Abstract
Netherton syndrome (NS) is a debilitating congenital skin disorder caused by mutations in the SPINK5 gene encoding the lymphoepithelial Kazal-type-related inhibitor (LEKTI). It is characterized by defective keratinization, recurrent infections, and hypernatraemic dehydration with a mortality rate of about 10% in the first year of life. Currently, there are no curative treatments for NS. We have developed a HIV-1 based, self-inactivating lentiviral vector to express SPINK5 in keratinocytes as part of an ex-vivo gene therapy strategy for NS. High transduction efficiency was achieved in NS keratinocytes and reconstitution of LEKTI expression was confirmed in previously deficient cells. These genetically corrected keratinocytes were further tested in an in vitro organotypic culture (OTC) system and in vivo mouse/human skin engraftment model. Results showed correction of epidermal architecture in both OTCs and regenerated skin grafts. Importantly, the results from corrected skin grafts indicated that even where detectable LEKTI expression was restored to a limited numbers of cells, a wider bystander benefit occurred around these small populations. As LEKTI is a secreted protein, the genetically modified graft may provide not only an immediate local protective barrier, but also act as a source of secreted LEKTI providing a generalized benefit following ex-vivo gene therapy.
Introduction
Netherton syndrome (NS) is a debilitating genetic condition caused by mutations in the SPINK5 gene, resulting in loss of activity of its encoded protein lymphoepithelial Kazal-type-related inhibitor (LEKTI).1,2 Clinically, NS is characterized by congenital ichthyosiform erythroderma, hair shaft defects, recurrent infections, and a defective skin barrier with histological features of a psoriasiform epidermal layer covered by a parakeratotic stratum corneum in the epidermis. LEKTI is one of the serine protease inhibitors expressed in the outermost layers of the skin, and plays a critical role in the regulation of serine proteases kallikrein 5, 7, and 14 which hydrolyze the extracellular corneodesmosomes in the skin.3,4,5 In NS patients, the inhibitory effect of LEKTI on kallikreins is abolished due to mutations in SPINK5 resulting in prematurely terminated or altered translation of SPINK5 gene and truncated expression of LEKTI protein. As a consequence, hyperactivated kallikreins cause premature degradation of corneodesmosomes resulting in a defective skin barrier.6 A more recent study has also shown that LEKTI can indirectly inhibit elastase 2, a new epidermal protease involved in profilaggrin processing and the lipid lamellae structure of the skin, by controlling kallikrein 5 mediated cleavage of proelastase 2.7 The prognosis of NS is poor with high mortality of 10% in the first year of life due to life-threatening complications such as bronchopneumonia, sepsis, and hypernatraemic dehydration. Currently, there are no specific treatments for NS except emollients to improve the compromised skin barrier. Gene therapy therefore, could provide a specific treatment for NS patients.
Keratinocytes, including keratinocyte stem cells or their derivatives (i.e., transit amplifying cells) can be cultured in vitro and sheets of epithelium can be generated for grafting.8 Current applications of cultured epidermal sheets and tissue engineered skin equivalents include treatment of patients with severe burns, skin ulcers, or impaired wound healing.9,10 These techniques can be readily adapted to patients with inherited skin diseases by adding a genetic modification step, with a view to generating autologous, gene-corrected skin grafts. Recently, such approaches have been used for the correction of laminin-5 expression, the gene defective in junctional epidermolysis bullosa. In that study, epidermal stem cells underwent retroviral transduction and the corrected epidermal sheets were subsequently used to treat areas of severely affected skin.11 This trial provided the first proof-of-principle confirmation that correction of skin stem cells can afford effective therapy of genodermatoses. Importantly, such ex-vivo correction of keratinocyte stem cells and the generation of autologous grafts circumvent the problems of host immune responses against viral vectors and avoid issues relating to in vivo vector biodistribution. In addition, areas of gene-modified skin can be easily monitored and readily biopsied if required. Combined with increasing expertise in the generation and application of keratinocyte skin sheets for tissue reconstruction, these advantages make ex-vivo gene therapy for skin disorders an attractive proposition.
Here, we report that lentiviral-mediated expression of SPINK5 can restore LEKTI activity in organotypic cultures (OTCs) derived from NS keratinocytes and is sufficiently robust to correct the architecture of NS epidermis in an in vivo humanized mouse skin model.
Results
NS histology and mutation analysis
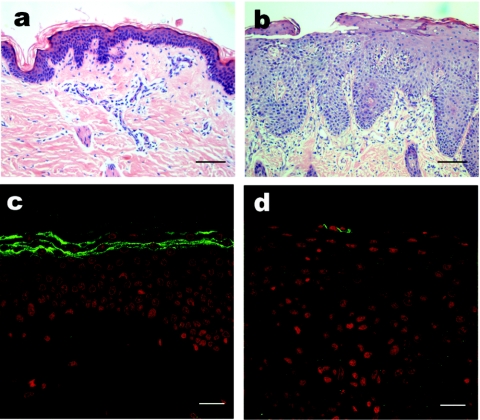
Histological examination of skin from a patient with clinical features of NS revealed nonspecific acanthosis, psoriasiform changes within the epidermis including hypogranulosis, and a parakeratotic stratum corneum. Immunofluorescence staining using LEKTI antibody confirmed absent expression of LEKT1 in the uppermost layers of the skin (Figure 1) and subsequent mutation analysis confirmed the presence of a homozygous mutation 2200delAA in the gene SPINK5 leading to a frameshift and a premature stop codon after a further 27 bp (data not shown).
Figure 1.
Morphology and LEKTI expression in the skin with Neterton syndrome (NS). (a,b) H&E staining and (c,d) immunofluorescence staining in healthy donor skin and NS skin. In NS, the acanthotic, psoriasiform epidermis was covered by parakeratotic stratum corneum (b) and LEKTI expression was absent in the uppermost layers of the skin (d), compared to healthy donor skin, where intense membranous staining (green color) was detectable in the granular and cornified layers (c). (a,b) Bar = 100 µm; (c,d) = 20 µm. LEKTI, lymphoepithelial Kazal-type-related inhibitor.
