Abstract
Some viral vectors are potent inducers of cellular and humoral responses; therefore, viral vectors can be used to vaccinate against cancer or infectious diseases. This report will focus on adenovirus (Ad)-based vectors. Traditional viral-vector vaccination embodies the concept that the vector uses the host-cell machinery to express antigens that are encoded as transgenes within the viral vector. Several preclinical successes have used this approach in animal model systems. However, in some instances, these conventional Ad-based vaccines have yielded suboptimal clinical results. These suboptimal results are ascribed, in part, to preexisting Ad serotype 5 (Ad5) immunity. To address this issue, the “antigen capsid-incorporation” strategy has been developed to circumvent the drawbacks associated with conventional transgene expression of antigens by Ad vectors. This strategy embodies the incorporation of antigenic peptides within the capsid structure of viral vectors. Incorporating immunogenic peptides into the Ad capsid offers potential advantages. Importantly, vaccination by means of the antigen capsid-incorporated approach results in a strong humoral response, similar to the response generated by native Ad capsid proteins. This strategy also allows for the boosting of antigenic specific responses. This strategy may be the way forward for improved vaccine schemes, especially for those infections requiring a strong humoral antigenic response.
Keywords: Vaccine, Antigen, Capsid-Incorporation, Adenovirus, Virus
Introduction
Some viral vectors are potent inducers of cellular and humoral responses; therefore, using recombinant viral vectors expressing tumor antigens to activate the immune system is a promising approach to prevent or treat cancer. In addition, viral vectors can be used to express protein from pathogens to vaccinate against infectious diseases. Several viral vectors have been successfully used for vaccination in experimental models. Some viral vectors that have been used for vaccination against cancer and infectious diseases include: alphaviruses, human rhinoviruses, adenoviruses (Ads), picornaviruses, poxviruses, measles viruses, and influenza viruses 1–12. Although each of these vectors has disadvantages and advantages with respect to vaccine development, this report will focus on those of Ad-based vectors.
Adenovirus Vectors as Vaccine Agents
In 2009, Ad-based vectors accounted for 23.9% of those in gene-therapy clinical trials13. The broad utility of these vectors is derived from several key characteristics: (a) the recombinant viral genome is readily manipulated; (b) replication-defective Ads can be propagated in complementing cell lines; (c) Ads infect a broad range of target cells 14,15; and (d) Ads can achieve high levels of in vivo gene transfer with concomitantly high levels of transgene expression 15,16. These concepts have also led to Ads being used as molecular vaccine agents.
Traditional viral-vector vaccination embodies the concept that the vector uses the host-cell machinery to express antigens that are encoded as transgenes within the viral vector. Cellular and humoral immune responses are generated against these antigens for a vaccine effect. Several preclinical successes have used this approach in animal model systems. However, in some instances, these conventional Ad-based vaccines have yielded suboptimal clinical results. These suboptimal results are ascribed, in part, to preexisting Ad serotype 5 (Ad5) immunity (vector liver sequestration is another major drawback, however; this will not be discussed in this review). It is estimated that 50% to 90% of the adult population has preexisting immunity (PEI) to Ad5; if one of these Ad5-immune individuals is vaccinated with an Ad vector for therapeutic purposes, there may be limited transgene or antigen expression due to Ad clearance by the immune system 17–21.
The “antigen capsid-incorporation” strategy has been developed to circumvent the drawbacks associated with conventional transgene expression of antigens by vectors. With respect to Ad, one drawback to conventional transgene expression of antigen is the inability of Ad-based vectors to produce a potent humoral response against certain antigens (as seen in the case of some malaria antigens) 22. In addition, this strategy may allow vectors to circumvent Ad5 PEI yielding a more robust immune response to either the antigen presented on the vector capsid or the antigen that is expressed as a transgene. The antigen capsid-incorporation strategy embodies the incorporation of antigenic peptides within the capsid structure of viral vectors. Incorporating immunogenic peptides into the Ad capsid offers potential advantages. Importantly, processing the capsid-incorporated antigen via the exogenous pathway could result in a strong humoral response, similar to the response generated by native Ad capsid proteins. In addition, because anti-Ad capsid responses are amplified by administering the vector repeatedly, immune responses against antigenic epitopes that are part of the Ad capsid should be increased by this approach as well, thus allowing boosting of the response 23–25. This strategy may have additional benefits of promoting immune cross-priming 26,27 and activation of CD8+ T cells by means of incorporating T cell helper epitopes into the Ad capsid proteins 28. Therefore, this novel antigen capsid-incorporation approach may offer exciting opportunities to create Ad-based vaccine vector strategies that circumvent the major limitations associated with Ad vectors.
Adenovirus Capsid Structure
With respect to Ad, the ability to circumvent normal host anti-vector immunity has been accomplished by genetically modifying the Ad capsid. This capacity has been greatly fostered by the intrinsic plasticity of the adenoviral capsid proteins 29,30.
The Ad capsid protein has 4 distinct domains available for antigen incorporation; these include: the hexon (polypeptide II), penton base (polypeptide III), fiber (polypeptide IV), and polypeptide IX (pIX)31 (Figure 1)32.
Figure 1.
