Abstract
Acute intermittent porphyria (AIP) is characterized by a hereditary deficiency of hepatic porphobilinogen deaminase (PBGD) activity. Clinical features are acute neurovisceral attacks accompanied by overproduction of porphyrin precursors in the liver. Recurrent life-threatening attacks can be cured only by liver transplantation. We developed recombinant adeno-associated virus (rAAV) vectors expressing human PBGD protein driven by a liver-specific promoter to provide sustained protection against induced attacks in a predictive model for AIP. Phenobarbital injections in AIP mice induced porphyrin precursor accumulation, functional block of nerve conduction, and progressive loss of large-caliber axons in the sciatic nerve. Hepatocyte transduction showed no gender variation after rAAV2/8 injection, while rAAV2/5 showed lower transduction efficiency in females than males. Full protection against induced phenobarbital-attacks was achieved in animals showing over 10% of hepatocytes expressing high amounts of PBGD. More importantly, sustained hepatic expression of hPBGD protected against loss of large-caliber axons in the sciatic nerve and disturbances in nerve conduction velocity as induced by recurrent phenobarbital administrations. These data show for the first time that porphyrin precursors generated in the liver interfere with motor function. rAAV2/5-hPBGD vector can be produced in sufficient quantity for an intended gene therapy trial in patients with recurrent life-threatening porphyria attacks.
Introduction
Acute intermittent porphyria (AIP) is an autosomal dominant metabolic disease characterized by a deficiency of porphobilinogen deaminase (EC 4.3.1.8; PBGD), the third enzyme of the heme-synthesis pathway.1,2,3 The dominant clinical feature consists of life-threatening acute intermittent attacks when hepatic heme synthesis is activated by endocrine and environmental factors, caloric restriction, or intercurrent infections. Among the endocrine factors, steroid hormones play an important role and women are affected more often than men.3,4 Drugs metabolized by microsomal cytochrome P450 enzymes greatly increase heme demand in the liver and thereby lead to an induction of hepatic aminolevulinate synthase (ALAS1), the first and rate-limiting enzyme, in the heme biosynthetic pathway in the liver.3 Genetic defect at the PBGD locus, in conjunction with impaired heme-mediated repression of hepatic ALAS1, leads to a marked overproduction and accumulation of aminolevulinate acid (ALA) and porphobilinogen (PBG) that are the substrates between both enzymes.
Symptoms of AIP are very heterogeneous and include abdominal pain and neurovisceral disturbances.1,2,3 Frequent acute attacks are associated with recurrent nerve damage,5,6 although the mechanisms of neurological damage are incompletely understood. It has been suggested that peripheral neuropathy originates from impaired hemoprotein metabolism in nervous tissue.7,8 A second hypothesis suggested a neurotoxic effect of the porphyrin precursors ALA and/or PBG.9 The latter idea arises from the marked elevation of such precursors during acute attacks and is in accordance with the observed remission of the disease after liver transplantation.5,10 Current treatments for AIP attacks are infusions of glucose and intravenous (i.v.) administration of Hemin,1 which restores the regulatory heme pool and suppresses hepatic ALAS1 induction. Nevertheless, hemin is rapidly metabolized and its effects are transient. Severe and recurrent porphyria attacks can be cured only by allogenic liver transplantation.5
Alternative approaches such as gene therapy have been proposed for those patients whose liver function is entirely normal, except for the PBGD deficiency. Specific reasons in favor of PBGD gene delivery therapy are that patients express half of the normal protein content that should be sufficient to induce tolerance. Furthermore, transgene regulation is not required, because PBGD is not the rate-limiting enzyme of the hepatic heme-synthesis pathway.3 The feasibility of gene delivery therapy has been already demonstrated in a murine model of AIP using a first generation adenoviral vector11 and also by hydrodynamic delivery of therapeutic plasmid into hepatocytes.12 However, these technologies do not result in long-term expression. Recently, recombinant adeno-associated virus (rAAV) vector serotype 8 demonstrated sustained and long-term murine PBGD expression in the liver.13 Indeed, the tropism of the AAV depends on the serotype of capsid protein where several authors have confirmed that rAAV serotypes 5 and 8 are suitable for liver transduction,14,15 although serotype 5 vector displays a narrower tissue tropism in wild-type mice.14
In this study, we developed a scalable manufacturing process of rAAV-mediated delivery of human PBGD protein, and obtain a proof-of-principle that sustained PBGD expression in the liver can restore the heme metabolic pathway and confer long-lasting protection against induced acute attacks in AIP mice. The use of liver-specific promoters in our rAAV vectors represents a novel experimental strategy to definitively document the role of the liver in peripheral neuropathy.
