Abstract
Two specific groups of neurons in the dorsolateral pons are activated by dietary sodium deprivation. These two groups are the pre-locus coeruleus (pre-LC) and the inner subdivision of the external lateral parabrachial nucleus (PBel-inner). In each site, after rats are fed an extremely low-sodium diet for over a week, neurons increase their expression of an activity-induced transcription factor, c-Fos. Here, we confirm this observation and extend it by demonstrating that these two groups of neurons express a common marker gene, the constitutively-expressed transcription factor Forkhead box protein 2 (FoxP2). That is, virtually all of the c-Fos activated neurons in both regions also express FoxP2. The expression of FoxP2 by both these groups of neurons suggests that they are developmentally-related subsets derived from the same basic population. Given that FoxP2, unlike c-Fos, is expressed independent of sodium deprivation, this marker may be useful in future studies of the pre-LC and PBel-inner. The molecular definition of these neurons, which project to circuits in the forebrain that influence visceral, appetitive, and hedonic functions, may allow direct experimental exploration of the functional role of these circuits using genetic tools.
Keywords: nucleus tractus solitarius, parabrachial nucleus, pre-locus coeruleus, salt appetite, sodium intake, transcription factor
1. Introduction
In the rostral, dorsolateral pons, two groups of neurons exhibit pronounced activation in rats after they have been deprived of dietary sodium (Geerling and Loewy, 2007). This complex region of the brainstem contains several well-defined populations of neurons, including the locus coeruleus (LC), a cluster of noradrenergic neurons adjacent to the fourth ventricle. It also contains the parabrachial nucleus (PB), a collection of subnuclei surrounding the superior cerebellar peduncle, which integrates information from the medulla and spinal cord related to visceral sensation, pain, and temperature and relays it to sites located primarily in the forebrain. In this region of the brainstem, the two groups of neurons with sodium deprivation-associated activity are found, first, in a small cluster immediately rostral to the LC, which we refer to as the pre-locus coeruleus (pre-LC), and second, in a thin band of neurons running along and within the ventrolateral aspect of the superior cerebellar peduncle as part of the inner subdivision of the external lateral PB (PBel-inner). These sodium deprivation-activated groups of neurons are described in anatomical detail in a previous study (Geerling and Loewy, 2007) and are shown in several images below.
Our laboratory identified this novel and highly restricted change in neuronal activity as a natural extension of work involving the expression of an activity-induced transcription factor, c-Fos, in the nucleus of the solitary tract (NTS) after dietary sodium deprivation. Sodium deprivation is a useful, non-invasive experimental manipulation for producing large physiological increases in aldosterone production by the adrenal glands, along with a behavioral change in salt intake (see Geerling and Loewy, 2008). Initially, this experimental paradigm was used to demonstrate activation of the aldosterone-sensitive HSD2 neurons in the NTS (Geerling et al., 2006). Then, several axonal tracing experiments established the dorsolateral pons as a major target of the efferent projections of HSD2 neurons; within this region, their axons appear to synapse primarily within the pre-LC and the PBel-inner (Geerling and Loewy, 2006). Finally, as mentioned above, dietary sodium deprivation – the experimental manipulation we found to induce c-Fos expression in HSD2 neurons in the NTS – was also found to induce a prominent c-Fos labeling in two specific regions of the dorsolateral pons, namely the pre-LC and PBel-inner (Geerling and Loewy, 2007). These combined pieces of evidence from tract-tracing and functional-anatomical experiments suggested, in combination, that HSD2 neurons in the NTS, which are activated by sodium deficiency, directly excite their post-synaptic target neurons in the pre-LC and PBel-inner, which integrate this information with other inputs and relay it to the forebrain (Geerling and Loewy, 2008).
The dorsolateral pons is a highly heterogeneous region of the brain, and while these small subsets of neurons do robustly express c-Fos after dietary sodium deprivation, the absence of a more general method to identify them is a major limitation to any further research on their functional and neuroanatomical properties. As these neurons show a distinct cytological response, we hypothesized they exhibit genetic similarities that could be used as markers to distinguish them from adjacent neurons. Thus, we analyzed a published database of transcription factor expression in the mouse brainstem (see Gray et al., 2004) to search for candidate genes with patterns of expression in the dorsolateral pons that might identify neurons in one or both of these groups. We observed that the transcription factor Forkhead box protein 2 (FoxP2), which is found in several regions of the brain, is expressed by a relatively specific subpopulations of neurons in the dorsolateral pons. Here, we show that expression of FoxP2 demarcates virtually all of the c-Fos-activated neurons in the pre-LC and PBel-inner after dietary sodium deprivation.
2. Results
2.1 FoxP2 in the dorsolateral pons
FoxP2 protein expression is robust in neuronal nuclei in several parts of the adult rat brainstem, in a pattern generally similar to previous anatomic work in p0 mouse pups (Gray, 2008). The overall pattern of FoxP2 expression in the brain is not the subject of this study, and will not be discussed further here (see Ferland et al., 2003; see Gray, 2008).
