Abstract
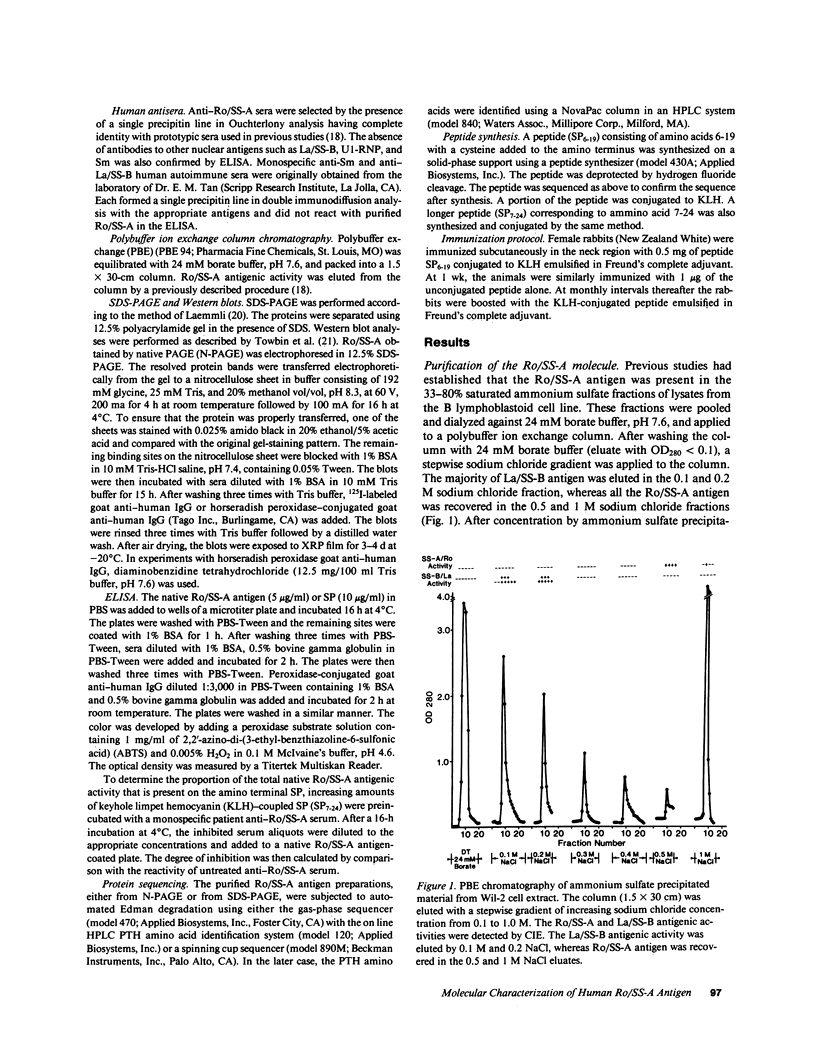
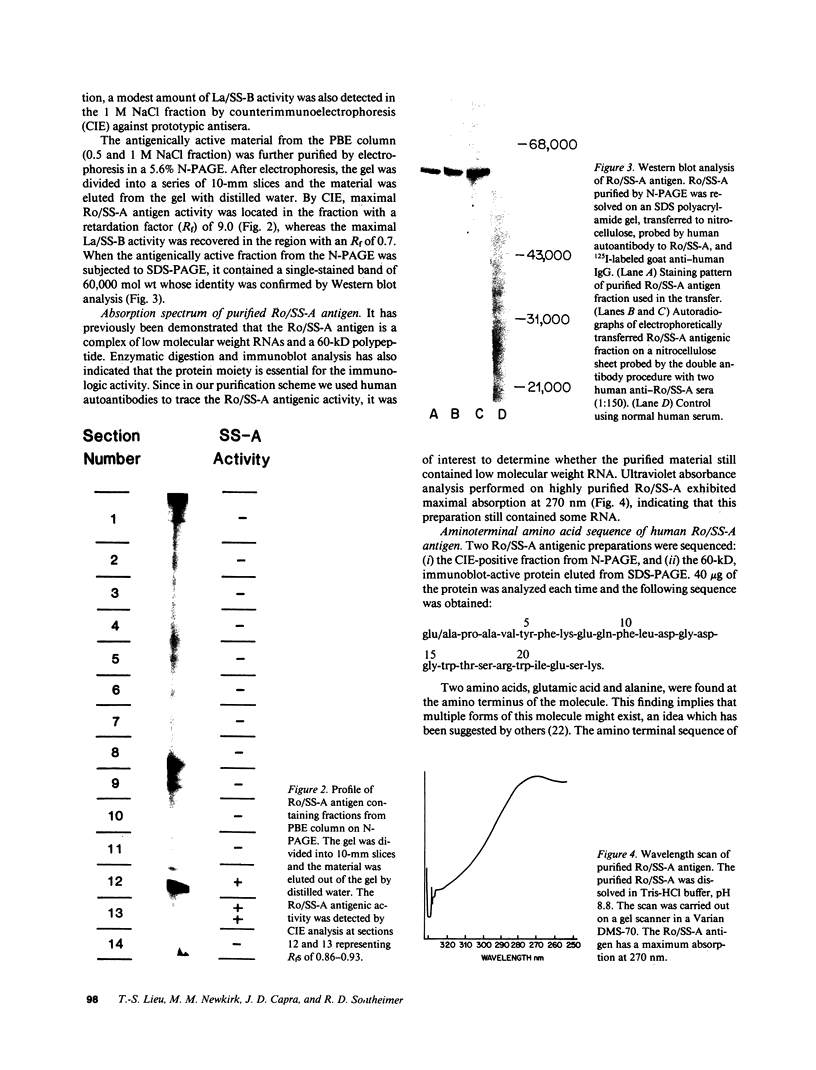
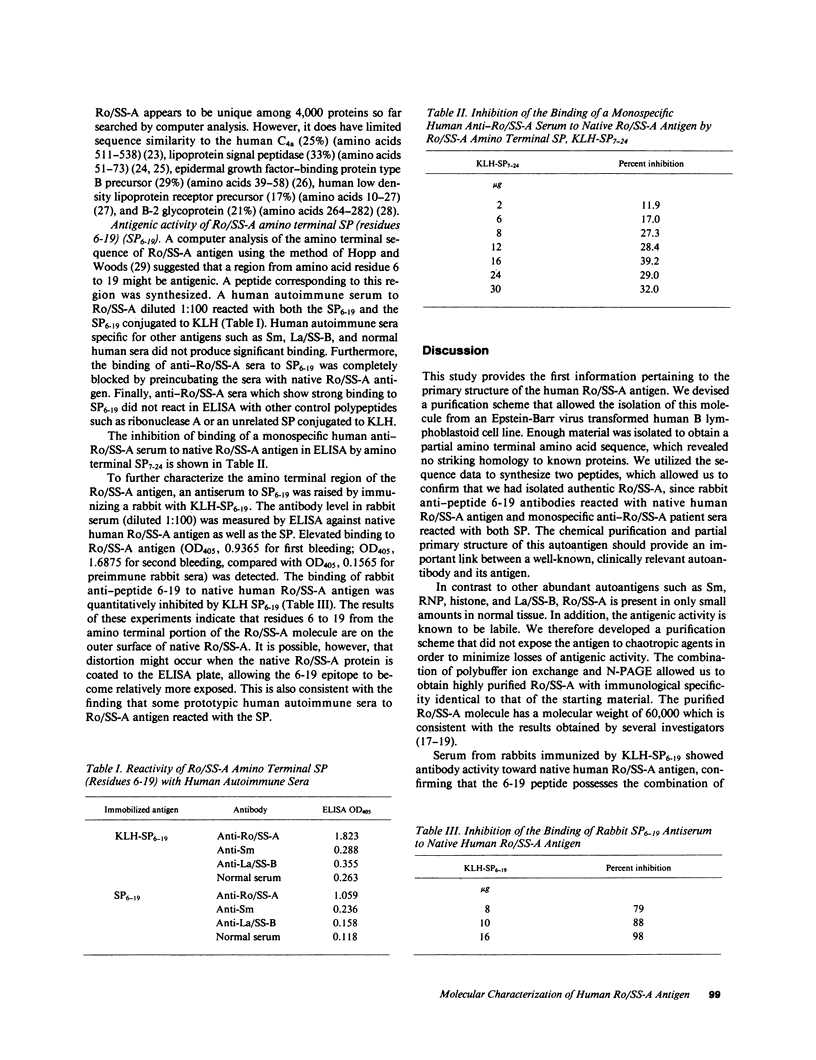
The Ro/SS-A antigen was purified from an Epstein-Barr virus-transformed human B lymphoblastoid cell line. The amino terminal amino acid sequence of the 60-kD polypeptide bearing this antigenic epitope was determined to be: (formula; see text) A peptide composed of residue 6-19 was synthesized by the solid-phase method. Immunodiffusion-defined monospecific autoimmune sera to Ro/SS-A reacted with this synthetic peptide in ELISA, whereas autoantibodies with other specificities such as anti-La/SS-B and anti-Sm, as well as normal human sera, were not reactive. In addition, rabbit anti-peptide 6-19 antisera reacted specifically with native human Ro/SS-A antigen in ELISA. Furthermore, this synthetic peptide inhibited the binding of rabbit anti-peptide antiserum to native human Ro/SS-A. An additional synthetic peptide corresponding to residues 7-24 partially inhibited the binding of a patient anti-Ro/SS-A serum to native Ro/SS-A. These results suggest that the amino terminal portion of the molecule represents a major epitope of Ro/SS-A. The determination of the amino acid sequence of Ro/SS-A and the availability of synthetic peptide(s) bearing this antigen should provide additional approaches to further characterize the autoimmune response to this antigen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Arnett F. C., Provost T. T., Stevens M. B. Sjögren's syndrome: association of anti-Ro(SS-A) antibodies with vasculitis, hematologic abnormalities, and serologic hyperreactivity. Ann Intern Med. 1983 Feb;98(2):155–159. doi: 10.7326/0003-4819-98-2-155. [DOI] [PubMed] [Google Scholar]
- Alspaugh M. A., Talal N., Tan E. M. Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum. 1976 Mar-Apr;19(2):216–222. doi: 10.1002/art.1780190214. [DOI] [PubMed] [Google Scholar]