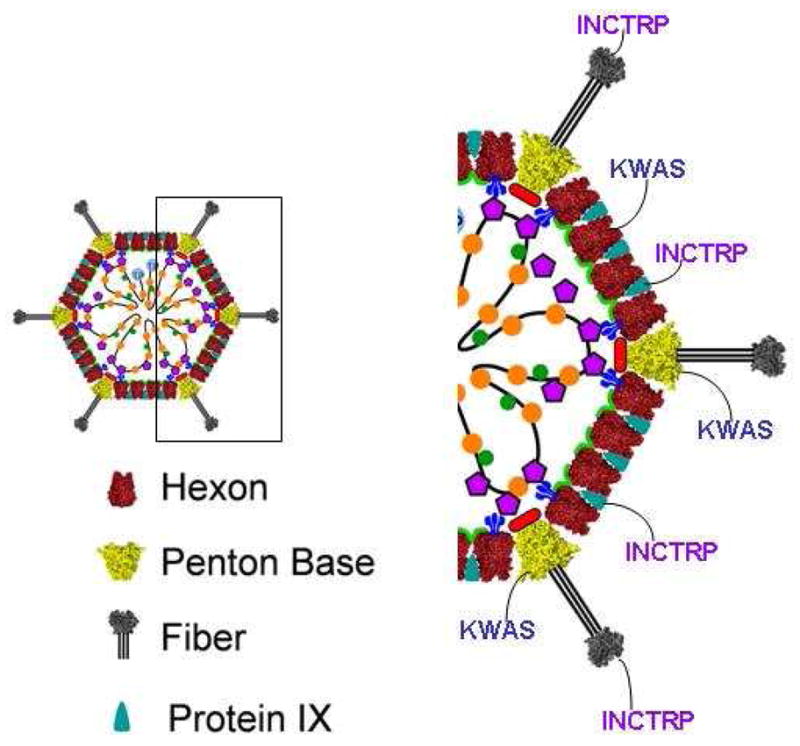
Antigen Capsid-incorporation within Adenovirus Structural Proteins. Adenoviral capsid protein consists of: Hexon (II), Penton Base (III), Fiber (IV), and protein IX (pIX). Antigenic epitopes can be incorporated into these capsid structural proteins to induce antigen-specific immune responses. For example, this figure depicts the incorporation of HIV antigens glycoproteins 41(KWAS) and 120 (INCTRP). This figure is adapted from Nemerow et al., 2009. Virology 384 (2009) 380–388, copyright Elsevier.
Hexon is the most abundant of the capsid’s structural proteins, accounting for 63% of the total protein mass 33,34. Ad2’s hexon polypeptide is 967 amino acids; the longest known molecular design of a hexon. Early analysis of the protein sequences of different hexon proteins revealed that, in addition to the conserved regions, there were 7 discrete hypervariable regions (HVRs) (now reclassified as 1–9). These HVRs do not appear to be involved in binding any other viral proteins 35. The HVRs of hexon contain serotype-specific epitopes 34,35. The loops at the top of the HVRs are the most amenable to modification by genetic engineering. Several groups have shown that short heterologous peptides can be incorporated within the HVRs of the hexon without affecting the virion’s stability or function. Of note, a subset of these modifiable loops were exposed on the surface of the capsid 36,37. HVR2 and HVR5 are the most flexible with respect to peptide or antigen incorporation 28,36–41. These studies highlight the high level of plasticity of hexon protein and suggest that additional vaccine utilities may be derived by exploiting these Ad capsid flexibilities.
Early electron microscopy (EM) studies of the penton base (a pentamer of polypeptide III) revealed that, the penton base has a polygonal cross-section with a central diameter having an approximately 30-Å diameter. There are approximately 571 residues of penton monomer. The penton base and fiber (a trimer of polypeptide IV) form the penton complex that seals the capsid protein at its 12 vertices. The fiber is responsible for attachment to the host cell, and the penton base is responsible for internalization of the virus. A series of complex events trigger membrane permeabilization and virus internalization. In brief, during the virus-entry process, the penton base binds αvβ3 integrins. The binding of penton base to αvβ3 integrins promotes virus infection by stimulating internalization into clathrin-coated vesicles rather than enhancing virus attachment 42–44. This interaction has been demonstrated for 4 adenovirus subgroups (A,B,C, and E), and is attributed to a conserved Arg-Gly-Asp (RGD) motif within a variable region of the penton base 45–48.
The penton base has been used for antigen incorporation strategies. The fiber is shed from the Ad capsid after internalization, exposing the penton base-modified Ad more efficiently to the host and allowing an immune response to be mounted 49,50. However, there have been very few attempts to incorporate peptides or antigen within the penton base 41. This fact may be due to the structural constraints related to antigen incorporation at the base of the capsid.
The fiber protein protrudes from 12 vertices of the capsid surface of the virion. Fiber exists as a trimeric structure composed of 3 domains: an N-terminal tail that attaches to the penton base, a central shaft with repeating motifs, and a C-terminal globular “knob” domain that is responsible for virus attachment to the host cell. With respect to Ad5, its fiber knob primarily binds the coxsackie-adenovirus receptor (CAR). The knob 3 structure of Ad3 51(a non-CAR binder) is similar to those of Ad5 52, Ad2 53, and Ad12 54. A wide range of variations exist in fiber length among the Ad serotypes because of the differences in the number of repeats in the fiber shaft, ranging from 6 residues in Ad3 55 to 22 residues in Ad2 and Ad5 56. With respect to fiber modifications, several groups have incorporated heterologous peptide motifs at the carboxy terminus and HI loop of fiber. These groups have also developed a more radical strategy to replace fiber with a substitute trimerization domain, allowing capsid incorporations of greater size and complexity 57–60.
One of the most characterized Ad minor capsid proteins is pIX. There are approximately 240 copies of this protein per virion, and each copy acts as a cement protein binding a group of nine hexons in each facet of the capsid 61. In addition to stabilizing the capsid structure, pIX promotes efficient virus proliferation. This protein has been a promising target for genetic manipulation; small and large epitopes have been incorporated into its C-terminus for imaging studies, cell targeting, and vaccination strategies 36,41,62–73.