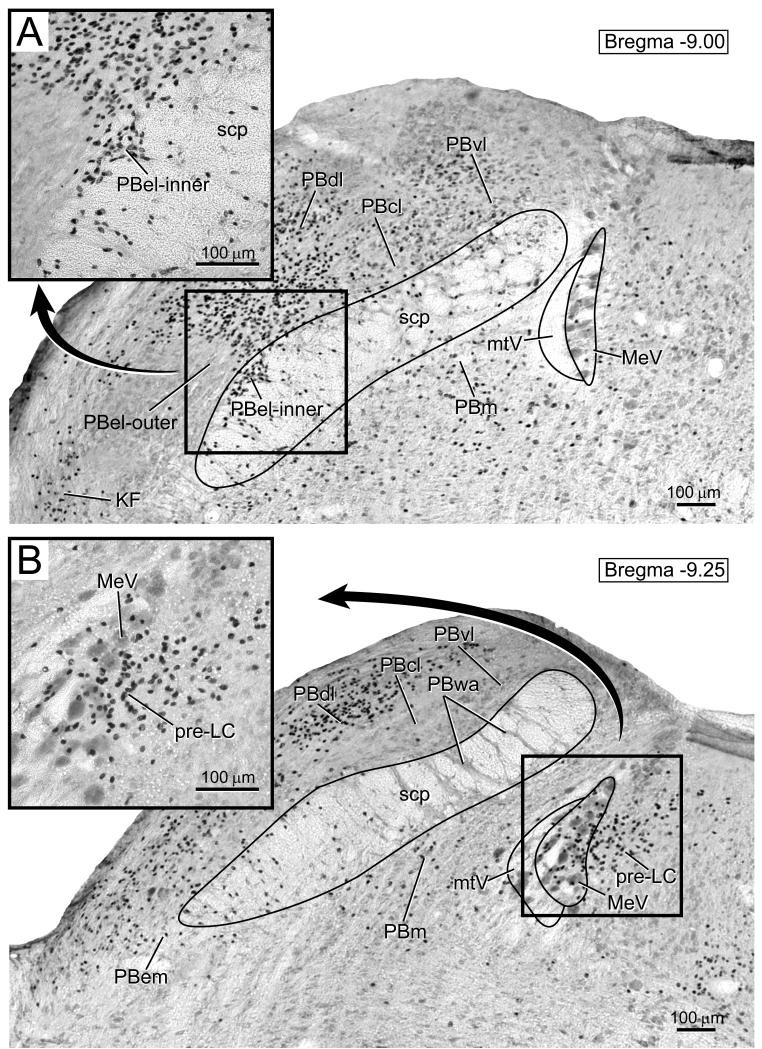
The region of interest for the present study, the rostral part of the dorsolateral pons, is shown in Figure 1 A and B. This figure shows immunohistochemical staining for FoxP2, seen as dark, punctate nuclear labeling in two transverse sections through the rostral dorsolateral pons. Within this region, FoxP2-immunoreactive (hereafter referred to as “FoxP2+”) nuclei were found only in specific neuronal populations, including several subnuclei of the PB. The dorsal lateral (PBdl) and central lateral (PBcl) subnuclei contained the largest and densest populations of FoxP2+ nuclei. Smaller numbers of FoxP2+ cells were found in several other PB subnuclei, including the medial (PBm), ventral lateral (PBvl), and Kolliker-Fuse (KF). Interestingly, FoxP2 was conspicuously absent from a large region of the external lateral PB (PBel, Saper and Loewy, 1980; Fulwiler and Saper, 1984), throughout its much larger “outer” subdivision (Herbert et al., 1990; Herbert and Saper, 1990).
Figure 1.
FoxP2 immunoreactivity in the rostral part of the dorsolateral pons is shown as dark, punctate nuclear staining. A. FoxP2+ nuclei in the pre-locus coeruleus (pre-LC), which is located just medial to and intermingled with the large neurons and tract of the mesencephalic trigeminal nucleus (MeV, mtV). Note insert showing pre-LC neurons (black). B. A more rostral section of the parabrachial nucleus showing the inner subdivision of the external lateral parabrachial nucleus (PBel-inner). Note the insert contains high magnification image of the PB-el inner showing labeled nuclei are distributed along and in the ventrolateral aspect of the superior cerebellar peduncle (scp). There was a prominent void of FoxP2-immunoreactivity throughout most of the PBel, which contrasts with the densely-packed FoxP2+ nuclei in the neighboring dorsal lateral (PBdl) and central lateral (PBcl) subnuclei. Other abbreviations: medial PB (PBm), external medial PB (PBem), ventral lateral PB (PBvl), Kölliker-Fuse nucleus (KF).
Figure 1A shows a cluster of FoxP2+ nuclei in the periventricular gray matter centered just medial to and intermingled with the giant, peripherally-projecting neurons of the mesencephalic trigeminal nucleus (MeV). This cluster of FoxP2+ nuclei overlaps the region containing a dense axon terminal field from the NTS (Geerling and Loewy, 2006) and is identical in appearance to the group of c-Fos-labeled nuclei found here after dietary sodium deprivation (Geerling and Loewy, 2007).
Figure 1B shows a dense concentration of FoxP2+ nuclei in the lateral parabrachial region, including the PBel-inner region. Note there is a conspicuous void of FoxP2 immunoreactivity in the PBel is surrounded on all sides by a dense rim of FoxP2+ nuclei. This rim of FoxP2+ nuclei includes, along the ventromedial aspect of PBel, a group of cells in what has been named its “inner” subdivision (PBel-inner, see Herbert and Saper, 1990; Geerling and Loewy, 2006; Geerling and Loewy, 2007), which is composed of a thin stripe of cells running along and cascading through the ventrolateral aspect of the superior cerebellar peduncle (scp). This region of PBel-inner is highlighted in the inset of Figure 1B. This is precisely the same location in which we found anterograde axonal projections from the NTS, as well as a prominent stripe of c-Fos-expressing nuclei after dietary sodium deprivation (Geerling and Loewy, 2007).
2.2 Dietary sodium deprivation selectively activates neurons which express FoxP2 in the pre-LC and PBel-inner
The number and distribution of FoxP2-expressing cells was similar between controls (n=5) and animals that were fed a sodium-deficient diet for eight days (n=6). We compared the number of FoxP2+ nuclei in the pre-LC and PBel-inner in sodium-deprived and non-deprived rats (see Table). In neither region of interest were the counts of FoxP2+ nuclei significantly different between between control and experimental animals (p = 0.8 and 0.5 for pre-LC and PBel-inner, respectively). Additionally, FoxP2 expression was stable after a variety of other osmotic, thermal, and nociceptive stimuli (not described here). These observations suggest that FoxP2 is a reliable marker for specific neurons within the dorsolateral pons.