- Alspaugh M. A., Tan E. M. Antibodies to cellular antigens in Sjögren's syndrome. J Clin Invest. 1975 May;55(5):1067–1073. doi: 10.1172/JCI108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Bixler G. S., Jr, Bean M., Atassi M. Z. Site recognition by protein-primed T cells shows a non-specific peptide size requirement beyond the essential residues of the site. Demonstration by defining an immunodominant T site in myoglobin. Biochem J. 1986 Nov 15;240(1):139–146. doi: 10.1042/bj2400139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruist M. F., Horvath S. J., Hood L. E., Steitz T. A., Simon M. I. Synthesis of a site-specific DNA-binding peptide. Science. 1987 Feb 13;235(4790):777–780. doi: 10.1126/science.3027895. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Chambers J. C., Keene J. D. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2115–2119. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G., Reichlin M., Tomasi T. B., Jr Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969 Jan;102(1):117–122. [PubMed] [Google Scholar]
- Deng J. S., Sontheimer R. D., Gilliam J. N. Expression of Ro/SS-A antigen in human skin and heart. J Invest Dermatol. 1985 Nov;85(5):412–416. doi: 10.1111/1523-1747.ep12277079. [DOI] [PubMed] [Google Scholar]
- Franco H. L., Weston W. L., Peebles C., Forstot S. L., Phanuphak P. Autoantibodies directed against sicca syndrome antigens in the neonatal lupus syndrome. J Am Acad Dermatol. 1981 Jan;4(1):67–72. doi: 10.1016/s0190-9622(81)70011-2. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Geysen H. M., Rodda S. J., Alexander H., Tainer J. A., Lerner R. A. Mechanisms of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1191–1196. doi: 10.1126/science.3823879. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Tokunaga M., Williams M. E., Loranger J. M., Chang S. Y., Chang S., Wu H. C. Nucleotide sequence of the Escherichia coli prolipoprotein signal peptidase (lsp) gene. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3708–3712. doi: 10.1073/pnas.81.12.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. A novel and comprehensive synthetic approach for the elucidation of protein antigenic structures. Determination of the full antigenic profile of the alpha-chain of human haemoglobin. Biochem J. 1980 Oct 1;191(1):261–264. doi: 10.1042/bj1910261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Structurally