Incorporation of Antigens into Viral Capsid Structures for a Vaccine Approach
Incorporating antigens into viral capsid structures has been used as a vaccination approach for several diseases 28,38,40,41,74. One of the first instances in which this strategy was used was in research performed by Crompton and colleagues in 1994. They genetically incorporated an 8 amino-acid sequence of the VP1 capsid protein of poliovirus type 3 into 2 regions of the adenovirus serotype 2 hexon. One of the chimeric vectors produced by using this method grew well in tissue culture, and antiserum raised against the Ad with the polio antigen specifically recognized the VP1 capsid of polio 74.
Similar studies have been performed by other research groups. For example, Worgall and colleagues used the antigen capsid-incorporation strategy to vaccinate against Pseudomonas aeruginosa (pseudomonas), a gram-negative bacterium that causes respiratory tract infections in individuals who have cystic fibrosis or are immunocompromised75 . Since pseudomonas is an extracellular pathogen, anti-pseudomonas humoral immunity should be sufficient to provide protective immunity. Thus, pseudomonas is a candidate for vaccine development. Several immunogenic peptides have been identified in the outer membrane protein F (OprF) of pseudomonas, including the immunodominant 14-mer peptide Epi8. This study describes genetic incorporations of a neutralizing epitope from the pseudomonas Epi8 into Ad5 HVR5 (AdZ.Epi8) 28. BALB/c mice vaccinated with the capsid-modified vectors showed an increase in antibody response consisting of both anti-pseudomonas IgG1 and IgG2a subtypes. Additionally, after mice immunized with the virus containing the OprF epitope were subjected to pulmonary challenge with pseudomonas, 60% to 80% of them survived.
The same group performed another study in which they constructed an optimized vaccine vector, AdOprF.RGD.Epi8. The 2 capsid modifications of the AdOprF. RGD.Epi8 vector included genetic incorporations of RGD into the fiber to enhance the infection of cells dendritic cells (DCs) 76,77 as well as the insertion of the 14-mer OprF epitope Epi8 into HVR5 of the Ad hexon to enable repeat administration of the same vector to boost the anti-OprF humoral response 76,77. Worgall’s study demonstrates that AdOprF.RGD.Epi8 expresses OprF, contains Epi8 in the hexon protein, and enhances gene transfer to dendritic cells more than AdOprF does (a similar Ad vector that expresses OprF with an unmodified capsid). Intramuscular immunization of C57BL/6 mice with AdOprF.RGD.Epi8 resulted in the generation of anti-OprF antibodies at similar levels to those induced by immunization with AdOprF; however, immunization with AdOprF.RGD.Epi8 was associated with increased CD4+ and CD8+ gamma interferon T-cell responses against OprF. The RGD-modified vector enhances expression of integrin-expressing cells, such as dendritic cells, and enhances the immune response generated against transgenes in animal model systems 76,77. Most importantly, immunization of mice with AdOprF.RGD.Epi8 increased their survival rates after pulmonary challenge with lethal doses of pseudomonas. Importantly, repeat administration of AdOprF.RGD.Epi8 boosted the humoral anti-OprF response and increased protection, whereas no boosting was achieved by repeat administration of AdOprF. These data suggest that the capsid-modified AdOprF.RGD.Epi8 vector is a more effective immunogen than the wild-type Ad capsid is, making this modified vector a good candidate for use in an anti-pseudomonas vaccine. This strategy whereby antigen-presenting cells are activated to elicit a robust immune response to capsid-incorporated antigens is a novel approach and requires extensive investigation.
In contrast to these results, McConnell et al., found that chimeric hexons containing incorporations of B anthracis’ protective antigen (PA) elicited antibodies against PA in mice but failed to yield protection against a challenge with anthrax toxin, lethal factor (B. anthracis secretes three subunits that make up the anthrax toxins, PA, lethal factor, and edema factor) 78. They speculated that the varying results could be a result of a variety of things. For instance, this lack of toxin neutralization could have been a result of inadequate neutralizing antibodies (Nab) generated by the immunization schedule. The authors also speculate that difference seen in their study compared to Worgall and colleagues could represent either a difference in the ability of the selected epitopes to elicit a Nab response in two different disease models or a difference in the antibody titers necessary to achieve protection against pseudomonas compared to those needed to protect against lethal factor challenge. In addition, the authors speculated that the latter may be related to the fact that, in the anthrax model, the response is directed against a secreted bacterial toxin, and in the pseudomonas model, the response is directed against the bacterium itself38.
Similar studies have been performed by Krause et al., 41. The focus of their study was determining which of the capsid proteins could be modified without affecting the infectivity of the Ad and while still inducing high anti-epitope immunity. This study compared the immune response generated by incorporating the hemagglutinin (HA) protein of the influenza A virus into the outer Ad capsid proteins: hexon, penton base, fiber knob, or pIX. The HA epitope was recognized by the anti-HA antibody in all 4 of the modified virions, although binding to the HA presented in hexon HVR5 was slightly stronger. It is a logical assumption that that genetic incorporation of antigens within the hexon protein would yield the most anti-epitope immune response because the hexon protein is more abundant than any other capsid protein. Of note, immunizations of mice with either the same Ad particle numbers, resulting in different amounts of HA copy numbers, or the same amount of HA copies, resulted in the highest humoral and cellular responses from the vector that contained HA within the fiber knob. As previously mentioned, fiber is approximately 60 times less abundant than hexon is on the Ad virion, and pIX is approximately 3 times less abundant than hexon is on the Ad capsid. These data suggest that, in this model system, antigenic epitopes that are incorporated within the fiber knob elicit the optimal response from an Ad-based vaccine. However, this study did not investigate whether or not the size of the incorporated epitopes also affected the immune response generated.