Table 1.
The number of FoxP2-immunoreactive, c-Fos-immunoreactive, and double-labeled FoxP2+ and c-Fos+ neurons from the control and sodium (Na)-deprived groups are presented in this table. The individual data, along with the mean +/− SEM for the pre-LC and the PBel-inner are presented. Asterisks denote significant differences versus control by t-test (see Results for exact p-values).
| pre-LC | PBel-inner | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal # |
FoxP2+ (total) |
c-Fos+ (total) |
FoxP2+ & c- Fos+ |
c-Fos+ (FoxP2−) |
FoxP2+ (total) |
c-Fos+ (total) |
FoxP2+ & c-Fos+ |
c-Fos+ (FoxP2− ) |
|
| Control | 3719 | 204 | 47 | 13 | 34 | 153 | 29 | 17 | 12 |
| Control | 3720 | 270 | 57 | 26 | 31 | 123 | 24 | 16 | 8 |
| Control | 3721 | 194 | 81 | 14 | 67 | 140 | 39 | 28 | 11 |
| Control | 3722 | 223 | 65 | 32 | 33 | 139 | 43 | 24 | 19 |
| Control | 3723 | 205 | 49 | 14 | 35 | 130 | 31 | 15 | 16 |
|
|
|
||||||||
| Control mean: | 219 | 60 | 20 | 40 | 137 | 33 | 20 | 13 | |
| SEM: | 15 | 7 | 4 | 8 | 6 | 4 | 3 | 2 | |
| pre-LC | PBel-inner | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal # |
FoxP2+ (total) |
c-Fos+ (total) |
FoxP2+ & c- Fos+ |
c-Fos+ (FoxP2−) |
FoxP2+ (total) |
c-Fos+ (total) |
FoxP2+ & c-Fos+ |
c-Fos+ (FoxP2− ) |
|
|
Na-
deprived |
3713 | 250 | 180 | 149 | 31 | 118 | 64 | 43 | 21 |
|
Na-
deprived |
3714 | 233 | 150 | 116 | 34 | 149 | 89 | 77 | 12 |
|
Na-
deprived |
3715 | 250 | 184 | 165 | 19 | 166 | 86 | 85 | 1 |
|
Na-
deprived |
3716 | 172 | 154 | 131 | 23 | 136 | 104 | 98 | 6 |
|
Na-
deprived |
3717 | 182 | 165 | 157 | 8 | 135 | 104 | 102 | 2 |
|
Na-
deprived |
3718 | 193 | 131 | 118 | 13 | 152 | 88 | 80 | 8 |
|
|
|
||||||||
| * | * | * | * | ||||||
| Na-deprived mean: |
213 | 161 | 139 | 21 | 143 | 89 | 81 | 8 | |
| SEM: | 16 | 9 | 9 | 5 | 7 | 7 | 9 | 3 | |
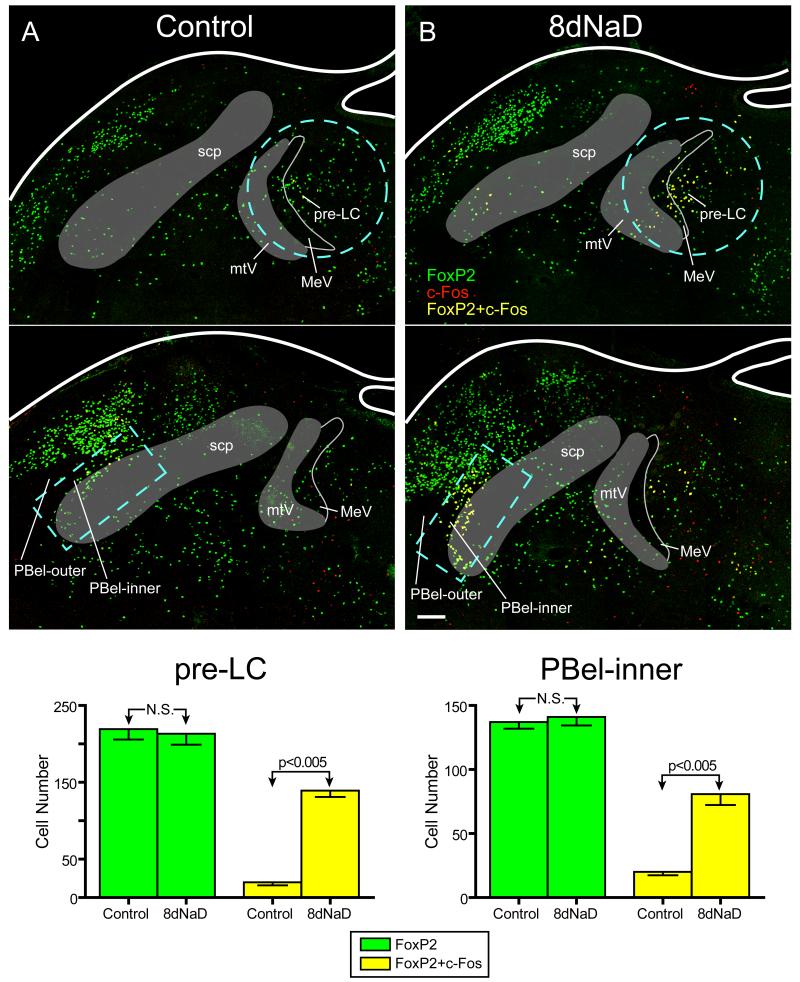
Unlike FoxP2, the number and pattern of c-Fos-labeled nuclei were unmistakably different between groups. Control animals (n=5) contained scattered c-Fos+ neuronal nuclei in small numbers in various regions of the dorsolateral pons, including the pre-LC and PBel-inner (red nuclei in Figure 2). Some of this c-Fos labeling in control animals occurred in neurons that also expressed FoxP2 (yellow nuclei in Figure 2), but qualitatively, no animal from the control group exhibited a noticeable cluster of c-Fos labeling in the pre-LC, PBel-inner, or any other site in the dorsolateral pons. In contrast, every animal in the sodium-deprived group (n=6) exhibited a conspicuous pattern of intense c-Fos labeling in both the pre-LC and PBel-inner, as shown previously (Geerling and Loewy, 2007).