High-level LEKTI expression in keratinocytes can be achieved by lentiviral transduction
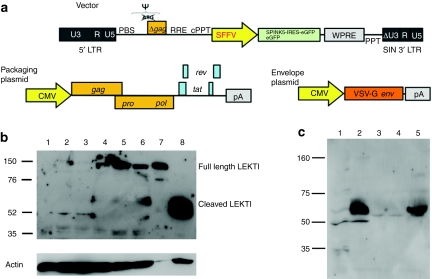
HIV-1 derived, self-inactivating, lentiviral vectors expressing enhanced green fluorescent protein (eGFP) alone, or in combination with SPINK5 were used to transduce keratinocytes (Figure 2a). These proof-of-principle experiments used constructs encoding internal promoter elements derived from the spleen focus-forming virus LTR, which are known to mediate high levels of transgene expression in a diverse variety of target tissues. In all experiments, the intensity of eGFP expression was greater from monocistronic (eGFP) constructs compared to bicistronic (SPINK5/eGFP) vectors. Codon optimization of the SPINK5 transgene resulted in increased levels of gene expression compared to SPINK5 transgene with native sequences and thus this form of the transgene was used in all subsequent experiments. Transduction efficiency in N-TERT cells, a keratinocyte cell line that has been shown to retain the normal growth and differentiation control mechanisms, was assessed by the detection of GFP+ cells using flow cytometry. As anticipated, increased proportions of GFP+ cells (ranging from 13 to 80%) were seen following transduction with increasing multiplicity of infection (0.1–25 infectious particles/cell). Analysis of LEKTI protein expression in transduced cells using an antibody specific for the C-terminus of the protein detected nonprocessed forms of LEKTI (~145 kd) as well as cleaved forms of the protein (~68 kd) (Figure 2b). In addition, western blot analysis undertaken on filtered supernatant collected from cells transduced to express SPINK5/eGFP, revealed increased levels of cleaved LEKTI fragment (~68 kd) compared to control cultures (Figure 2c), indicating that LEKTI was being efficiently expressed, processed, and secreted following lentiviral transduction.
Figure 2.
SPINK5/eGFP lentiviral vector mediate LEKTI expression in keratinocytes. (a) Schematic representation of lentiviral vectors used in this study. The vectors used were self-inactivating HIV-1-based viruses, encoding the spleen focus-forming virus (SFFV) LTR promoter, the HIV-1 cPPT element, and a mutated WPRE. The accessory plasmids mediated vector packaging and pseudotyping with the vesicular stomatitis virus (vsv) envelope. (b) Western blotting of N-TERT cells following transduction with increasing MOIs of SPINK5/eGFP lentivirus. Lane 1 shows nontransduced N-TERT cells in which full length, unprocessed LEKTI is not detectable. Lanes 2–6 show cells transduced with an increasing MOI of vector-encoding SPINK5/eGFP (0.1, 0.6, 3.2, 16, and 25). Lane 7 was loaded 1 µg of recombinant LEKTI and lane 8 was loaded tissue lysate from human skin. Increasing levels of full length (~145 kd) and cleaved LEKTI bands (~68) were detected in transduced cells. Lane 7 and 8 are used as positive controls showing the size of full-length LEKTI and processed LEKTI. (c) Western blotting of culture media to detect secreted LEKTI fragments. Lane 1 was N-TERT cell lysate; lane 2 was health donor skin lysate; lane 3 was culture medium of nontransduced N-TERT cells; lane 4 was culture medium of cells transduced with eGFP alone; and lane 5 was culture medium of cells transduced with SPINK5/eGFP. Results showed an intensive band at size ~68 kd, representing secreted LEKTI in lane 5, but not lane 3 or 4 which were derived from media of nontransduced cells and cells transduced with eGFP alone. Thus, gene-modified cells expressed, processed, and secreted LEKTI. CMV, cytomegalovirus; cPPT, central polypurine tract; eGFP, enhanced green fluorescent protein; LEKTI, lymphoepithelial Kazal-type-related inhibitor; LTR, long-terminal repeat; MOI, multiplicity of infection; SIN, self-inactivating; WPRE, Woodchuck postregulatory element.
Lentiviral-mediated expression of LEKTI does not disrupt keratinocyte survival
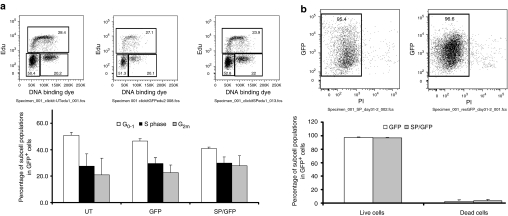
As SPINK5 is expressed in differentiated keratinocytes, upregulation or overexpression of LEKTI in proliferating keratinocytes transduced with the SPINK5/eGFP vectors may alter proliferation kinetics, resulting in early differentiation in cells. We therefore assessed DNA synthesis, cell cycling, and cell death in transduced N-TERT cells using 5-ethynyl-2′-deoxyuridine (EdU) and propidium iodide (PI) staining. Nontransduced and transduced cells were cultured from day 0 up to day 50 post-transduction. Cells collected on days 9, 18, and 50 were treated with EdU and assayed. These cells exhibited expected patterns of growth and differentiation before and after transduction at different time points. No significant differences were observed between nontransduced and transduced cells as well. Analysis after transduction with the SPINK5/eGFP vector, detected a small, nonsignificant increase in the proportion of cells in the G2m phase of the cell cycle compared to nontransduced cells or cells transduced with vector-encoding eGFP alone. There was also no significant difference in the proportion of cells in G0–1 or S phase of the cell cycle (Figure 3a). In addition, on the basis of PI staining, there was no detectable change in the levels of cell death between the test groups (Figure 3b). Thus, lentiviral-mediated expression of SPINK5 neither confers a survival advantage nor adversely influences keratinocyte proliferation.
Figure 3.