Results
rAAV2/8-mediated hepatic hPBGD expression in AIP mice provides prolonged enzymatic correction and prevents phenobarbital-induced acute attacks
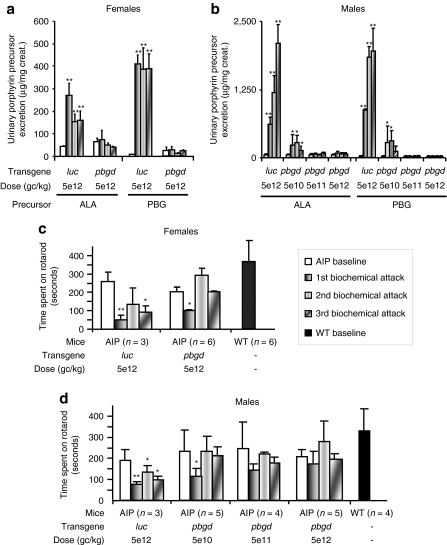
The safety of a single i.v. administration of rAAV was evaluated in AIP mice. The administration of 5 × 1012 genome copies (gc)/kg of rAAV2/8-hPBGD did not lead to increased porphyrin precursor excretion or significant inflammatory response, as determined by an absence of increased serum IL-6 within 6 hours after the vector injection (data not shown). In accordance with Lindberg et al.,16 phenobarbital challenge increased urine levels of ALA and PBG in AIP mice, which had received the reporter rAAV2/8-luciferase vector (Figure 1a,b). In striking contrast, no ALA or PBG overexcretion was found in mice injected with the highest doses of rAAV2/8-hPBGD in either female or male mice. Phenobarbital also exacerbated motor disturbances in AIP mice, as evaluated by the rotarod test (Figure 1c,d). Of note, AIP mice injected with the therapeutic vector did not suffer from phenobarbital-induced motor disturbance. Liver function tests were normal in all cohorts of AIP animals and were not modified after repetitive administration of phenobarbital (data not shown).
Figure 1.
Therapeutic efficacy of the rAAV2/8-hPBGD vector in AIP mice. Porphyrin precursor excretion [(a) females, (b) males] and motor coordination [(c) females, (d) males] were determined in mice before (baseline) and after biochemical induction of porphyrin precursors with increasing doses of phenobarbital for four consecutive days. Motor coordination was determined by the time that mice can stay on a rotating dowel turning at a positive acceleration. The first bar is baseline; the second bar represents measurements performed 15 days after the administration of a respective dose of rAAV2/8 vector and after phenobarbital administration. The third and forth bars show the same procedure performed 28 and 90 days after respective dose of gene therapy, respectively. Panels c and d have extra bars corresponding to baseline values from noninjected wild-type mice. The Wilcoxon signed-rank test was used for comparison of means before and after phenobarbital administrations. *P < 0.05; **P < 0.01; ***P < 0.001 versus baseline values in each group. AIP, acute intermittent porphyria; ALA, δ-aminolevulinic acid; PBG, porphobilinogen; rAAV, recombinant adeno-associated virus; WT, wild type.