Figure 2.
FoxP2 expression (green nuclei) is shown in combination with c-Fos immunoreactive cells (red nuclei, representing activated neurons) in two sections through the dorsolateral pons in a control and a sodium-deprived rat. A. Photoimages through the pre-LC (top panel) and PB-el inner (bottom panel) showing FoxP2 immunoreactive neurons (green). Only an occasional neuron was found to express c-Fos (red) or both c-Fos and FoxP2 (yellow). Note that the outlined areas (light blue dashed lines) are the regions of interest selected for quantitative analysis. B. Photoimages from the dorsolateral pons from an 8 day sodium deprived (8dNaD) rat. In the pre-LC (top) and the PBel-inner (bottom panel), the number of c-Fos-expressing neurons increased significantly compared to the control animals. A large number of neurons co-expressing both c-Fos (red) and FoxP2 (green) appear yellow. C. Bar graphs comparing the number of FoxP2-expressing (green bars) and double-labeled (c-Fos+, FoxP2+, yellow bars) neurons in each area of interest. In both regions, the total number of FoxP2-expressing neurons remained constant between groups. However, in 8dNaD animals, the number of double labeled (FoxP2+ and c-Fos +) neurons increased dramatically (p<0.005 by t-test in both region). For absolute numbers and exact p-values see Results and Table.
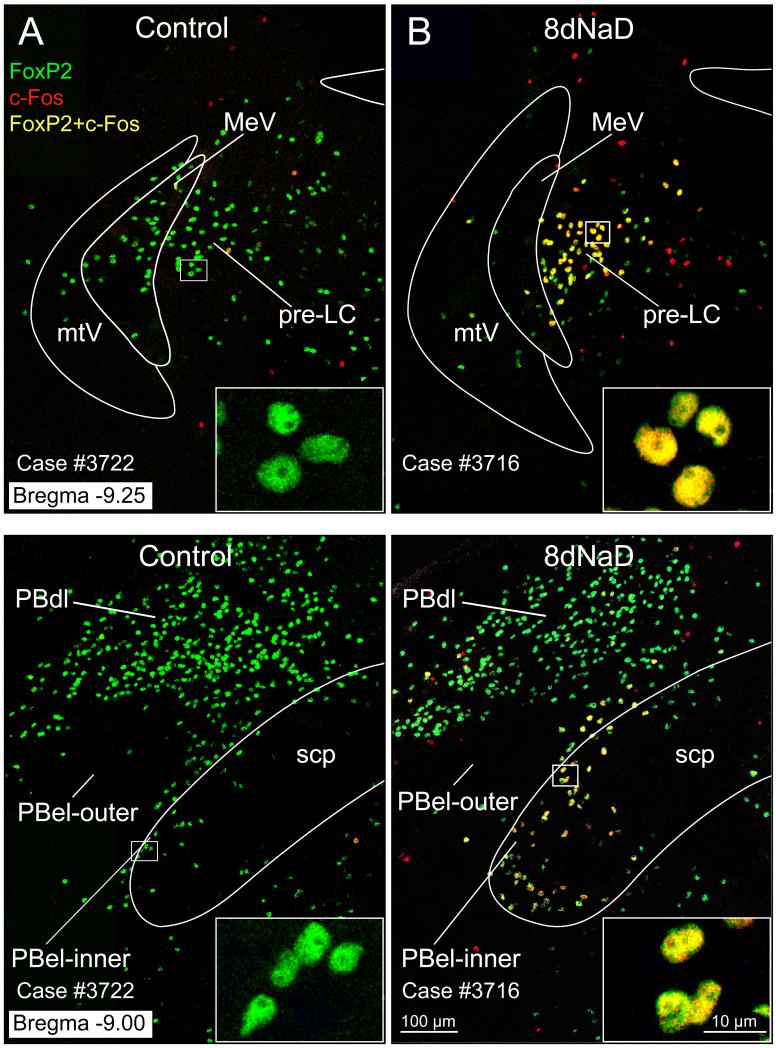
Virtually all of the increased c-Fos labeling in the dorsolateral pons that was induced by dietary sodium deprivation was restricted to neurons that also expressed FoxP2 (c-Fos+, FoxP2+; yellow nuclei in Figures 2 and 3). In every sodium-deprived animal, this intense clustering of double-labeled (c-Fos+, FoxP2+) nuclei was readily apparent in both the pre-LC and PBel-inner. This increase in c-Fos+ FoxP2+ double-labeling was statistically significant in both sites. The mean ± SEM increase from control to sodium-deprived rats in the pre-LC was 20 ± 4 to 139 ± 9 (p=0.0000008), and in PBel-inner it was 20 ± 3 to 81 ± 9 (p=0.00002). Likewise, if c-Fos labeling is considered alone, the numbers and differences were similarly significant (pre-LC 60 ± 7 to 161 ± 9, p=0.000005; PBel-inner 33 ± 4 to 89 ± 7, p=0.00003). Outside the prominent clusters of double-labeled neurons found directly within the pre-LC and PBel-inner, there was no consistent pattern or clustering of activated neurons in this region of the brainstem, with the exception of a few neurons in a thin line extending from the pre-LC laterally and rostrally towards the PBel-inner. This pattern was consistent among sodium-deprived animals, but cannot be appreciated readily from the selected images shown here, and their small numbers and distribution made it impossible to quantify accurately with the counting technique used for the pre-LC and PBel-inner.