Flow cytometry analysis of cell proliferation and death in transduced N-TERT cells. (a) Cells transduced with eGFP or SPINK5/eGFP expressing viral vector were treated with EdU. Examples of the distribution of cell populations in different stages of the cell cycles are shown. There were no significant differences between groups, suggesting that lentiviral transduction and LEKTI expression do not affect cell turnover. (b) Cell viability of transduced N-TERT cells was analyzed using propidium iodide staining and flow cytometry. The distribution of live and dead cells in the eGFP+, transduced cell populations is shown. There were no significant differences in cell death or survival between groups indicating that transduction with vectors encoding SPINK5/eGFP does not influence cell survival. EdU, 5-ethynyl-2′-deoxyuridine; eGFP, enhanced green fluorescent protein; LEKTI, lymphoepithelial Kazal-type-related inhibitor.
Lentiviral transduction of primary keratinocytes restores LEKTI expression in three-dimensional OTCs
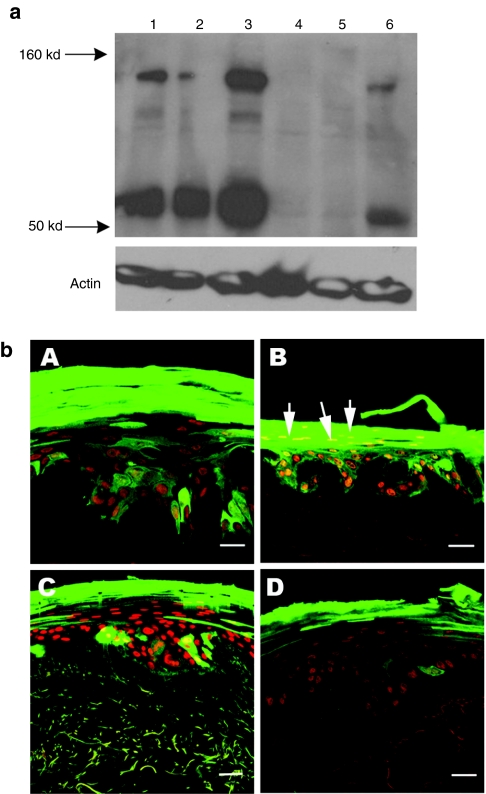
Before transduction, western blot analysis detected LEKTI expression in healthy donor keratinocytes but not in NS keratinocytes. Following exposure to one round of lentiviral infection at multiplicity of infection 30, over 40–60% of primary keratinocytes were transduced (on the basis of eGFP expression) in the case of both healthy donor and NS cultures. Subsequent western blot analysis confirmed that expression of LEKTI had been restored in NS cells following transduction with vectors encoding SPINK5/eGFP but not in control groups expressing eGFP alone (Figure 4a).To determine the transduction efficiency of the stem cell compartment, the clonal assay was performed and large cell colonies (holoclones, >5 mm) which showed high clonogenic potential were examined for eGFP expression. The percentage of GFP+ holoclones formed from heterogeneous NS keratinocytes transduced with SPINK5/eGFP was 43%, which was similar to the percentage of eGFP+ cells in the initial transduced cells (40–60%).
Figure 4.
Lentiviral vector delivery of SPINK5 transgene into primary keratinocytes. (a) Transduced healthy donor or NS primary keratinocytes were harvested and the LEKTI expression was assessed using anti-LEKTI antibody by western blot analysis. Lane 1 (healthy donor) and lane 4 (NS) were nontransduced cells. Endogenous full length (145 kd) and cleaved (~68 kd) LEKTI could be detected in normal keratinocytes nontransduced or transduced with eGFP alone expressing vector (lane 1 and 2), but not in NS cells (lanes 4 and 5). In contrast, in cells transduced with SPINK5/eGFP vector, increased expression of LEKTI was observed in both healthy donor (lane 3) and NS cells (lane 6) compared to the cells transduced with eGFP alone (lanes 2 and 5). These results demonstrate successful reconstitution of LEKTI expression in NS keratinocytes following lentiviral transduction. (b) Primary keratinocytes from health donors or NS were transduced with lentiviral vector expressing SPINK5/eGFP or eGFP alone. GFP+ cells were sorted by flow cytometry to enrich eGFP+ cells and then deployed in organotypic cultures (OTC). eGFP in frozen sections from (OTC) was directly detected under fluorescence microscopy. (A,C) Cultures derived from healthy donor keratinocytes, and (B,D) cultures derived from NS keratinocytes. Cells transduced with eGFP alone (A,B) or SPINK5/eGFP (C,D) all expressed high levels of eGFP (green) with an intense staining pattern especially in the uppermost layers of the epidermis. NS keratinocytes transduced with SPINK5/eGFP showed reduced numbers of nuclei in the cornified layer of the epidermis (D) compared to the culture generated by NS cells transduced with eGFP alone (B, arrowed). Red shows nuclei staining by propidium iodide. Bar = 40 µm. eGFP, enhanced green fluorescent protein; LEKTI, lymphoepithelial Kazal-type-related inhibitor; NS, Netherton syndrome.
To further confirm the clonogenic potential of transduced cells, eGFP+ cells in transduced primary keratinocytes were enriched by flow cytometry sorting and used to generate three-dimensional OTCs. Matured cultures were fixed and sectioned to examine morphology as well as eGFP and LEKTI protein expression. Results showed fully developed epidermal structures in cultures generated by normal or NS keratinocytes. eGFP fluorescence could be visualized directly in sections of both cultures particularly within the upper layers of the epidermis, reflecting accumulation of eGFP in these layers (Figure 4b). eGFP was actually expressed in every layer of the OTCs including cultures transduced with SPINK5/eGFP. However, due to the accumulation of eGFP in the uppermost layers, the eGFP intensity in these layers resulted in bleaching effects across other compartments.