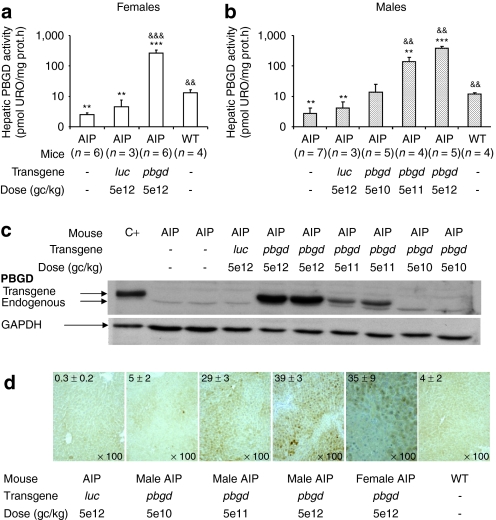
Three months after the vector injection, enzymatic activity measurements (Figure 2a,b) and western blots (Figure 2c) demonstrated functional hPBGD expression in liver homogenates. A dose-dependent increase in expression was observed in male AIP mice treated with the therapeutic rAAV2/8-hPBGD vector. Immunohistochemical analysis of liver sections from AIP mice injected with saline or rAAV2/8 luciferase vector exhibited weak brown signal in the cytoplasm of whole hepatocytes (Figure 2d). Intense immunostaining for PBGD, attributable to the transgene, was detected in 39, 29, and 5% of hepatocytes from male mice injected with high, medium, or low doses of rAAV2/8-hPBGD, respectively. Comparable levels of active hPBGD enzyme and hepatocyte transduction were observed between males and females injected with the same dose of therapeutic vector. The proportion of PBGD-stained cells negatively correlated with urine PBG excretion (y = 260x−0.9862, Spearman's rank correlation r = −0.8, P < 0.0001) and urine ALA excretion (y = 2157x−1.24, r = −0.72, P < 0.0001). The threshold for therapeutic efficiency, measured in terms of preventing porphyrin precursor accumulation after phenobarbital administration, was reached when ~10% of hepatocytes expressed high amounts of PBGD.
Figure 2.
Dose-dependent increase of functionally active hepatic hPBGD 3 months after the administration of rAAV2/8-hPBGD. The figure shows the expression of PBGD obtained after different gene doses of recombinant PBGD; measured as enzyme activity in (a) female and (b) male, by (c) immunoblot assay and (d) immunohistochemistry. A polyclonal antibody against PBGD, generated in our laboratory, recognized both human and murine PBGD. Both proteins show a different pattern of migration when separated on a 12% sodium dodecyl sulfate–polyacrylamide gel. It allows for differentiation between endogenous mouse and exogenous human PBGD, as illustrated in c. The micrographs illustrated in d, are representative for immunochemical analysis of livers from animals injected with increasing doses of rAAV2/8-hPBGD (and controls). Values in the upper left corner represent the mean percentage (±SD) of brown stained PBGD-positive cells calculated in each group of mice (additional detail in Supplementary Materials and Methods). All the analyses are performed in mice 3 months after gene therapy. The results obtained with different rAAV2/8 doses are statistically compared to the noninjected AIP mice (&&P < 0.01; &&&P < 0.01) and also to the wild type animals (**P < 0.01; ***P < 0.001) using the Mann–Whitney test. AIP, acute intermittent porphyria; URO, uroporphyrin; rAAV, recombinant adeno-associated virus; WT, wild type.
Long-lasting hPBGD expression in the liver prevents loss of large-caliber axons in the sciatic nerve
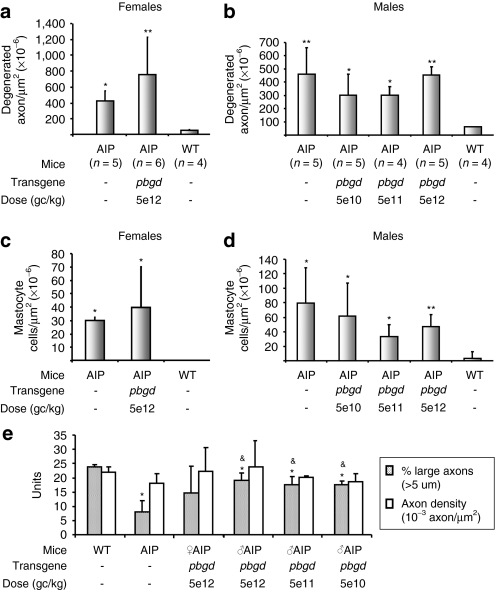
Light microphotographs taken from cross-sections of sciatic nerves from AIP mice demonstrated abundant myelin damage with different breakdown steps of the myelin sheath (broken myelin, lipid phagocytosis, and fat granule cells; data not shown), a high degree of axonal degeneration (Figure 3a,b) and elevated numbers of mastocytes (Figure 3c,d) compared to wild-type animals. AIP mice injected with the therapeutic vector exhibited a comparable number of degenerated axons and mastocytes to untreated AIP animals. Interestingly, AIP mice exhibited normal axon density when compared with wild-type mice, but also a decreased percentage of large-caliber axons (Figure 3e). Because all cohorts of AIP animals receiving the therapeutic vector displayed normal percentages of large axons, we hypothesize that specific damage and loss of large-caliber axons are related to hepatic PBGD deficiency and/or porphyrin precursor accumulation resulting from an acute attack. Given that atrophy of large, myelinated motor axons selectively affects nerve conduction velocity, neurophysiological studies were performed in AIP mice before and after seven consecutive acute attacks induced by phenobarbital.