Figure 3.
Confocal images of c-Fos + FoxP2 immunofluorescence neurons in the pre-LC and PBel-inner. A. Comparison of FoxP2 (green), c-Fos (red), and combined FoxP2 and c-Fos (yellow) labeling in the pre-LC from a control rat (left) and an 8dNaD rat (right). Insert images in lower right hand corner show enlargements of these respective neurons. B. Images shown here compare the FoxP2, c-Fos, and combined FoxP2 and c-Fos labeling found in the PBel-inner region. Inserts show examples of these neurons at higher magnification.
In contrast to the pronounced c-Fos activation found in FoxP2+ neurons in these two sites, the number of neurons that expressed c-Fos, but not FoxP2 (c-Fos+, FoxP2−) was small, and even decreased slightly between controls and sodium deprived animals. In the pre-LC, the number of c-Fos-activated, FoxP2-negative neurons decreased from 40 ± 8 in controls to 21 ± 5 in sodium-deprived animals (p=0.04), and a parallel decrease occurred in PBel-inner, from 13 ± 2 to 8 ± 3 (p=0.23). Thus, the significant increase in c-Fos labeling between control and sodium-deprived animals occurred specifically in FoxP2+ neurons and not in surrounding neurons.
Finally, as is evident from the large number of green nuclei (FoxP2+, c-Fos−) in Figures 2 and 3, many FoxP2-expressing neurons adjacent to the pre-LC and in particular the PBel-inner were not activated by sodium deprivation. The neurons outside PBel-inner are plainly evident as a large, dense population of FoxP2+ cells in PBcl and PBdl; these appear to be anatomically continuous with PBel-inner, with the only apparent separation being the c-Fos labeling that appears after sodium deprivation in PBel-inner, but not in these other two subnuclei. Around the c-Fos+ cluster in the pre-LC, there were relatively fewer other FoxP2-expressing cells, with the exception of a small caudal-medial continuation of FoxP2+ nuclei which extend roughly 200μm behind the pre-LC back to the rostralmost level of the LC (not shown here). If FoxP2 labeling were considered alone, this caudal trail of nuclei behind the pre-LC would appear to be a continuation of same cell population; they are distinguished only by their slightly more caudal and medial location and by their total and consistent lack of c-Fos expression in sodium-deprived animals.
3. Discussion
The key finding of this study is that, within the dorsolateral pons, the neurons activated by dietary sodium deprivation appear to share a common developmental phenotype, marked by the persistent expression of FoxP2 in adult rats. Anatomically, neuronal activation in this region was already known to be limited to the pre-LC and PBel-inner. Now, the discovery of FoxP2+ nuclei in this region is a unifying characteristic of these two populations, raising the possibility that they share a functional relationship that may be greater than what is suggested by their spatial separation.
The identification of FoxP2 as a stable marker for essentially all the dorsolateral pontine neurons that are activated by dietary sodium deprivation is important for at least three reasons. First, the fact that they express FoxP2 provides insight into their developmental history, and consequently, their specific neuronal phenotype, as discussed below. Second, this stable molecular marker (viz., FoxP2) may be a useful tool for future neuroanatomical studies of both of these cell groups. Also, the constitutive expression of FoxP2 from postnatal development through adulthood, in contrast to the phasic, activity-induced expression of c-Fos in these neurons, eliminates the need for an extra experimental manipulation (dietary sodium deprivation) that was necessary previously to determine with certainty the identity of a neuron of interest in the pre-LC or PBel-inner. Third, the identification of this developmentally-stable marker offers the potential for genetic access to these neurons.
The following discussion reviews the phenotypic information implied by their FoxP2 expression, as well as the small amount of background information we have at this time regarding the connections and possible functions of neurons in the pre-LC and PBel-inner.
3.1 FoxP2
The transcription factor FoxP2 initially gained prominence because of its importance for human language development. A rare, autosomal-dominant speech and language disorder was found to result from a point mutation in a single allele of the foxp2 gene (Fisher et al., 1998; Lai et al., 2001). Subsequently, recent evolutionary changes in this gene have been posited as an important factor in the language gap between humans and other primates (Enard et al., 2002).
An overview of FoxP2 expression in the rodent brain was published, but the brainstem was not shown in that article, and there was no mention of expression in the dorsolateral pons (Ferland et al., 2003). A later anatomic study in p0 mouse pups identified FoxP2 expression in several pontine nuclei, focusing on a caudal group of FoxP2+ cells, which appear to define the Kölliker-Fuse nucleus (Gray, 2008). On a macroscopic level, the pattern of FoxP2 expression we found in adult rats is not substantially different from p0 mouse pups, when minor differences in the geometry of several pontine nuclei between age groups and small species differences are taken into account. It is also important to point out the FoxP2 alone may not be a specific marker for the Kölliker-Fuse neurons, but it can be used to define multiple subsets of parabrachial subnuclei, such as the PBel-inner.
In mice, using a transgenic labeling strategy in combination with FoxP2-immunofluorescence, Gray showed that most of the FoxP2-labeled neurons in the dorsolateral pons are derived from a well-characterized precursor population in the rhombic lip that transiently express Atoh1 during early development (2008). If this holds true for FoxP2-expressing neurons in adult rats, as appears to be the case in mouse, it is likely that these neurons share a glutamatergic phenotype (Rose et al., 2009). Additional information about these cells, their origins, and their likely complement of expressed genes in the adult may be obtained by comparison with emerging data involving other Atoh1-derived brainstem populations.