To expand on knowledge gained from previous antigen capsid-incorporation studies, our group set out to create novel vaccine vectors that would yield optimal vaccine efficacy. Therefore, our focus was to develop vectors that could aid in the development of multivalent vaccine vectors. Our 2008 manuscript explored the use of either Ad5 HVR2, HVR5, or both to create vectors containing identical antigenic epitopes in either region. To compare the flexibility and capacities of Ad5 HVR2 to those of HVR5, we genetically incorporated identical epitopes of increasing size within HVR2 or HVR5 of the Ad5 hexon. These epitopes ranged in size from 33–83 amino acids. Stable viruses were produced with incorporations of 33 amino acids plus a 12 amino acid linker at HVR2 or HVR5. In addition, stable viruses were produced with incorporations up to 53 amino acids plus a 12 amino acid linker in HVR5. With respect to the selected antigen incorporations in Ad5 HVR2 or HVR5, HVR5 was more permissive, allowing an epitope incorporation of 65 amino acids. Whole virus ELISA analysis revealed that these model antigens were surface-exposed, and in vivo immunization with these vectors elicited antigen-specific immune responses 40.
Recently, Shiratsuchi, et. al, tested the antigen capsid-incorporation strategy to develop a vaccine against malaria. In an effort to enhance humoral response to Plasmodium yoelii circumsporozoite (CS) protein and circumvent Ad vector PEI, they constructed and analyzed various vectors containing the CS protein of malaria. These vectors contained the CS protein in the hexon and/or fiber region of the Ad capsid as well expressed the CS protein as a transgene. Several vaccinations with the antigen capsid-modified vectors induced a substantially increased level of protection against subsequent malaria challenge in mice when compared with unmodified WT/CS-GFP. However, in brief they illustrated that CS incorporation at HVR1, but not other portions of the capsid proteins, could circumvent Ad PEI, while maintaining the immunogenicity of a B cell epitope of the CS protein expressed on the capsid and expressed as a transgene. This is an important finding because of two reasons: this is the first published report of the antigen capsid-incorporation strategy for malaria and this is the first report of antigen incorporated into Ad HVR1 for vaccine purposes 22.
In 2010, Bayer and colleagues constructed an Ad vector that could be used to vaccinate against Friend virus (FV), a murine virus that causes robust polyclonal erythroblast proliferation, leading to spleen enlargement, erythroleukemia, and death in adult mice 79. The FV infection model allows the study of retrovirus infection in a model with similarities to HIV 80. Using the FV model, Bayer’s group evaluated a vaccine strategy that combined genetic and protein vaccination by using an Ad vector that vaccinated by means of transgene antigen expression and capsid-incorporation of antigen (Ad antigen expression/ antigen display vector). This study by Bayer, et. al, demonstrates that the Ad antigen expression/antigen display vector lead to better protection against FV challenge as compared to (a) conventional antigen expression vector or (b) antigen display-only vector. In this regard, this improved protection correlated with neutralizing antibody levels and CD4+ responses. Using a vector that displays gp70 (without encoding it), they found that antigen display on the capsid alone was sufficient to induce high levels of binding antibodies. However, for the induction of neutralizing antibodies, the display on the particle alone was not sufficient. They speculated that antigen expression by in vivo transduction of the Ad antigen expression/antigen display vector lead to increase Nab response and CD4+ T cell responses.
Bayer and group speculate that there are several advantages to pIX incorporation as compared to incorporation in hexon, fiber, or penton base, which have been previously explored for antigen presentation 28,39–41,74. For example, pIX allows large proteins to be incorporated with minimal loss of function or viral integrity 64–67,69,71. These researchers also speculate that whole-protein incorporation is a promising approach to capsid-incorporation; the presence of several relevant epitopes due to expression of a whole protein would allow for a broader vaccine application. Furthermore, displaying an entire protein rather than just the antigenic epitopes on the vector surface may be advantageous because the B-cell epitopes would be more likely to be in the proper confirmation when incorporated as part of the original protein rather than as a scaffold protein. This problem was encountered when incorporating polio virus epitopes within hexon protein 74. Additional advantages of pIX display include its arrangement into trimers on the Ad capsid surface 81; antigens presented on pIX would be in close proximity to one another and may be able to form trimers on the Ad capsid, possibly allowing for the induction of conformation-dependent antibodies. Similar results were obtained by Boyer et al.; they showed that Ad vectors displaying Y. pestis’ V antigen or F1 capsular antigen on the virion surface elicit a higher V- or F1-specific antibody response, allowing boosting and better protection against a lethal challenge than that produced by vaccination with V or F1 proteins that are paired with conventional adjuvants 82.
Antigen Capsid-Incorporation for HIV Vaccination
Since its discovery and characterization in the early 1980s, there have been several advancements as scientists continue to search for ways to eradicate HIV from the human population. The majority of the effort and nearly all of the success have come in the area of patient treatment rather than in inhibiting contraction or spread of the virus. However, treatments are often costly and disruptive to the daily lives of patients. Therefore, a safe and effective HIV vaccine is urgently needed.