Figure 3.
Number and caliber of axons in the sciatic nerve from wild-type and AIP mice injected with different doses of rAAV2/8-hPBGD. The number of degenerated axons [(a) female, (b) males] and the mastocyte counts [(c) females, (d) male] were measured in light micrographs sections (see Supplementary Materials and Methods) of sciatic nerves from mice 90 days after respective dose of gene therapy. Mastocyte cells were readily differentiated based upon morphological criteria and the presence of metachromatic granules. (e) Axon density and percentage of large axons were also measured in the same animals. The results obtained with different rAAV2/8 doses are statistically compared to the baseline level in the AIP group (&P < 0.05; &&P < 0.01) and also to the wild-type animals (*P < 0.05; **P < 0.01) using the nonparametric Mann–Whitney test. AIP, acute intermittent porphyria; rAAV, recombinant adeno-associated virus; WT, wild type.
rAAV-mediated liver expression of hPBGD provides sustained protection against motor dysfunction secondary to repeated acute attacks in young and old AIP mice
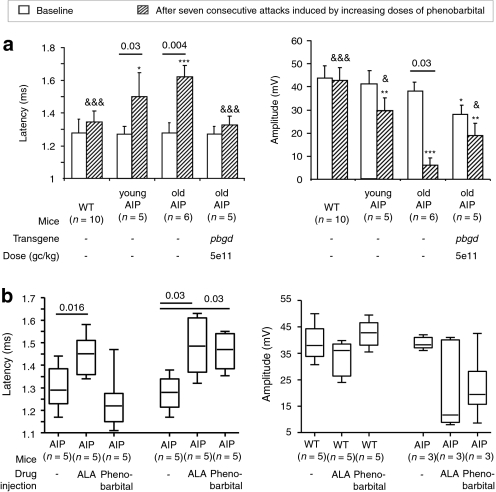
Neurophysiological studies regarding sciatic nerve conduction velocity demonstrated latency, amplitude, and duration differences between the different groups, but only after repeated induction of acute porphyric attacks (Figure 4a). Latency values were longer for AIP than for wild-type mice, but were within normal limits in the rAAV2/8-hPBGD-treated mice (Figure 4a, left). Similarly, the duration was shorter in the AIP mice injected with rAAV2/8-hPBGD than in old AIP mice (3.38 ± 0.30 ms versus 3.99 ± 0.42 ms, respectively; P = 0.002, Mann–Whitney test) and was similar to young AIP mice (3.48 ± 0.33 ms; P = 0.66, Mann–Whitney test) or wild-type animals (3.22 ± 0.31 ms, P = 0.24, Mann–Whitney test). The protective effect of rAAV2/8-hPBGD was evident, but not total, in the recorded amplitude values (Figure 4a, right). The amplitude values obtained in the older group of AIP mice receiving therapeutic vector were larger than in the AIP mice of a similar age, but smaller than the amplitude observed in young AIP mice or controls.
Figure 4.
The impact on sciatic nerve function caused by high levels of porphyrin precursors. (a) The compound-muscle action potentials evoked by proximal stimulation of the sciatic nerve (left, latency; right, amplitude) were measured before and after seven consecutive attacks (induced biweekly by the administration of four increasing doses of phenobarbital). AIP animals at different ages are compared to wild-type and to those AIP mice receiving rAAV2/8-hPBGD, using the Mann–Whitney test (&P < 0.05; &&&P < 0.001 versus old AIP mice and *P < 0.05; **P < 0.01; ***P < 0.001 versus wild-type animals). (b) The figure shows the impact on the sciatic nerve function (left, latency; right, amplitude) caused solely by PBGD deficiency, by intravenous (i.v.) administration of ALA (3 mmol/kg/day for 4 consecutive days) or by high levels of porphyrin precursors induced by phenobarbital (after four consecutive doses). The compound-muscle action potentials were measured in the two hind legs of each of the animals. Comparisons are performed between animals with increased levels of porphyrin precursors induced by phenobarbital and those receiving ALA i.v., using the Wilcoxon signed-rank test. AIP, acute intermittent porphyria; ALA, δ-aminolevulinic acid; rAAV, recombinant adeno-associated virus; WT, wild type.