3.2 Dietary sodium deprivation
Eliminating sodium from the diet for a week is a relatively simple and non-invasive experimental manipulation for activating homeostatic defense mechanisms aimed at maintaining the body’s extracellular fluid volume. These defense mechanisms include a large increase in the production of aldosterone by the adrenal glands and, in many animal species, a dramatic increase in sodium appetite (reviewed in Geerling and Loewy, 2008). Initially, when a rat is switched to a sodium-restricted diet, its kidneys continue excreting for one or two days the same baseline amount of sodium related roughly to its previous level of dietary sodium intake. Then, after 1-2d, increased aldosterone levels effectively reduce excretory sodium losses to zero (Contreras and Hatton, 1975; Stricker et al., 1991), and after several days to a week – we use eight days as an empirically useful time-point in our laboratory because by this time rats exhibit maximum and uniform adaptations – rats exhibit a large increase in voluntary salt ingestion, with a time course that roughly parallels prominent activation of the aldosterone-sensitive HSD2 neurons in the NTS, which is evidenced by their increased expression of c-Fos (Geerling et al., 2006; Geerling and Loewy, 2008).
A prominent increase in neuronal activation was also observed in the two pontine sites targeted by axonal projections from the HSD2 neurons in the NTS – the pre-LC and PBel-inner (Geerling and Loewy, 2007), although initially we had no other information about the properties of these cells. This experimental paradigm was used again in the present study to help establish the identity of these c-Fos-expressing cells in the dorsolateral pons.
Beyond its obvious utility as a highly specific tool for highlighting the activation of aldosterone-sensitive neurons in the NTS and identifying their ascending relay nuclei in the pons, sodium deprivation may have important medical and psychiatric implications, based on the projections of these activated neurons to forebrain regions that control basic appetitive, hedonic, and arousal processes (reviewed in Geerling and Loewy, 2006; Geerling and Loewy, 2008). Rats and humans alike, in response to challenges to their extracellular fluid space (or to pathologic increases in aldosterone production), exhibit behavioral changes that go beyond simply increased thirst and/or sodium appetite, including anxiety, depressed mood, and anhedonia (Emanuele et al., 2005; Sonino et al., 2006; Hlavacova and Jezova, 2008; Hlavacova et al., 2010; Morris et al., 2010). Thus, further investigation of the sodium deprivation-activated relay neurons in the pre-LC and PBel-inner, which are key nodes in the transmission of sodium- and aldosterone- related information from the medulla to the forebrain, may provide important insights into how changes in hydromineral and mineralocorticoid internal state may influence mood and, ultimately, behavior.
3.3 FoxP2+ neurons in the pre-LC and PBel-inner are activated by sodium deprivation
The identification of a common genetic marker for sodium deprivation-activated neurons in the pre-LC and PBel-inner suggests that they share a common developmental origin. They may even represent two parts of the same basic population, separated in space only incidentally, perhaps by the developmental expansion of the superior cerebellar peduncle and the medial PB subnucleus, by a gap of a few hundred microns. This latter proposition is supported by, first, their specific expression of FoxP2; second, a trail of FoxP2+ c-Fos+ double-labeled cells we observed between these two groups beneath the medial PB in sodium-deprived animals (see Results); third, their similar sources of axonal input from afferent sites such as the HSD2 neurons in the NTS (Geerling and Loewy, 2006; Geerling and Loewy, 2007) and the paraventricular hypothalamic nucleus (Geerling et al., 2010); and fourth, their coincidental c-Fos activation patterns after dietary sodium deprivation.
What, if any, physiological or behavioral changes activate these neurons besides dietary sodium deprivation are unknown. Beyond their volume- and aldosterone-related ascending input from the HSD2 neurons in the NTS, the relatively strong descending input projections they receive from the paraventricular hypothalamic nucleus (recently identified in Geerling et al., 2010) suggest that they may respond to a wider variety of stimuli than just dietary sodium deficiency, possibly including stimuli for thirst and other general physiological and psychological stressors that activate neurons in the PVH. We do not yet have complete information about the full set of input connections to these two sites, let alone the degree to which they are influenced functionally by these afferents or the relevant role(s) they play in physiological regulation, mood, or behavior. In this regard, a detailed investigation of the axonal projections from FoxP2-expressing cells in each site would be highly informative.
Hopefully, the full complement of information about efferent projections from FoxP2-expressing neurons in the pre-LC and PBel-inner, once available, will narrow the number of possibilities for their possible functional roles. At this point, suffice it to say that these FoxP2-expressing neurons are a key link in a novel ascending circuit that delivers visceral information including dietary sodium deficiency to subcortical regions of the forebrain with unknown functional relevance, which may include increasing the hunger for salt, but may involve much more than this.
3.4 Conclusions
Virtually all the neurons activated by dietary sodium deprivation in the dorsolateral pons express the transcription factor FoxP2. These neurons are distributed into two small sites – the pre-LC and the PBel-inner – which may share developmental origins and functional properties with one another and even with larger, adjacent populations of FoxP2-expressing neurons in the parabrachial nucleus. Together, they appear to form an important relay node for the transmission of aldosterone- and sodium/volume-related viscerosensory information from the NTS to subcortical sites in the forebrain, although their specific functional role remains to be determined. The identification of FoxP2 expression in these neurons should increase our ability to decipher their behavioral function(s).
4. Experimental procedure
All experimental protocols were approved by the Washington University School of Medicine Institutional Animal Care and Use Committee and conformed to NIH guidelines. Throughout these experiments, an automatically controlled room lighting system with a 12/12 hour light-dark schedule was used (lights on at 6:30AM; lights off at 6:30 PM).