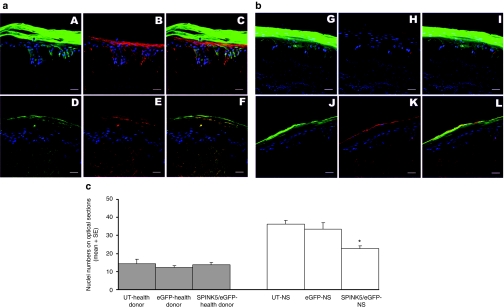
To ensure that eGFP expression overlapped with the therapeutic protein in OTCs generated from keratinocytes transduced with SPINK5/eGFP, the in situ expression of LEKTI was confirmed by immunofluorescence staining. The results confirmed overlapping expression of LEKTI and eGFP proteins in OTCs transduced with the bicistronic vectors (Figure 5a,b). In addition, the OTCs generated by gene-corrected NS keratinocytes exhibited reduced numbers of nuclei in the stratum corneum (Figure 4b, image D) compared to the OTCs generated by nontransduced cells or cells transduced with eGFP alone (Figure 4b, image B). Nuclei numbers in the stratum corneum on optical sections were counted and were significantly reduced in the OTC generated from NS cells transduced with SPINK5/eGFP (mean ± SE, 22.6 ± 6.19) compared to OTCs derived from nontransduced NS cells (36.2 ± 2.15) and NS cells transduced with eGFP alone (33.4 ± 3.61) (analysis of variance test, P < 0.01) (Figure 5c). There were no differences in OTCs generated from normal donor cells transduced with SPINK5/eGFP (13.8 ± 1.28) or transduced with eGFP alone (12.2 ± 1.11) or nontransduced cells (14.4 ± 2.38) (analysis of variance test, P > 0.05). Parakarotosis is one of the histopathological features in the skin with NS, and thus reversal of this morphological change in the gene-corrected OTCs highlighted restoration of LEKTI function following gene correction.
Figure 5.
LEKTI expression in OTCs transduced with SPINK5/eGFP. (a) OTC generated from healthy donor keratinocytes and (b) is the OTC generated from NS keratinocytes. Cells were transduced with (A–C, G–I) vector-encoding eGFP vector or (D–F, J–L) SPINK5/eGFP vector and used in OTCs. Sections from OTCs were stained with anti-LEKTI antibody and the expression of LEKTI (red) and eGFP (green) was verified by Confocal microscopy. Endogenous LEKTI expression could be detected in OTCs derived from normal cells (B) but not in OTC generated from NS cells (H). In contrast, both eGFP and LEKTI were detected in OTCs derived from healthy donor (E) or NS cells (K) transduced with SPINK5/eGFP expressing vectors. Merged images showed the overlapped expression of SPINK5 and eGFP in (C), (F), and (L) but not where NS cells had been transduced with vector expressing eGFP alone (I). These results demonstrate that LEKTI expression can be restored in NS using lentiviral vector technology and that the corrected cells can grow and differentiate as seen in healthy donor cells. Bar = 40 µm. (c) Nuclei numbers in the stratum corneum of OCTs in optical sections were analyzed by Image-Pro and results showed a significant reduction in the OTC using NS keratinocytes transduced with SPINK5/eGFP compared to nontransduced or eGFP transduced cells (*single factor ANOVA, P < 0.01). eGFP, enhanced green fluorescent protein; LEKTI, lymphoepithelial Kazal-type-related inhibitor; NS, Netherton syndrome; OTC, organotypic culture.
Lentiviral-mediated correction of NS: epidermal morphology of in vivo skin grafts
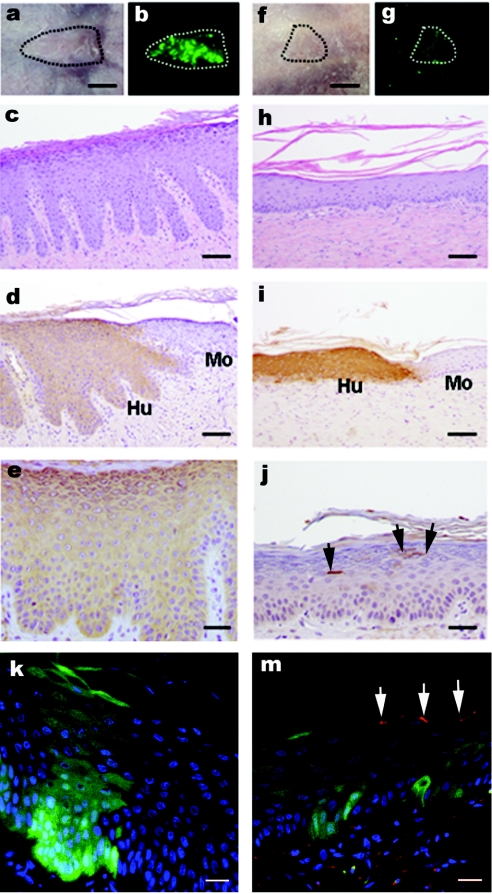
Although OTC experiments were highly informative and validated the feasibility of this approach, they were not suitable for longer-term studies required to demonstrate that the NS skin defect (as illustrated in Figure 1) can be corrected by ex-vivo gene transfer. Thus, in further studies, NS keratinocytes were transduced by exposure to one round of eGFP or SPINK5/eGFP-expressing lentiviral vector (multiplicity of infection 30). The transduction efficiency as assessed by flow cytometry for GFP expression was 40–50% for both groups and proviral copy number measured by quantitative PCR and found to be 0.59/cell in the eGFP-transduced cultures and 0.68/cell for SPINK5/eGFP-modified cultures. The transduced cells were expanded for another 4–5 days before passaging them on a fibrin matrix populated with live fibroblasts. Two days after the seeding, the bioengineered cultures were then grafted onto immunodeficient recipient mice and the animals tracked for a minimal period of 8 weeks allowing epidermal regeneration at the expense of engrafted stem cells and graft maturation. Examination of animals under fluorescence stereomicroscope revealed eGFP expression as a sign of sustained engraftment. Grafts transduced with vectors encoding eGFP alone were much brighter and this is in keeping with our observation that the intensity of eGFP expression was reduced in bicistronic vectors compared to monocistronic constructs (Figure 6b,g). At postmortem, no macroscopic abnormalities were detected and histological analysis of the graft and surrounding murine tissue was undertaken. Staining for human involucrin allowed areas of human and murine epidermis to be clearly delineated. NS grafts transduced with eGFP alone were found to have histological features of NS, including psoriasiform changes and hypergranulosis with a parakeratotic stratum corneum and exfoliated corneocytes (Figure 6c). In contrast, in grafts generated from cells transduced with SPINK5/eGFP, the epidermis exhibited corrected morphology with reduced rate ridges and almost complete restoration of normal epidermal architecture (Figure 6h). Importantly, immunohistochemical staining of LEKTI detected only a limited number of cells expressing the protein in these grafts (Figure 6j). This result was further confirmed by the overlapping expression of the LEKTI with eGFP in frozen sections where eGFP was directly visualized under blue light (488 nm) and LEKTI expression was detected by immunofluorescence staining (Figure 6m). As noted for OTCs, bright eGFP expression reflects accumulation of the marker protein and relatively small numbers of LEKTI-expressing cells appear capable of mediating wider bystander benefits. LEKTI is a secreted protein, and thus the genetically modified grafts may not only provide an immediate local protective barrier, but can also act as source of secreted LEKTI sufficient to mediate more generalized effects.