inherent antigenic sites. Localization of the antigenic sites of the alpha-chain of human haemoglobin in three host species by a comprehensive synthetic approach. Biochem J. 1982 Apr 1;203(1):201–208. doi: 10.1042/bj2030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kephart D. C., Hood A. F., Provost T. T. Neonatal lupus erythematosus: new serologic findings. J Invest Dermatol. 1981 Sep;77(3):331–333. doi: 10.1111/1523-1747.ep12482531. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeFeber W. P., Norris D. A., Ryan S. R., Huff J. C., Lee L. A., Kubo M., Boyce S. T., Kotzin B. L., Weston W. L. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984 Oct;74(4):1545–1551. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. A., Harmon C. E., Huff J. C., Norris D. A., Weston W. L. The demonstration of SS-A/Ro antigen in human fetal tissues and in neonatal and adult skin. J Invest Dermatol. 1985 Aug;85(2):143–146. doi: 10.1111/1523-1747.ep12276566. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Jiang M., Steigerwald J. C., Tan E. M. Identification of the SS-A/Ro intracellular antigen with autoimmune sera. J Immunol Methods. 1984 Jul 6;71(2):217–228. doi: 10.1016/0022-1759(84)90068-1. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Reimer C. B., Sontheimer R. D. Immunoglobulin class and subclass profile of the Ro/SS-A autoantibody response. J Invest Dermatol. 1988 Feb;90(2):158–164. doi: 10.1111/1523-1747.ep12462142. [DOI] [PubMed] [Google Scholar]
- Lozier J., Takahashi N., Putnam F. W. Complete amino acid sequence of human plasma beta 2-glycoprotein I. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin L. R., 3rd, Hall J., Hogan J. D., Tucker S. B., Jordon R. E. Neonatal lupus erythematosus. A report of three cases associated with anti-Ro/SSA antibodies. Arch Dermatol. 1985 Mar;121(3):377–381. doi: 10.1001/archderm.121.3.377. [DOI] [PubMed] [Google Scholar]
- Lundgren S., Ronne H., Rask L., Peterson P. A. Sequence of an epidermal growth factor-binding protein. J Biol Chem. 1984 Jun 25;259(12):7780–7784. [PubMed] [Google Scholar]