4.1 Normal rat brainstem: FoxP2 Immunohistochemistry
Brainstems (n= 4) from female rats (250-350g; Harlan, Indianapolis, IN) were cut in the coronal plane at 75 μm on a freezing microtome. Serial sections were processed for immunohistochemical detection of FoxP2 by the ABC-DAB method.
Free-floating sections were incubated overnight in rabbit antibodies to FoxP2 (1:8,000; ab6046, Abcam, Cambridge, MA) made in a solution containing 0.3% Triton-X (Sigma, St. Louis, MO) and 5% donkey serum in 0.1M sodium phosphate (pH = 7.4, washed in potassium phosphate buffered saline (KPBS; 0.01M, pH = 7.4), transferred to a biotinylated donkey anti-rabbit (1:250; Jackson ImmunoResearch, West Grove, PA) solution which was the same one used with the primary antiserum, then washed in KPBS, treated for 1 hour in the avidin-biotin complex (ABC, Vectastain kit, Vector Labs, Burlingame, CA) solution, washed in KPBS, and colorized in a cobalt-diaminobenzidine (Co-DAB) solution (D-0426, Sigma). A single Co-DAB tablet was dissolved in 20-25 ml of distilled water containing one tablet of urea. The sections were reacted for 15 min in this solution, washed three times in KPBS, mounted on gelatinized slides, and air dried. The sections were dehydrated and coverslipped directly without any counterstaining.
The rabbit polyclonal antibody to FoxP2 was made against a synthetic peptide made from residues 700 to the C-terminus of human FoxP2 which was conjugated to keyhole limpet hemocyanin. This antibody showed a single band in Western blots performed by the manufacturers and were specific to human and mouse FoxP2. Based on sequence homology, this antibody was predicted by the vendor to be specific against rat FoxP2 as well.
4.2 Dietary sodium deprivation
Male Sprague-Dawley rats weighing 220-270g (n=10; Harlan, Indianapolis, IN) underwent dietary sodium deprivation as previously described. Rats were housed individually during sodium deprivation with daily cage changes to prevent any re-ingestion of excreted sodium. They were kept on a 12h/12h automated light/dark cycle. At all times, they had ad libitum access to chow and water. Prior to the experimental sodium deprivation period, they were given tap water and standard rat chow (PicoLab rodent diet #20, containing 0.33% sodium; Lab-Diet, Richmond, IN).
During the eight-day deprivation period, fluid in the drinking bottle of each experimental animal was switched to distilled water (dH2O) and their food was replaced with a chow that is similar in nutrient content except that it is extremely low in sodium (<0.01% Na, diet #85292, Harlan-Teklad, Madison, WI, USA). Control animals retained their tap water and normal rodent chow. For the eight days that constituted the experimental period, all rats in both experimental and control groups were provided with clean cage bedding every day in order to prevent re-ingestion of any excreted sodium by the sodium-deprived (experimental) group and to ensure the consistently of environmental stressors (cage-change stress) between groups. Rats were run in pairs, with an experimental and a control animal run alongside one another, and then perfused together on the morning after the eighth day of the experimental period. Matched pairs of rats (experimental and control) were perfused within 15-20 minutes of each other. The first pair of rats was killed at 7:30 AM, and the last at 9:00 AM. Each rat was deeply anesthetized with i.p. pentobarbital and killed by perfusion through the ascending aorta with a saline solution, followed by 4% paraformaldehyde. The brains were dissected out and placed 4% paraformaldehyde for several weeks before blocking and sectioning.
4.3 Combined FoxP2 and c-Fos Immunohistochemistry
Using a freezing microtome, sections were cut 50 μm thick in the transverse plane through the rostral pons. These sections were collected and stored in a one-in-five series (each section spaced 200μm apart) in 0.1 M sodium phosphate buffer containing 1% sodium azide. Later, they were washed in KPBS solution and then incubated in mixed primary antibody solution.
To label c-Fos, we used an antiserum raised in rabbit diluted at 1:10,000 (c-Fos “Ab-5” from Oncogene; now PC38, Calbiochem, San Diego, CA). This antiserum was raised against a synthetic peptide (SGFNADYEASSSRC) corresponding to amino acids 4–17 of human c-Fos (Calbiochem data sheet).
To label FoxP2, we used an antiserum raised in sheep against AA 640-715 of a recombinant human FoxP2 protein, isoform 1 (accession #O15409, 100% sequence conservation between human and rat); this antiserum was diluted 1:5,000 (product # AF5647; R&D Systems, Minneapolis, MN), and was shown by the manufacturer to be specific for human and mouse FoxP2 (rat FoxP2 was not available for testing).
Sections were immunostained by a double-direct immunofluorescence procedure. First, free-floating sections were incubated a mixture of anti-c-Fos (1:10,000) and anti-FoxP2 (1:5,000) antibodies overnight. The following morning, they were washed in KBPS buffer and transferred to a mixed secondary antibody solution consisting of Cy2-donkey-anti-sheep and Cy3-donkey-anti-rabbit (both 1:250; Jackson) for two hours, again washed in buffer, and then mounted on glass slides. After drying, slides were coverslipped using a fade-retardant glycerol mounting solution containing sodium azide and n-propyl gallate, and secured around the edges with fingernail polish.
In a separate test (n=2), both rabbit and sheep anti-FoxP2 antibodies were used together in a dual immunofluorescence procedure to analyze the parabrachial region. For the PB dorsal lateral region, a 99% co-localization was found. The sheep antibody tended to cause more background staining than the rabbit antibody.
