally have low distention thresholds of activation, while spinal afferents are classified as low-threshold, high-threshold, or silent mechanoreceptors. Low-threshold afferents respond to physiologic levels of distention, whereas high-threshold afferents respond to higher levels of distention that are in the noxious range. Silent nociceptors do not respond at all in the normal intestine but become responsive to distention when the intestine is injured or inflamed (Gebhart, 2000). Injury (i.e., high pressure distention) and inflammation may produce peripheral sensitization by decreasing the threshold and increasing the magnitude of the response for a given stimulus (Cervero et al., 2003). Our findings showing different effects and mechanisms of gastric distention at different pressure levels were in agreement with these concepts.
Various methods of electrical stimulation have been derived from the variations of electrical stimuli, including long-pulse stimulation, short-pulse stimulation, pulse train stimulation, dual pulse (several short pulses followed by one long pulse) stimulation and synchronized dual pulse stimulation as described in a recent review (Zhang et al., 2006b). Long pulse GES and dual pulse GES were found to improve gastric distention-induced discomfort in this study. Since it was the common component of these two GES methods, the long pulse was regarded as the sole effective component in reducing gastric distention-induced behavioral score. Accordingly, the further mechanistic experiment was performed using only GES of long pulses. The results of the study indicated that GES of long pulses was not only effective in reducing the TBS in the control dogs but also in the vagotomized dogs. The comparable effects of GES with vagotomy and guanethidine suggested a possibility of the involvement of the vagal and sympathetic pathway; however, this could not be confirmed in the current studies. The vagal and sympathetic mechanisms of GES have been previously investigated and positively reported (Ouyang et al., 2005; Zhu et al., 2007).
Additional experiment was performed in this study to investigate whether the ameliorating effect of GES on gastric distention-induced increase in the animal behavioral score was attributed to its inhibitory effect on gastric tone. In a number of previous studies (Sun et al., 2006; Xing et al., 2003; Xing et al., 2006), GES with long pulses was shown to inhibit gastric tone reflected as an increase in gastric volume. In this study, however, GES did not alter gastric volumes, suggesting that the ameliorating effect of GES on the gastric distention-induced behavioral changes was not attributed to its effect on gastric tone. The difference in their effects on gastric tone between this current study and previous studies was attributed to the difference in stimulation parameters, such as pulse width. Although long pulses were used in this and previous studies, the pulse width and stimulation frequency were both lower in this study than those used in the previous experiments (Sun et al., 2006; Xing et al., 2006). It has been well documented that GES with higher output is often inhibitory (Zhang et al., 2006a).
GES has been applied to treat gastrointestinal motility disorders as well as obesity (Abell et al., 2003; Anand et al., 2007; Andersson et al., 2006; Cigaina, 2002; Gourcerol et al., 2007a; Gourcerol et al., 2007b; Lin et al., 2007; Maranki et al., 2007; Nie et al., 2006; Sanmiguel et al., 2006; Tack, 2007; Zhang et al., 2006b). A number of animal and preliminary clinical studies have shown the effectiveness of long pulse GES or GES with higher output (Liu et al., 2006a; Yao et al., 2005; Yin et al., 2005; Zhang et al., 2007). The findings of this study seem to suggest the potential of GES with appropriate parameters for ameliorating gastrointestinal distention-induced symptoms. Future studies are needed to prove this anticipated new application.
In conclusion, gastric distention-induced visceral discomfort is mediated via the vagal pathway at low distention pressures but via both vagal and sympathetic pathways at high distention pressures. GES with long but not short pulses reduces gastric distention-induced visceral sensitivity.