Figure 6.
In vivo assessment of NS keratinocyte correction in a humanized mouse model: macroscopic and histological examination following lentiviral transduction. NS keratinocytes were transduced with (a–e,k) eGFP or (f–j,m) SPINK5/eGFP and grafted onto nude mice in two independent series of experiments. Regenerated skin grafts were examined 8 weeks after grafting. (a,f) macroscopic appearance of the graft under transmit light and (b,g) a real-time eGFP expression under 488 nm light. (c,h) Histological appearance of grafts. (d,i) Human involucrin expression using antihuman involucrin antibody to indicate mouse (Mo)—human (Hu) skin boundary. (e) eGFP expression using GFP antibody. (j) The LEKTI expression using antihuman LEKTI antibody and arrows indicate isolated or clustered LEKTI+ cells within a wider area of skin. (k,m) Both frozen sections represent the overlapping between real-time eGFP (green) expression and LEKTI expression (red) stained with LEKTI antibody. These images showed that there was reversal of papillary changes in the graft transduced with SPINK5/eGFP (h) compared to the graft transduced with control vector eGFP (c). Low numbers of LEKTI expression cells in the grafts transduced with SPINK5/eGFP vector in both paraffin and frozen sections (j and m, arrowed areas) suggest small population of corrected cells sufficient to mediate wider correction of the epidermal architecture. bars: (a,b,f,g) = 5 µm; (c,d,h,i) = 200 µm; (e,j) = 100 µm; (k,m) = 40 µm. eGFP, enhanced green fluorescent protein; NS, Netherton syndrome.
Discussion
The ability to target holoclone-forming keratinocyte stem cells for genetic correction offers tremendous therapeutic possibilities.12,13 A relatively small number of such gene-modified cells have the capability of generating epithelial grafts for treatment of large areas of damaged or diseased skin. Proof-of-principle studies in junctional epidermolysis bullosa have used a murine gamma retroviral vector to stably transfer the LAMB3 complementary DNA to autologous keratinocyte stem cells harvested from an affected patient.11 High levels of gene transfer (>95%) were achieved, with LAM5-β3 protein expression being mediated by the strong viral promoter within the long-terminal repeat regions of the vector. Transduced epithelial sheets were grafted and have remained stable, without blistering, over a period of almost 4 years. There has been no evidence of immunological reaction to the graft, and no vector-mediated adverse events. The latter has become an important concern in relation to integrating vector systems after leukemia secondary to vector-mediated insertional mutagenesis was detected in five children after retroviral gene therapy for severe combined immunodeficiency.14,15,16 Similar complications have also arisen in a retroviral gene therapy study of chronic granulomatous disease,17 and although problems have not been detected in other studies including those involving the modification of differentiated cells such as T cells,18 improvements in vector safety are required. The development of self-inactivating vectors should reduce the risk of transactivation, and a switch to lentiviral vectors may further improve safety.19,20
In this study, we have demonstrated that replication defective viral vectors, derived from HIV-1 and devoid of pathogenic genes, can efficiently mediate stable gene transfer to keratinocyte stem cells without evidence of toxicity. Transfer of marker genes (eGFP), either alone or in combination with a therapeutic SPINK5 gene, was readily achieved by exposure of cultured keratinocyte stem cells to a single round of virus infection. The proportion of transduced cells confirmed by vector copy numbers was similar in both cases, although the levels of eGFP transgene expression from vectors encoding SPINK/eGFP were notably lower than from control vectors expressing eGFP alone. Vector copy number was similar in both cell populations and thus the reduced levels of eGFP in bicistronic constructs was probably due to the large (>3 kbp) size of the SPINK5 transgene and/or the inclusion of an internal ribosomal entry sequence in these constructs. Importantly, western blot analysis confirmed robust levels of LEKTI expression, with both correctly processed and unprocessed forms being detectable in transduced cells.
Previous studies have revealed that the full-length LEKTI (pro-LEKTI) synthesized in cells is rapidly processed into proteolytic fragments and secreted into extracellular compartments.21 More than five secreted LEKTI fragments with heterogeneous molecular weights have been found in the extracellular compartment of the epidermis including LEKTI domain 5 and 6. It has been confirmed that these domains can effectively inhibit KLK5, 7 and 14.3,5 We have shown that processed LEKTI is detectable in culture medium, indicating effective SPINK5 transgene expression, processing, and secretion of LEKTI.
As well as correction of LEKTI expression in cell lines in culture, we aimed to demonstrate correction of epidermal skin sheets generated from NS tissue. Transduction of NS patient derived keratinocytes including keratinocyte stem cells and the generation of epidermal sheets for use in three-dimensional OTCs was shown to be feasible. We have demonstrated that the viral promoter spleen focus-forming virus mediated stable gene expression in all epidermal compartments with pronounced accumulation of eGFP in the most superficial cornified layers. For clinical applications, replacement of spleen focus-forming virus with alternative, nonviral promoter elements are being investigated to improve the safety profile of the vector.
For other skin conditions, such as junctional epidermolysis bullosa, it may be desirable to restrict gene expression to the basal epidermal layers and alternative promoters, such as the keratin 14 promoter have been incorporated into lentiviral vectors to help achieve this.22 In the context of NS, expression throughout the epidermis may be acceptable and as discussed below, may have advantages in terms of supporting local paracrine effects.