Abe and colleagues evaluated the ability of Ad5-based vectors expressing an HIV transgene to induce antigen-specific immune responses under Ad5 pre-immune conditions. To overcome limitations that are generally experienced because of pre-existing immunity to Ad5, they constructed vectors that have a modification in the HVR5. Their study, characterized various immunological parameters generated by these vectors such as vector neutralization, acquisition of adaptive immune response, and comparison of protective immunity. First, in order to evaluate the utility of the modified Ad vector, they measured the neutralizing activity of sera by a modified-Ad vector. They administered Ad-Luc (luciferase protein expressed as a transgene in the Ad E1 deleted region), Ad-HisLuc (His6 epitope presented in the HVR5 region and luciferase protein expressed as a transgene in the E1 deleted region), or Ad-END/AAALuc vector (containing 3 amino-acid mutations in HVR5 and expressing luciferase protein in the E1 deleted region) to mice intramuscularly. After administration of these vectors, neutralizing activity against Ad5 was observed from 0–8 weeks. The hexon-modified vector (i.e., Ad-HisLuc) generated the lowest Ad5-specific neutralizing activity, which was significantly lower than that generated by Ad-Luc at weeks 6 and 8 or by Ad-End/AAALuc at week 8. The individual neutralizing activity of Ad-HisLuc immunization was significantly lower than that of Ad-Luc immunization. Studies performed by this research group indicate that a change in the immunogenic epitope is necessary to avoid neutralization by pre-existing Nabs. Additional studies performed by Abe and colleagues support the concept that modified hexon inhibits Ad5 NAbs and promotes cellular immune responses by inducing antigen-specific immune responses after transgene expression 83.
Our most recent study exploits the antigen capsid-incorporation strategy further by using novel vectors that were constructed to provide cellular and humoral HIV immunity 84. Our study is the first of its kind to genetically incorporate an HIV antigen within the Ad5 hexon’s HVR2 alone or in combination with the genomic incorporation of a Gag transgene (Ad5/HVR2-MPER-L15(Gag)). In this study, we successfully incorporated a 24 amino-acid epitope of HIV within HVR2. The HIV region selected for HVR2 incorporation was the membrane proximal ectodomain region (MPER) derived from HIV glycoprotein (gp)41. When the MPER epitope was incorporated into HVR2 in combination with transgene expression, we observed growth kinetics and thermostability changes similar to those observed in other studies after using capsid-incorporated vectors, 67,85 indicating that incorporation of the MPER epitope within HVR2 was not dramatically detrimental to virus characteristics 66,85. In addition, we demonstrated that the MPER epitope is surface-exposed within HVR2. Most importantly, we observed a humoral anti-HIV response in mice vaccinated with the hexon-modified vector. The MPER-modified vector allows boosting compared to AdCMVGag, possibly because the Ad5/HVR2-MPER-L15(Gag) Ad elicits less of an anti-Ad5 immune response. It is possible that the MPER epitope reduced the immunogenicity of the Ad5 vector. This finding is noteworthy because HVR2 has not been fully explored for use in antigen capsid-incorporation strategies.
With respect to HIV vaccination, the antigen capsid-incorporation strategy has also been evaluated within the context of human rhinovirus vectors. Research groups have constructed human rhinovirus:HIV chimeras to stimulate immunity against HIV-1 4,86. Additionally, researchers have generated combinatorial libraries of human rhinovirus capsid-incorporated HIV-1 gp41 epitope, eliciting antibodies whose activity can mimic the Nab effect 4,86.
Commercial and clinical development of Ad-based HIV vaccines have progressed more than the development of vector systems such as human rhinovirus because the flexibility of Ad generally exceeds that of current rhinovirus systems. For example, because human rhinovirus is a relatively small RNA virus, the human rhinovirus platform can only display 60 copies of a single HIV-1 epitope 4,86. In contrast, the Ad vector capsid platform could allow incorporation of HIV-1 epitopes into 4 structurally distinct domains including: hexon (HVR2 and HVR5)83, fiber, penton base, and pIX, similar to the illustration depicted in Figure 1.
In comparison, the Ad MPER antigen capsid-incorporation display platform can present an array of 720 HIV-1 epitope copies within Ad hexon and 240 HIV-1 epitope copies within pIX. If a multivalent Ad vector is generated with HIV epitopes within the hexon and pIX domains this would represent 960 HIV epitopes within one Ad particle. Another significant difference between the Ad and human rhinovirus platforms is in the number of locales that have been successfully used to insert heterologous epitopes. Lastly, in contrast to the rhinoviruses, which lack this capacity, the Ad platform has sufficient coding capacity, allowing for HIV-1 transgene expression and presentation of either the same or a different antigen on the viral capsid surface. This latter finding is important because it provides the basis for constructing vectors that will provide both cellular and humoral HIV immunity potentially leading to a prophylactic HIV vaccine. Based on setbacks and progress from recent HIV clinical trial findings, there has been a shift in the vaccine paradigm. There is a urgent need for vectors that provide both cellular and humoral HIV immunity. Vectors and strategies described in this review could potentially be the way forward for safe and effective HIV vaccination as well as vaccination for other diseases.
Acknowledgments
Grant support was provided by NIH: 2P30AI027767-21A1.
I would like to thank Dr. Phoebe L. Stewart for allowing us to reproduce her figure (Nemerow et al., 2009. Virology 384 (2009) 380–388), copyright Elsevier. I would like to thank Drs. Glenn C. Rowe and Cherise M. Guess for their thoughtful insight and review of this article.