- Maddison P. J., Reichlin M. Deposition of antibodies to a soluble cytoplasmic antigen in the kidneys of patients with systemic lupus erythematosus. Arthritis Rheum. 1979 Aug;22(8):858–863. doi: 10.1002/art.1780220808. [DOI] [PubMed] [Google Scholar]
- Mamula M. J., Fox O. F., Yamagata H., Harley J. B. The Ro/SSA autoantigen as an immunogen. Some anti-Ro/SSA antibody binds IgG. J Exp Med. 1986 Dec 1;164(6):1889–1901. doi: 10.1084/jem.164.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S., Kitamura W., Yoshioka J., Sakamoto K. Placental transfer of anticytoplasmic antibodies in annular erythema of newborns. Arch Dermatol. 1981 Sep;117(9):569–572. [PubMed] [Google Scholar]
- Scott J. S., Maddison P. J., Taylor P. V., Esscher E., Scott O., Skinner R. P. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983 Jul 28;309(4):209–212. doi: 10.1056/NEJM198307283090403. [DOI] [PubMed] [Google Scholar]
- Sontheimer R. D., Maddison P. J., Reichlin M., Jordon R. E., Stastny P., Gilliam J. N. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982 Nov;97(5):664–671. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Gariépy J., Schoolnik G. K., McConnell H. M. T-cell activation by peptide antigen: effect of peptide sequence and method of antigen presentation. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5480–5484. doi: 10.1073/pnas.82.16.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston W. L., Harmon C., Peebles C., Manchester D., Franco H. L., Huff J. C., Norris D. A. A serological marker for neonatal lupus erythematosus. Br J Dermatol. 1982 Oct;107(4):377–382. doi: 10.1111/j.1365-2133.1982.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Harley J. B., Reichlin M. Molecular properties of the Ro/SSA antigen and enzyme-linked immunosorbent assay for quantitation of antibody. J Clin Invest. 1984 Aug;74(2):625–633. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Yu F., Yamada H., Daishima K., Mizushima S. Nucleotide sequence of the lspA gene, the structural gene for lipoprotein signal peptidase of Escherichia coli. FEBS Lett. 1984 Jul 23;173(1):264–268. doi: 10.1016/0014-5793(84)81060-1. [DOI] [PubMed] [Google Scholar]