Although in vitro OTCs have proven to be valuable for three-dimensional characterization of gene expression, they are not suitable to assess persistence of therapeutic effects. Thus, we have used a previously established skin humanized mouse model23 to investigate the durability of transgene expression and to evaluate the reconstitution of LEKTI expression in regenerated NS skin in vivo. The model was first validated using eGFP-expressing healthy donor grafts exhibiting high levels of expression readily detectable by fluorescence microscopy. Subsequently, we have demonstrated for the first time the feasibility of grafting human NS bioengineered skin onto immunodeficient mice. Characteristic histological features of the disease could be demonstrated, with full differentiated stratum corneum and undulating papillary changes. Remarkably, in grafts transduced with SPINK5/eGFP, there was clear reversal of these abnormalities, even where intracellular LEKTI staining revealed that a small number of epidermal cells were expressing the protein at levels detectable by immunohistochemistry. The much wider area of histological correction raises the possibility that secreted LEKTI may mediate paracrine effects over a wide area.
Further studies using this model will aim to characterize the clonal diversity of cells populating the grafts. High throughput sequencing mapping of vector integration sites at different time points facilitates the detection of dominant clones, allowing determination of survival advantage and screening for transformational changes.24
Our finding that limited numbers of LEKTI-expressing cells mediate valuable beneficial effects would support the use of emerging techniques that aim to edit gene mutations in situ rather than add additional corrected copies of a gene.25,26 Such approaches use DNA nucleases to create double-stranded DNA breaks which can then be targeted for repair by homologous recombination. Recently, meganucleases have been generated to target the xeroderma pigmentosum group C gene and in hematopoietic cell studies,27 Zinc-finger nucleases have been delivered by integration-deficient lentiviral vectors.26 Although these approaches have low efficiency, they hold out the prospect of gene repair without the risks associated with integrating viral vector systems.
The vector systems described here can be readily be adapted for clinical application and phase 1 testing in human patients with NS.28,29 Replacement of the viral promoter with a human system appropriate for expression in skin tissue is underway, and regulatory issues will have to be addressed. In the first instance, approvals for grafting of small areas of easily accessible skin with ex-vivo gene-corrected NS epidermal sheets will be sought to allow the feasibility, safety, and therapeutic potential of the strategy to be investigated.
Materials and Methods
Skin biopsies and primary keratinocyte cultures. Skin biopsies were obtained by punch biopsy from healthy volunteer donors or patients with NS under a protocol approved by our local ethics committee and with informed consent from parents or volunteers before the procedure. Primary keratinocytes were isolated from skin biopsies by incubation with 0.25% trypsin–EDTA for 3 hours, seeded on lethally irradiated 3T3 cells and grown in the keratinocyte culture media.10 After reaching confluence, cells were frozen and stored in liquid nitrogen for further use. Culture media were obtained from Invitrogen (Paisley, UK) and chemicals were obtained from Sigma (Poole, UK).
Construction of lentiviral vectors, lentiviral vectors production, and keratinocyte transduction. Codon optimized SPINK5 complementary DNA (GENEART, Regensburg, Germany) was cloned into a previously described replication deficient self-inactivating HV-1 lentiviral vector.30 The vector encoded the HIV-1 central polypurine tract, start site mutated Woodchuck postregulatory element and spleen focus-forming virus promoter.31 Vectors encoded eGFP alone (eGFP) or linked to SPINK5 via an internal ribosomal entry sequence from the endomyocarditis virus (SPINK5/eGFP).
Lentivirus vector preparations were produced by transfecting 293T cells with a packaging plasmid encoding viral gag, pol and accessory proteins (pCMV8.74), a plasmid encoding the envelope of vesicular stomatitis virus (pMDG2), a plasmid vector SPINK5/eGFP or eGFP and 1 × 10−7 mol/l of polyethylenimine (Sigma). Infectious lentiviruses were harvested at 72 hours post-transfection, filtered through 0.45-µm pore cellulose acetate filters and concentrated by ultracentrifugation (2 hours at 50,000g).32 The final viral pellets were resuspended in plain Dulbecco's modified Eagle's medium for 30 minutes at ice and aliquots of viral stocks were kept at −80 °C until further use. The viral titer was assessed on human 293T cells by flow cytometry and the typical viral titers were 0.8–1 × 108 UI/ml for SPINK5/eGFP vector and 1–2 × 109 UI/ml for eGFP vector.
Primary keratinocytes or cells from the N-TERT cell line which are derived from normal keratinocytes33 were infected with SPINK5/eGFP or eGFP lentiviral stock at various multiplicities of infection without addition of a cationic polymer.
Quantitative PCR. Integrated vector copy number was assessed in transduced cells after multiple passages by quantitative PCR using primers and probe designed to target the Woodchuck postregulatory element region of the vector in comparison to signal generated for the housekeeping gene β-actin. Integrated copy number was calculated with the aid of standard curves generated using plasmids encoding the vector and complementary DNA for human β-actin.
OTC. Following a single passage, primary keratinocytes were cultivated in vitro on de-epidermalized dermal matrix. Briefly, glycerol-preserved de-epidermalized dermal matrix (Euro Skin Bank, Beverwijk, the Netherlands) was prepared in phosphate-buffered saline containing antibiotics by incubation at 37 °C for up to 10 days and then cut into 1.5 × 1.5 cm2. Dermal fibroblasts derived from normal human skin biopsies were seeded onto the reticular side of the de-epidermalized dermal matrix overnight and keratinocytes were seeded on the papillary side next day. After keratinocytes reached confluence (about 2–3 days), the culture was lifted at the air–liquid interface for 14 days to allow keratinocyte differentiation. Primary human fibroblasts (passages 3–7) were used for the culture. For histological examination, matured cultures were fixed in 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. For direct eGFP protein detection and immunofluorescence staining, matured cultures were fixed in 4% paraformaldehyde in phosphate-buffered saline for 4 hours at 4 °C, followed by treatment with 0.1 mol/l ice-cold glycine for 1 hour and overnight incubation in 0.6 mol/l sucrose/phosphate-buffered saline solution at 4 °C. Tissues were then embedded in OCT (Thermo Scientific, Hampshire, UK) and stored at −80 °C for further use.