4.3 Cell counts and data analysis
Cell counts were made of the FoxP2, c-Fos, and double-labeled (FoxP2+, c-Fos+) neurons in both the pre-LC and the PBel-inner. Counts were performed unilaterally (right side) in a series of three total sections selected from a one-in-five series through the dorsolateral pons. These sections encompassed the back of the pre-LC caudally through the front of the PBel-inner rostrally, a span of roughly 450μm (the thickness of three 50μm sections plus the two 200μm sampling-gaps between them). The caudal-most two of these three sections were used for cell counts in the pre-LC, and the rostral-most two sections were used for the cell counts for the pre-LC (the middle section typically contained both the rostral cells of pre-LC and the caudal cells of PBel-inner).
A calibrated reticule in the microscope eyepiece was used to demarcate a counting boundary for each region of interest (ROI), superimposed over the fluorescence-labeled neurons in each two regions. For the pre-LC, a circle with a diameter of 500 μm was positioned with its lateral edge bordering the convex (medial) surface of mesencephalic tract of the trigeminal nerve. For the PBel-inner, a rectangle measuring 450 μm × 200 μm was positioned along the ventrolateral border of the superior cerebellar peduncle. FoxP2, c-Fos, and co-labeled neurons were counted separately. Counting was performed at 200× magnification with continual adjustment of focal depth to ensure that any nucleus counted as double-labeled was in fact labeled with both fluorophores at the same focal z-axis position. These data were recorded with a MD3 Microscope Digitizer using MDPlot software (AccuStage, Shorewood, MN).
4.4 Imaging and data analysis
All confocal imaging was performed using an Olympus Fluoview FV500b laser-scanning microscope. Montage images were acquired as multiple individual stacks using a 20× oil objective (NA 1.17) in 1 ſm z-steps throughout the full tissue depth. Each z-frame from each image tile in the montage was collapsed into a single two-dimensional, maximum-projection image to produce individual tiles each at a resolution of 1024 × 1024 pixels (roughly 642 × 642 μm). Manipulation of confocal stacks and z-frame compression was performed with MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Individual 2D panels were then aligned into larger-area photomontages after brightness and contrast normalization and pseudocoloration of each channel in Adobe Photoshop. Brightfield images were captured using a standard CCD camera from Nikon and ACT-1 imaging software; brightness and contrast adjustments and cropping were performed in Photoshop. Final figure layouts were prepared using Adobe Illustrator.
Quantitative data were entered into a Microsoft Excel spreadsheet. All comparisons shown here were compared by Student’s t-test (two-tailed) with no correction for multiple comparisons. A p-value of less than 0.005 by t-test was chosen arbitrarily as signifying statistical significance for this study. The primary comparison for this study was the number of neurons double-labeled for c-Fos and FoxP2 between controls and sodium-deprived animals. Individual data from each animal and group data are shown in Table 1. All group data are presented as mean ± SEM.
Research Highlights.
FoxP2 neurons of pre-locus coeruleus are activated by sodium deprivation
FoxP2 neurons in the lateral parabrachial nucleus are activated by sodium deprivation
Acknowledgements
Special thanks to Xay Van Nguyen for his expert surgical and histological assistance. We also thank Marcy Hartstein for the computer graphics and Dennis Oakley of the Bakewell Neuroimaging Laboratory at Washington University for assistance with confocal imaging hardware and software.
Grant Support.
(1) National Institute of Heart, Lung, and Blood of the NIH, Grant #: HL-25449 (ADL)
(2) National Institute of Heart, Lung, and Blood of the NIH, Grant #: HL-089742 (PAG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Contreras RJ, Hatton GI. Gustatory Adaptation as an Explanation for Dietary-Induced Sodium Appetite. Physiol Behav. 1975;15:569–576. [Google Scholar]
- Emanuele E, Geroldi D, Minoretti P, Coen E, Politi P. Increased plasma aldosterone in patients with clinical depression. Arch Med Res. 2005;36:544–548. doi: 10.1016/j.arcmed.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Paabo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Vargha-Khadem F, Watkins KE, Monaco AP, Pembrey ME. Localisation of a gene implicated in a severe speech and language disorder. Nat Genet. 1998;18:168–170. doi: 10.1038/ng0298-168. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci. 2006;26:411–417. doi: 10.1523/JNEUROSCI.3115-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J Comp Neurol. 2007;504:379–403. doi: 10.1002/cne.21452. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2008;93:177–209. doi: 10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Herbert H, Saper CB. Cholecystokinin-, galanin-, and corticotropin-releasing factor-like immunoreactive projections from the nucleus of the solitary tract to the parabrachial nucleus in the rat. J Comp Neurol. 1990;293:581–598. doi: 10.1002/cne.902930405. [DOI] [PubMed] [Google Scholar]
- Hlavacova N, Bakos J, Jezova D. Eplerenone, a selective mineralocorticoid receptor blocker, exerts anxiolytic effects accompanied by changes in stress hormone release. J Psychopharmacol. 2010;24:779–786. doi: 10.1177/0269881109106955. [DOI] [PubMed] [Google Scholar]
- Hlavacova N, Jezova D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety-like behavior. Horm Behav. 2008;54:90–97. doi: 10.1016/j.yhbeh.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Na ES, Johnson AK. Mineralocorticoid receptor antagonism prevents hedonic deficits induced by a chronic sodium appetite. Behav Neurosci. 2010;124:211–224. doi: 10.1037/a0018910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MF, Ahmad KA, Thaller C, Zoghbi HY. Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Acad Sci U S A. 2009;106:22462–22467. doi: 10.1073/pnas.0911579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Sonino N, Fallo F, Fava GA. Psychological aspects of primary aldosteronism. Psychother Psychosom. 2006;75:327–330. doi: 10.1159/000093956. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Thiels E, Verbalis JG. Sodium appetite in rats after prolonged dietary sodium deprivation: a sexually dimorphic phenomenon. Am J Physiol. 1991;260:R1082–1088. doi: 10.1152/ajpregu.1991.260.6.R1082. [DOI] [PubMed] [Google Scholar]