Reference List
- 1.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, Moog C, Liljestrom P, Wagner R, Wolf H, Kraehenbuhl JP, Esteban M, Heeney J, Frachette MJ, Tartaglia J, McCormack S, Babiker A, Weber J, Pantaleo G. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205 (1):63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, Than S, Adams EM, Graham BS, Koup RA, Bailer RT, Smith C, Dally L, Farah B, Anzala O, Muvunyi CM, Bizimana J, Tarragona-Fiol T, Bergin PJ, Hayes P, Ho M, Loughran K, Komaroff W, Stevens G, Thomson H, Boaz MJ, Cox JH, Schmidt C, Gilmour J, Nabel GJ, Fast P, Bwayo J. Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One. 2010;5 (9):e12873. doi: 10.1371/journal.pone.0012873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Locke E, Bruder J, Clarke D, Doolan DL, Havenga MJ, Hill AV, Liljestrom P, Monath TP, Naim HY, Ockenhouse C, Tang DC, Van Kampen KR, Viret JF, Zavala F, Dubovsky F. Viral vectors for malaria vaccine development. Vaccine. 2007;25 (14):2567–2574. doi: 10.1016/j.vaccine.2006.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AD, Geisler SC, Chen AA, Resnick DA, Roy BM, Lewi PJ, Arnold E, Arnold GF. Human rhinovirus type 14:human immunodeficiency virus type 1 (HIV-1) V3 loop chimeras from a combinatorial library induce potent neutralizing antibody responses against HIV-1. J Virol. 1998;72 (1):651–659. doi: 10.1128/jvi.72.1.651-659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold GF, Velasco PK, Holmes AK, Wrin T, Geisler SC, Phung P, Tian Y, Resnick DA, Ma X, Mariano TM, Petropoulos CJ, Taylor JW, Katinger H, Arnold E. Broad neutralization of human immunodeficiency virus type 1 (HIV-1) elicited from human rhinoviruses that display the HIV-1 gp41 ELDKWA epitope. J Virol. 2009;83 (10):5087–5100. doi: 10.1128/JVI.00184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson S, Papagatsias T, Benlahrech A. Use of adenovirus in vaccines for HIV. Handb Exp Pharmacol. 2009;(188):275–293. doi: 10.1007/978-3-540-71029-5_13. [DOI] [PubMed] [Google Scholar]
- 7.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24 (7):849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crotty S, Andino R. Poliovirus vaccine strains as mucosal vaccine vectors and their potential use to develop an AIDS vaccine. Adv Drug Deliv Rev. 2004;56 (6):835–852. doi: 10.1016/j.addr.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292 (5514):69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 10.Gherardi MM, Esteban M. Recombinant poxviruses as mucosal vaccine vectors. J Gen Virol. 2005;86 (Pt 11):2925–2936. doi: 10.1099/vir.0.81181-0. [DOI] [PubMed] [Google Scholar]
- 11.Langley WA, Bradley KC, Li ZN, Smith ME, Schnell MJ, Steinhauer DA. Induction of neutralizing antibody responses to anthrax protective antigen by using influenza virus vectors: implications for disparate immune system priming pathways. J Virol. 2010;84 (16):8300–8307. doi: 10.1128/JVI.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slobod KS, Lockey TD, Howlett N, Srinivas RV, Rencher SD, Freiden PJ, Doherty PC, Hurwitz JL. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur J Clin Microbiol Infect Dis. 2004;23 (2):106–110. doi: 10.1007/s10096-003-1075-3. [DOI] [PubMed] [Google Scholar]
- 13.Journal Gene Therapy. Worldwide web site . www.wiley.co.uk/genetherapy/clinical. Ref Type: Generic.
- 14.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7 (1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 15.Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta. 2002;1575 (1–3):1–14. doi: 10.1016/s0167-4781(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 16.Curiel DT. Strategies to adapt adenoviral vectors for targeted delivery. Ann N Y Acad Sci. 1999;886:158–171. doi: 10.1111/j.1749-6632.1999.tb09409.x. [DOI] [PubMed] [Google Scholar]
- 17.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6 (9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 18.Kass-Eisler A, Falck-Pedersen E, Elfenbein DH, Alvira M, Buttrick PM, Leinwand LA. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1 (6):395–402. [PubMed] [Google Scholar]
- 19.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3 (2):154–162. [PubMed] [Google Scholar]
- 20.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62 (7):2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schagen FH, Ossevoort M, Toes RE, Hoeben RC. Immune responses against adenoviral vectors and their transgene products: a review of strategies for evasion. Crit Rev Oncol Hematol. 2004;50 (1):51–70. doi: 10.1016/S1040-8428(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 22.Shiratsuchi T, Rai U, Krause A, Worgall S, Tsuji M. Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J Clin Invest. 2010;120 (10):3688–3701. doi: 10.1172/JCI39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69 (4):2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10 (11):955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 25.Sette A, Fikes J. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr Opin Immunol. 2003;15 (4):461–470. doi: 10.1016/s0952-7915(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 26.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 27.Carbone FR, Heath WR. Cross-priming: its beginnings. J Immunol. 2010;185 (3):1353–1354. doi: 10.4049/jimmunol.1090065. [DOI] [PubMed] [Google Scholar]
- 28.Worgall S, Krause A, Rivara M, Hee KK, Vintayen EV, Hackett NR, Roelvink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J Clin Invest. 2005;115 (5):1281–1289. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70 (10):6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol. 2002;76 (17):8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rux JJ, Burnett RM. Adenovirus structure. Hum Gene Ther. 2004;15 (12):1167–1176. doi: 10.1089/hum.2004.15.1167. [DOI] [PubMed] [Google Scholar]