Immunostaining and immunoblotting. Immunofluorescence staining and immunohistochemistry were performed on frozen or paraffin tissue sections (6 µm thickness) using methods previously described.34 A custom-made affinity purified rabbit polyclonal antibody directed against the C-terminus of LEKTI was produced by Eurogentec (Southamton, UK). This antibody was used at 0.58 ng/ml for both immunostaining and immunoblotting. The anti-GFP antibody (Molecular Probes, Eugene, OR) and human-specific involucrin monoclonal antibody (clone SY5, Sigma) were used in 1:100 dilution for immunohistochemistry. Images taken from stained tissues were recorded using a Leica SP2 laser confocal microscope (Leica, Milton Keynes, UK) and processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA).
For immunoblotting, cells were suspended in a cooled buffer composed of 50 mmol/l Tris–HCl pH 8.0, 150 mmol/l NaCl, 5 mmol/l EDTA, cocktail protease inhibitors, and 1 mmol/l phenylmethanesulfonylfluoride. Samples were lysed by sonicating and incubated for 15 minutes at 4 °C. The sonicated samples were then centrifuged at 12,000 r.p.m. for 10 minutes to pellet the insoluble material. Filtered culture media were freeze-dried and lyophilized proteins were then dissolved in the same buffer. The total protein concentration in the supernatant was determined using Bio-Rad protein assay kit (Hertfordshire, UK). Samples from cell lysate or lyophilized protein suspension were further diluted in 5× sample buffer containing 100 mmol/l dithiothreitol, 10% sodium dodecyl sulfate, 30% glycerol, 0.001% bromophenol blue, and 0.5 mmol/l Tris–HCl pH 6.8. Equal quantities of total protein were loaded in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred to polyvinylidene fluoride membranes and incubated with LEKTI antibody overnight at room temperature with shaking. On the next day, membranes were further incubated with mouse anti-rabbit immunoglobulin G antibody conjugated with horse-radish peroxidase (Sigma) and signals were detected using the ECLplus System (GE Healthcare, Bucks, UK).
Flow cytometry analysis and cell sorting. Cell viability of tranduced N-TERT cells was evaluated using flow cytometry. Briefly, a suspension of nonfixed cells was incubated with 50 µl PI in 50 µg/ml (Invitrogen) at room temperature for 5 minutes and cells were then analyzed on a BD LSRII FACS machine (Becton Dickinson, San Jose, CA). Forward and side scatter were used to exclude debris and aggregates and eGFP fluorescence was measured by filter 530/30 nm and PI fluorescence was measured above 670 nm. In all cases, 10,000 eGFP+ cells were collected. Transduced cells were identified by eGFP fluorescence and cell death was quantified within this population by PI uptake. Analysis was performed using FlowJo (Tree Star, Stanford University, San Francisco, CA).
To enriching eGFP+ cells for OTC cultures, primary cells transduced with eGFP or SPINK/eGFP vector were sorted using a MoFlo cell sorter (DakoCytomation, Fort Collins, CO). Approximate 106 eGFP+ cells were sorted for each sample. Sorted eGFP+ cells were cultured in media described before immediately after sorting, and harvested for OTCs when reaching confluence.
Cell proliferation assays. Cell proliferation was assessed using Click-iT EdU Flow Cytometry Assay kit (Invitrogen). EdU is a nucleoside analogue of thymidine that is incorporated into DNA during active DNA synthesis. N-TERT cells were plated in 60 mm dishes 24 hours prior assay. On the next day, cells were incubated with 20 µmol/l of EdU for 1 hour at 37 °C. As negative control, cells from the same population were not treated with EdU. After incubation, cells were harvested and the incorporated EdU was labeled by Pacific blue azide following the manufacturer's protocols. As the fluorescent protein eGFP is not compatible with the Click-iT detection reaction regent, an anti-GFP rabbit antibody (Invitrogen) was used for labeling eGFP protein expression in transduced cells. Labeled samples were measured at 405 nm excitation for S phase cell population labeled with Pacific blue, 488 nm excitation for eGFP+ cell population labeled with Alexa 488, and 633 nm excitation for G0–1 and G2m cell populations labeled with 633-red DNA binding dye. A LSRII FACS machine was used for the assay and data was analyzed by FlowJo.
Bioengineered skin preparation and grafting to immunodeficient mice. The methods for preparing and grafting bioengineered skin in nude mice have been previously described.23 Briefly, primary NS keratinocytes infected with lentiviral vectors encoding eGFP or SPINK/eGFP genes were seeded on top of a fibrin matrix populated with live primary human fibroblasts (dermal equivalent). After keratinocytes reached confluence, the bioengineered skin constructs were grafted onto the dorsum of 6-weeks-old female immunodeficient nu/nu mice (NMRI strain; Elevage-Janvier, Le Genest Saint-Isle, France). Six mice were grafted for each condition. Successfully grafted animals were anesthetized and the presence of eGFP fluorescent grafts in the whole animals was monitored using a Kodak ISO 2000 MM live imaging apparatus (Kodak, Rochester, NY) under appropriate illumination/filters pairs. Close-up photographs of fluorescent grafted areas were obtained in a stereomicroscope equipped with fluorescent illumination and a digital camera (Olympus, Barcelona, Spain). Eight weeks after grafting, skin samples from grafts were taken postmortem, embedded in paraffin and sectioned for histological and immunohistochemical examination.
Quantification of nuclei number. Images from OTC cultures were taken using a Leica DMLB upright microscope (Leica) and the numbers of nuclei on optical sections were quantified using Image-Pro 6.2 (MediaCybernetics, Marlow, UK). Briefly, five nonoverlapped but adjacent optical sections were recorded and saved digitally from each slide using a 10× objective. To ensure relative reliability the light level on the microscope was set to a fixed level and the camera acquisition settings were kept constant for each slide. Following image recording, the stratum corneum of the epidermis on each optical section was highlighted manually as an area of interest. The numbers of nuclei were obtained by measuring the optical counts within the area of interest based on intensity threshold. The data of counts from optical sections were analyzed and compared statistically using single factor analysis of variance test.
Colonal analysis. Heterogeneous primary NS keratinocytes were transduced with SPINK5/eGFP vector and plated in ten 100 mm culture dish at a density of 600–800 cells on 3T3 feeder layers. The cells were allowed to grow for 14 days to identify holoclones, and then fixed with 4% paraformaldehyde in phosphate-buffered saline.35 Plates were examined under an inverted fluorescence microscope (Leica) to mark GFP+ colonies. The dishes were then stained with hematoxylin and photographed at a fixed distance using Camera Canon EOS-4150D (Canon, Tokyo, Japan). The images were analyzed using ImageJ (version 4.2; NIH, Bethesda, MD) to identify >5 mm colonies. The percentage of positive colonies was calculated based on these large colonies.