Acknowledgments
This study was partially supported by a grant from National Institutes of Health (1R43CA121489).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- Anand C, Al-Juburi A, Familoni B, Rashed H, Cutts T, Abidi N, Johnson WD, Minocha A, Abell TL. Gastric electrical stimulation is safe and effective: a long-term study in patients with drug-refractory gastroparesis in three regional centers. Digestion. 2007;75:83–89. doi: 10.1159/000102961. [DOI] [PubMed] [Google Scholar]
- Andersson S, Lonroth H, Simren M, Ringstrom G, Elfvin A, Abrahamsson H. Gastric electrical stimulation for intractable vomiting in patients with chronic intestinal pseudoobstruction. Neurogastroenterol Motil. 2006;18:823–830. doi: 10.1111/j.1365-2982.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Grundy D, Scratcherd T. Vagal afferent discharge from mechanoreceptors in different regions of the ferret stomach. J Physiol. 1980;298:513–524. doi: 10.1113/jphysiol.1980.sp013098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgutay AM, Lillehei CW, Wingrove R, Griffen WO, Bonnabeau RC. Gastrointestinal Pacing: A New Concept in the Treatment of Ileus. Biomed Sci Instrum. 1963;1:377–383. [PubMed] [Google Scholar]
- Cervero F, Laird JM. Role of ion channels in mechanisms controlling gastrointestinal pain pathways. Curr Opin Pharmacol. 2003;3:608–612. doi: 10.1016/j.coph.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12(Suppl 1):12S–16S. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Gonzalez MF, Deutsch JA. Vagotomy abolishes cues ofsatiety produced by gastric distention. Science. 1981;212:1283–1284. doi: 10.1126/science.7233218. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Gallas S, Mounien L, Leblanc I, Bizet P, Boutelet I, Leroi AM, Ducrotte P, Vaudry H, Jegou S. Gastric electrical stimulation modulates hypothalamic corticotropin-releasing factor-producing neurons during post-operative ileus in rat. Neuroscience. 2007a;148:775–781. doi: 10.1016/j.neuroscience.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Leblanc I, Leroi AM, Denis P, Ducrotte P. Gastric electrical stimulation in medically refractory nausea and vomiting. Eur J Gastroenterol Hepatol. 2007b;19:29–35. doi: 10.1097/01.meg.0000250584.15490.b4. [DOI] [PubMed] [Google Scholar]
- Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Tache Y, Wood JD. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- Hiyama T, Yoshihara M, Matsuo K, Kusunoki H, Kamada T, Ito M, Tanaka S, Chayama K, Haruma K. Treatment of functional dyspepsia with serotonin agonists: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2007;22:1566–1570. doi: 10.1111/j.1440-1746.2006.04723.x. [DOI] [PubMed] [Google Scholar]
- Holtmann G, Gschossmann J, Neufang-Huber J, Gerken G, Talley NJ. Differences in gastric mechanosensory function after repeated ramp distensions in non-consulters with dyspepsia and healthy controls. Gut. 2000;47:332–336. doi: 10.1136/gut.47.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupcinskas L, Lafolie P, Lignell A, Kiudelis G, Jonaitis L, Adamonis K, Andersen LP, Wadstrom T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine. 2008;15:391–399. doi: 10.1016/j.phymed.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Lin Z, Sarosiek I, McCallum RW. Gastrointestinal electrical stimulation for treatment of gastrointestinal disorders: gastroparesis, obesity, fecal incontinence, and constipation. Gastroenterol Clin North Am. 2007;36:713–734. x–xi. doi: 10.1016/j.gtc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274:G186–191. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- Liu J, Hou X, Song G, Cha H, Yang B, Chen JD. Gastric electrical stimulation using endoscopically placed mucosal electrodes reduces food intake in humans. Am J Gastroenterol. 2006a;101:798–803. doi: 10.1111/j.1572-0241.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu J, Chen JD. Neural mechanisms involved in the inhibition of intestinal motility induced by intestinal electrical stimulation in conscious dogs. Neurogastroenterol Motil. 2006b;18:62–68. doi: 10.1111/j.1365-2982.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- Madisch A, Miehlke S. How effective is itopride for the treatment of patients with functional dyspepsia? Nat Clin Pract Gastroenterol Hepatol. 2006;3:490–491. doi: 10.1038/ncpgasthep0579. [DOI] [PubMed] [Google Scholar]
- Maranki J, Parkman HP. Gastric electric stimulation for the treatment of gastroparesis. Curr Gastroenterol Rep. 2007;9:286–294. doi: 10.1007/s11894-007-0032-1. [DOI] [PubMed] [Google Scholar]
- McCallum RW, Dusing RW, Sarosiek I, Cocjin J, Forster J, Lin Z. Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients. Neurogastroenterol Motil. 2010 Feb;22(2):161–7. e50–1. doi: 10.1111/j.1365-2982.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- Nie Y, Chen JD. Effects and mechanisms of anal electrical stimulation on anorectal compliance and tone in dogs. Dis Colon Rectum. 2006;49:1414–1421. doi: 10.1007/s10350-006-0599-x. [DOI] [PubMed] [Google Scholar]