- 32.Nemerow GR, Pache L, Reddy V, Stewart PL. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384 (2):380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van OJ, Burnett RM. Molecular composition of the adenovirus type 2 virion. J Virol. 1985;56 (2):439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rux JJ, Kuser PR, Burnett RM. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J Virol. 2003;77 (17):9553–9566. doi: 10.1128/JVI.77.17.9553-9566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford-Miksza L, Schnurr DP. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol. 1996;70 (3):1836–1844. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vigne E, Mahfouz I, Dedieu JF, Brie A, Perricaudet M, Yeh P. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J Virol. 1999;73 (6):5156–5161. doi: 10.1128/jvi.73.6.5156-5161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Han T, Belousova N, Krasnykh V, Kashentseva E, Dmitriev I, Kataram M, Mahasreshti PJ, Curiel DT. Identification of sites in adenovirus hexon for foreign peptide incorporation. J Virol. 2005;79 (6):3382–3390. doi: 10.1128/JVI.79.6.3382-3390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConnell MJ, Danthinne X, Imperiale MJ. Characterization of a permissive epitope insertion site in adenovirus hexon. J Virol. 2006;80 (11):5361–5370. doi: 10.1128/JVI.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worgall S, Krause A, Qiu J, Joh J, Hackett NR, Crystal RG. Protective immunity to pseudomonas aeruginosa induced with a capsid-modified adenovirus expressing P. aeruginosa OprF. J Virol. 2007;81 (24):13801–13808. doi: 10.1128/JVI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews QL, Yang P, Wu Q, Belousova N, Rivera AA, Stoff-Khalili MA, Waehler R, Hsu HC, Li Z, Li J, Mountz JD, Wu H, Curiel DT. Optimization of capsid-incorporated antigens for a novel adenovirus vaccine approach. Virol J. 2008;5:98. doi: 10.1186/1743-422X-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krause A, Joh JH, Hackett NR, Roelvink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG, Worgall S. Epitopes expressed in different adenovirus capsid proteins induce different levels of epitope-specific immunity. J Virol. 2006;80 (11):5523–5530. doi: 10.1128/JVI.02667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73 (2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 43.Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127 (1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai M, Campisi L, Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J Virol. 1994;68 (9):5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67 (9):5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use alpha v integrins for infection. J Virol. 1994;68 (10):6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart PL, Chiu CY, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow GR. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 1997;16 (6):1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu CY, Mathias P, Nemerow GR, Stewart PL. Structure of adenovirus complexed with its internalization receptor, alphavbeta5 integrin. J Virol. 1999;73 (8):6759–6768. doi: 10.1128/jvi.73.8.6759-6768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong SS, Habib NA, Franqueville L, Jensen S, Boulanger PA. Identification of adenovirus (ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative ad (addl1520) for treatment of liver tumors. J Virol. 2003;77 (19):10366–10375. doi: 10.1128/JVI.77.19.10366-10375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol. 2000;74 (15):7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durmort C, Stehlin C, Schoehn G, Mitraki A, Drouet E, Cusack S, Burmeister WP. Structure of the fiber head of Ad3, a non-CAR-binding serotype of adenovirus. Virology. 2001;285 (2):302–312. doi: 10.1006/viro.2001.0967. [DOI] [PubMed] [Google Scholar]
- 52.Xia D, Henry LJ, Gerard RD, Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure. 1994;2 (12):1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]
- 53.van Raaij MJ, Louis N, Chroboczek J, Cusack S. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 A resolution. Virology. 1999;262 (2):333–343. doi: 10.1006/viro.1999.9849. [DOI] [PubMed] [Google Scholar]
- 54.Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286 (5444):1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- 55.Signas C, Akusjarvi G, Pettersson U. Adenovirus 3 fiber polypeptide gene: implications for the structure of the fiber protein. J Virol. 1985;53 (2):672–678. doi: 10.1128/jvi.53.2.672-678.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green NM, Wrigley NG, Russell WC, Martin SR, McLachlan AD. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983;2 (8):1357–1365. doi: 10.1002/j.1460-2075.1983.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jerry LB, Hui L, Jesus G, Igor D, Victor K, Christy AR, Denise RS, Ronald DA, David TC, Theresa VS. Using a Tropism-Modified Adenoviral Vector to Circumvent Inhibitory Factors in Ascites Fluid. Human Gene Therapy. 2000;11 (12):1657–1669. doi: 10.1089/10430340050111313. [DOI] [PubMed] [Google Scholar]
- 58.Michael SI, Hong JS, Curiel DT, Engler JA. Addition of a short peptide ligand to the adenovirus fiber protein. Gene Ther. 1995;2 (9):660–668. [PubMed] [Google Scholar]
- 59.Wickham TJ, Tzeng E, Shears LL, Roelvink PW, Li Y, Lee GM, Brough DE, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71 (11):8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72 (3):1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van OJ, Smith PR, Mohraz M, Burnett RM. Interpretation of electron micrographs of adenovirus hexon arrays using a crystallographic molecular model. J Ultrastruct Mol Struct Res. 1986;96 (1–3):77–90. doi: 10.1016/0889-1605(86)90009-1. [DOI] [PubMed] [Google Scholar]