Acknowledgments
Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the UK Department of Health. This study was also supported by the Newlife Foundation for Disabled Children and Ichthyosis Support Group. W.Q. is supported by Leukemia Research Senior Lecturership and A.J.T. is a Wellcome Trust Senor Fellow in Clinical Science. F.L. and M.D.R. are supported by grants P-BIO-0306-2006, PI081054 and SAF-2007-61019 from Spanish CAM and MICINN, respectively. We are indebted to Mrs Almudena Holguin, Blanca Duarte, and Nuria Illera for grafting experiments. We gratefully acknowledge Mr Prabhjoat Chana, Dr Ayad Eddaoudi, and Dr Bertrand Vernay (ICH Confocal Core Facility) for their expertise in flow cytometry analysis and ImageJ application for colonal analysis. The authors declared no conflict of interest.
REFERENCES
- Bitoun E, Micheloni A, Lamant L, Bonnart C, Tartaglia-Polcini A, Cobbold C, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Hum Mol Genet. 2003;12:2417–2430. doi: 10.1093/hmg/ddg247. [DOI] [PubMed] [Google Scholar]
- Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- Borgoño CA, Michael IP, Komatsu N, Jayakumar A, Kapadia R, Clayman GL, et al. A potential role for multiple tissue kallikrein serine proteases in epidermal desquamation. J Biol Chem. 2007;282:3640–3652. doi: 10.1074/jbc.M607567200. [DOI] [PubMed] [Google Scholar]
- Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D'Alessio M, et al. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Invest Dermatol. 2006;126:1622–1632. doi: 10.1038/sj.jid.5700284. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Deraison C, Bonnart C, Bitoun E, Robinson R, O'Brien TJ, et al. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005;124:360–366. doi: 10.1111/j.0022-202X.2004.23583.x. [DOI] [PubMed] [Google Scholar]
- Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, Ishida-Yamamoto A, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- Bonnart C, Deraison C, Lacroix M, Uchida Y, Besson C, Robin A, et al. Elastase 2 is expressed in human and mouse epidermis and impairs skin barrier function in Netherton syndrome through filaggrin and lipid misprocessing. J Clin Invest. 2010;120:871–882. doi: 10.1172/JCI41440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. The stem cell compartment in human interfollicular epidermis. J Dermatol Sci. 2002;28:173–180. doi: 10.1016/s0923-1811(02)00003-8. [DOI] [PubMed] [Google Scholar]
- MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG. Baserga R. A Practical Approach. IRL Press: Oxford, p 81; 1989. Methods for clonal growth and serial cultuvation of normal human epidermal keratinocytes and mesothelial cells. [Google Scholar]
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- De Luca M, Pellegrini G., and, Mavilio F. Gene therapy of inherited skin adhesion disorders: a critical overview. Br J Dermatol. 2009;161:19–24. doi: 10.1111/j.1365-2133.2009.09243.x. [DOI] [PubMed] [Google Scholar]
- Mavilio F., and, Ferrari G. Genetic modification of somatic stem cells. The progress, problems and prospects of a new therapeutic technology. EMBO Rep. 2008;9 Suppl 1:S64–S69. doi: 10.1038/embor.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mägert HJ, Ständker L, Kreutzmann P, Zucht HD, Reinecke M, Sommerhoff CP, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274:21499–21502. doi: 10.1074/jbc.274.31.21499. [DOI] [PubMed] [Google Scholar]
- Di Nunzio F, Maruggi G, Ferrari S, Di Iorio E, Poletti V, Garcia M, et al. Correction of laminin-5 deficiency in human epidermal stem cells by transcriptionally targeted lentiviral vectors. Mol Ther. 2008;16:1977–1985. doi: 10.1038/mt.2008.204. [DOI] [PubMed] [Google Scholar]
- Larcher F, Dellambra E, Rico L, Bondanza S, Murillas R, Cattoglio C, et al. Long-term engraftment of single genetically modified human epidermal holoclones enables safety pre-assessment of cutaneous gene therapy. Mol Ther. 2007;15:1670–1676. doi: 10.1038/sj.mt.6300238. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Hacein-Bey-Abina S, Wissler M, Carlier F, Lim A, Prinz C, et al. Clonal evidence for the transduction of CD34+ cells with lymphomyeloid differentiation potential and self-renewal capacity in the SCID-X1 gene therapy trial. Blood. 2005;105:2699–2706. doi: 10.1182/blood-2004-07-2648. [DOI] [PubMed] [Google Scholar]
- Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- Redondo P, Prieto J, Muñoz IG, Alibés A, Stricher F, Serrano L, et al. Molecular basis of xeroderma pigmentosum group C DNA recognition by engineered meganucleases. Nature. 2008;456:107–111. doi: 10.1038/nature07343. [DOI] [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- Qasim W, Mackey T, Sinclair J, Chatziandreou I, Kinnon C, Thrasher AJ, et al. Lentiviral vectors for T-cell suicide gene therapy: preservation of T-cell effector function after cytokine-mediated transduction. Mol Ther. 2007;15:355–360. doi: 10.1038/sj.mt.6300042. [DOI] [PubMed] [Google Scholar]
- Yáñez-Muñoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Doumeng C., and, Taichman LB. Durable and stratum-specific gene expression in epidermis. Gene Ther. 2002;9:1278–1285. doi: 10.1038/sj.gt.3301800. [DOI] [PubMed] [Google Scholar]
- Welm BE, Dijkgraaf GJ, Bledau AS, Welm AL., and, Werb Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell. 2008;2:90–102. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di WL, Hennekam RC, Callard RE., and, Harper JI. A heterozygous null mutation combined with the G1258A polymorphism of SPINK5 causes impaired LEKTI function and abnormal expression of skin barrier proteins. Br J Dermatol. 2009;161:404–412. doi: 10.1111/j.1365-2133.2009.09231.x. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., and, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]