- Oshima T, Miwa H. Treatment of functional dyspepsia: where to go and what to do. J Gastroenterol. 2006;41:718–719. doi: 10.1007/s00535-006-1865-3. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Xing J, Chen J. Electroacupuncture restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci. 2004;49:1418–1424. doi: 10.1023/b:ddas.0000042240.05247.01. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Xing J, Chen JD. Tachygastria induced by gastric electrical stimulation is mediated via alpha- and beta-adrenergic pathway and inhibits antral motility in dogs. Neurogastroenterol Motil. 2005;17:846–853. doi: 10.1111/j.1365-2982.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- Peles S, Petersen J, Aviv R, Policker S, Abu-Hatoum O, Ben-Haim SA, Gutterman DD, Sengupta JN. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285(3):G577–85. doi: 10.1152/ajpgi.00109.2003. [DOI] [PubMed] [Google Scholar]
- Qin C, Sun Y, Chen JD, Foreman RD. Gastric electrical stimulation modulates neuronal activity in nucleus tractus solitarii in rats. Auton Neurosci. 2005;29, 119(1):1–8. doi: 10.1016/j.autneu.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sanmiguel CP, Casillas S, Senagore A, Mintchev MP, Soffer EE. Neural gastrointestinal electrical stimulation enhances colonic motility in a chronic canine model of delayed colonic transit. Neurogastroenterol Motil. 2006;18:647–653. doi: 10.1111/j.1365-2982.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Smith ML. Functional dyspepsia pathogenesis and therapeutic options--implications for management. Dig Liver Dis. 2005;37:547–558. doi: 10.1016/j.dld.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Song G, Hou X, Yang B, Sun Y, Liu J, Qian W, Chen JD. Efficacy and efficiency of gastric electrical stimulation with short pulses in the treatment of vasopressin-induced emetic responses in dogs. Neurogastroenterol Motil. 2006;18:385–391. doi: 10.1111/j.1365-2982.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen JD. Gastric electrical stimulation inhibits postprandial antral tone partially via nitrergic pathway in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2006;290:R904–908. doi: 10.1152/ajpregu.00842.2004. [DOI] [PubMed] [Google Scholar]
- Sun Y, Qin C, Chen JDZ. Effects and Mechanisms of Gastric Electrical Stimulation on Visceral Hypersensitivity in Rats with Gastric Ulcers. Gastroenterology. 2010 supplement (DDW abstract) [Google Scholar]
- Tack J. The difficult patient with gastroparesis. Best Pract Res Clin Gastroenterol. 2007;21:379–391. doi: 10.1016/j.bpg.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- Vandenberghe J, Dupont P, Van Oudenhove L, Bormans G, Demyttenaere K, Fischler B, Geeraerts B, Janssens J, Tack J. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology. 2007;132:1684–1693. doi: 10.1053/j.gastro.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–324. [PubMed] [Google Scholar]
- Weinland SR, Drossman DA. Should clinicians integrate medical and psychological interventions for the treatment of functional dyspepsia? Nat Clin Pract Gastroenterol Hepatol. 2008;5:68–69. doi: 10.1038/ncpgasthep1006. [DOI] [PubMed] [Google Scholar]
- Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13:1313–1332. doi: 10.3748/wjg.v13.i9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing JH, Brody F, Brodsky J, Larive B, Ponsky J, Soffer E. Gastric electrical stimulation at proximal stomach induces gastric relaxation in dogs. Neurogastroenterol Motil. 2003;15:15–23. doi: 10.1046/j.1365-2982.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- Xing JH, Chen JD. Effects and mechanisms of long-pulse gastric electrical stimulation on canine gastric tone and accommodation. Neurogastroenterol Motil. 2006;18:136–143. doi: 10.1111/j.1365-2982.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Brining DL, Chen JD. Effects of vasopressin and long pulse-low frequency gastric electrical stimulation on gastric emptying, gastric and intestinal myoelectrical activity and symptoms in dogs. Neurogastroenterol Motil. 2005;17:236–244. doi: 10.1111/j.1365-2982.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Qian L, Chen JD. Anti-dysrhythmic effects of long-pulse gastric electrical stimulation in dogs. Digestion. 2004;69:63–70. doi: 10.1159/000077390. [DOI] [PubMed] [Google Scholar]
- Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Visceral sensitivity to gastric stimulation and its correlation with alterations in gastric emptying and accommodation in humans. Obes Surg. 2005;15:247–253. doi: 10.1381/0960892053268363. [DOI] [PubMed] [Google Scholar]
- Yin J, Chen JD. Retrograde gastric electrical stimulation reduces food intake and weight in obese rats. Obes Res. 2005;13:1580–1587. doi: 10.1038/oby.2005.194. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen JD. Pacing the gut in motility disorders. Curr Treat Options Gastroenterol. 2006a;9:351–360. doi: 10.1007/s11938-006-0017-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006b;24:991–1002. doi: 10.1111/j.1365-2036.2006.03087.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu X, Chen JD. Chronic tachygastrial electrical stimulation reduces food intake in dogs. Obesity (Silver Spring) 2007;15:330–339. doi: 10.1038/oby.2007.557. [DOI] [PubMed] [Google Scholar]
- Zhu H, Sallam H, Chen DD, Chen JD. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1875–1881. doi: 10.1152/ajpregu.00821.2006. [DOI] [PubMed] [Google Scholar]