- 62.Dmitriev IP, Kashentseva EA, Curiel DT. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J Virol. 2002;76 (14):6893–6899. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parks RJ. Adenovirus protein IX: a new look at an old protein. Mol Ther. 2005;11 (1):19–25. doi: 10.1016/j.ymthe.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Meulenbroek RA, Sargent KL, Lunde J, Jasmin BJ, Parks RJ. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion--generation of fluorescent virus through the incorporation of pIX-GFP. Mol Ther. 2004;9 (4):617–624. doi: 10.1016/j.ymthe.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Le LP, Everts M, Dmitriev IP, Davydova JG, Yamamoto M, Curiel DT. Fluorescently labeled adenovirus with pIX-EGFP for vector detection. Mol Imaging. 2004;3 (2):105–116. doi: 10.1162/15353500200404100. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Le L, Sibley DA, Mathis JM, Curiel DT. Genetic incorporation of HSV-1 thymidine kinase into the adenovirus protein IX for functional display on the virion. Virology. 2005;338 (2):247–258. doi: 10.1016/j.virol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Matthews QL, Sibley DA, Wu H, Li J, Stoff-Khalili MA, Waehler R, Mathis JM, Curiel DT. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol Imaging. 2006;5 (4):510–519. [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Fatima A, Komarova S, Ugai H, Uprety P, Roth JC, Wang M, Oster RA, Curiel DT, Matthews QL. Evaluation of adenovirus capsid labeling versus transgene expression. Virol J. 2010;7:21. doi: 10.1186/1743-422X-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimball KJ, Rivera AA, Zinn KR, Icyuz M, Saini V, Li J, Zhu ZB, Siegal GP, Douglas JT, Curiel DT, Alvarez RD, Borovjagin AV. Novel infectivity-enhanced oncolytic adenovirus with a capsid-incorporated dual-imaging moiety for monitoring virotherapy in ovarian cancer. Mol Imaging. 2009;8 (5):264–277. [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez M, Matthews Q, Curiel D, Borovjagin A. Imaging in Vector Development. In: Weissleder, Gambhir, Ross, Rehemtulla, editors. Molecular Imaging: Principles and Practice. BC Decker Inc; 2010. [Google Scholar]
- 71.Borovjagin AV, McNally LR, Wang M, Curiel DT, MacDougall MJ, Zinn KR. Noninvasive monitoring of m. Mol Imaging. 2010;9 (2):59–75. [PMC free article] [PubMed] [Google Scholar]
- 72.de VJ, Uil TG, van den Hengel SK, Cramer SJ, Koppers-Lalic D, Verweij MC, Wiertz EJ, Vellinga J, Willemsen RA, Hoeben RC. Adenovirus targeting to HLA-A1/MAGE-A1-positive tumor cells by fusing a single-chain T-cell receptor with minor capsid protein IX. Gene Ther. 2008;15 (13):978–989. doi: 10.1038/gt.2008.26. [DOI] [PubMed] [Google Scholar]
- 73.Campos SK, Parrott MB, Barry MA. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol Ther. 2004;9 (6):942–954. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crompton J, Toogood CI, Wallis N, Hay RT. Expression of a foreign epitope on the surface of the adenovirus hexon. J Gen Virol. 1994;75(Pt 1):133–139. doi: 10.1099/0022-1317-75-1-133. [DOI] [PubMed] [Google Scholar]
- 75.Garau J, Gomez L. Pseudomonas aeruginosa pneumonia. Curr Opin Infect Dis. 2003;16 (2):135–143. doi: 10.1097/00001432-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Okada N, Saito T, Masunaga Y, Tsukada Y, Nakagawa S, Mizuguchi H, Mori K, Okada Y, Fujita T, Hayakawa T, Mayumi T, Yamamoto A. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res. 2001;61 (21):7913–7919. [PubMed] [Google Scholar]
- 77.Worgall S, Busch A, Rivara M, Bonnyay D, Leopold PL, Merritt R, Hackett NR, Rovelink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J Virol. 2004;78 (5):2572–2580. doi: 10.1128/JVI.78.5.2572-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N Engl J Med. 1999;341 (11):815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 79.FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957;105 (4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dittmer U, Brooks DM, Hasenkrug KJ. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J Virol. 1998;72 (8):6554–6558. doi: 10.1128/jvi.72.8.6554-6558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fabry CM, Rosa-Calatrava M, Conway JF, Zubieta C, Cusack S, Ruigrok RW, Schoehn G. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005;24 (9):1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyer JL, Sofer-Podesta C, Ang J, Hackett NR, Chiuchiolo MJ, Senina S, Perlin D, Crystal RG. Protective Immunity against a Lethal Respiratory Yersinia pestis Challenge Induced by V Antigen or the F1 Capsular Antigen Incorporated into Adenovirus Capsid. Hum Gene Ther. 2010 doi: 10.1089/hum.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abe S, Okuda K, Ura T, Kondo A, Yoshida A, Yoshizaki S, Mizuguchi H, Klinman D, Shimada M. Adenovirus type 5 with modified hexons induces robust transgene-specific immune responses in mice with pre-existing immunity against adenovirus type 5. J Gene Med. 2009;11 (7):570–579. doi: 10.1002/jgm.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matthews QL, Fatima A, Tang Y, Perry BA, Tsuruta Y, Komarova S, Timares L, Zhao C, Makarova N, Borovjagin AV, Stewart PL, Wu H, Blackwell JL, Curiel DT. HIV antigen incorporation within adenovirus hexon hypervariable 2 for a novel HIV vaccine approach. PLoS One. 2010;5 (7):e11815. doi: 10.1371/journal.pone.0011815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang Y, Le LP, Matthews QL, Han T, Wu H, Curiel DT. Derivation of a triple mosaic adenovirus based on modification of the minor capsid protein IX. Virology. 2008;377 (2):391–400. doi: 10.1016/j.virol.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 86.Smith AD, Resnick DA, Zhang A, Geisler SC, Arnold E, Arnold GF. Use of random systematic mutagenesis to generate viable human rhinovirus 14 chimeras displaying human immunodeficiency virus type 1 V3 loop sequences. J Virol. 1994;68 (1):575–579. doi: 10.1128/jvi.68.1.575-579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