Interestingly, successive ALA injections increased the latency of the compound-muscle action potentials in both AIP and wild-type mice (Figure 4b, left). As a control, phenobarbital administration to the same animals only modified these parameters in AIP mice. Duration was not significantly modified after ALA administration in both AIP and wild-type mice (data not shown). Finally, several AIP mice (two out of three animals) showed reduced amplitudes of the compound-muscle action potentials after successive administrations of ALA (Figure 4b, right). Collectively, these results suggested that rAAV-mediated liver gene therapy protects against functional block and motor-axonal degeneration induced by porphyrin precursors generated in the liver from AIP mice.
Development of a safe, efficient, and cost-effective manufacturing process of rAAV2/5 vector for preclinical and clinical studies
A critical step was to develop a scalable manufacturing process of rAAV for translational research. Major advantages in terms of safety using rAAV vectors produced in the SF9 insect cell system include: (i) the nonrequirement for adenoviral helper sequences; (ii) the use of baculovirus that cannot replicate in mammalian cells; and (iii) the use of serum-free amplification. The upscalable production and purification protocol for rAAV2/5 provide a considerable advance for their clinical application. Following specific affinity chromatography processing of 25 l from a working volume of SF9 cells, ~1 × 1014 vector genomes can be obtained. By contrast, classical production using HEK-293 cells requires a production from 10,000 roller bottles.
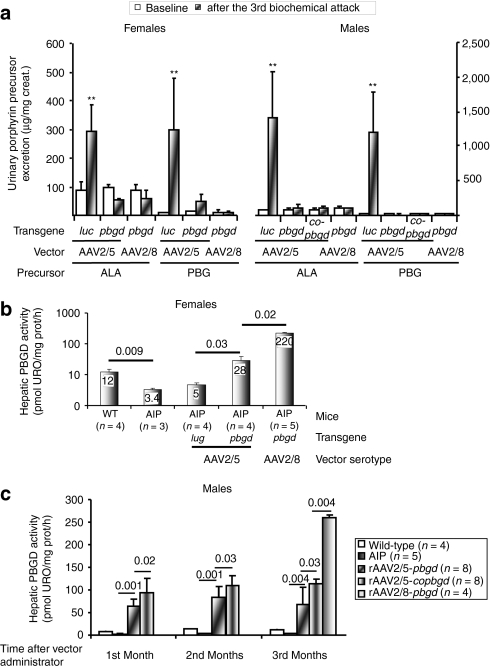
Both rAAV2/5-hPBGD and rAAV2/8-hPBGD demonstrated equal efficiency preventing ALA and PBG accumulation in AIP mice after biochemical induction with increasing doses of phenobarbital for four consecutive days (Figure 5a). Moreover, rAAV-hPBGD vectors protect against peripheral motor disturbance induced by phenobarbital. Rotarod tests were performed before (considered as 100%) and 3 months after administration of rAAV2/5-luc. The time that female AIP mice were able to stay on the rotarod decreased to 53 ± 8%. However, if female animals had received rAAV-PBGD vector, the time spent on rotarod was maintained or even increased (132 ± 30% with serotype 5 and 115 ± 30% with serotype 8). Similar results were obtained in male AIP animals (65 ± 11% in mice injected with rAAV2/5-luc, 101 ± 10% with rAAV2/5-hPBGD, and 94 ± 19% with rAAV2/8-hPBGD; data not shown).
Figure 5.
Full protection against phenobarbital-induced attacks of porphyria in AIP mice injected with therapeutic rAAV2/5 or rAAV2/8 vectors. (a) Porphyrin precursor excretion in 24-hour urine samples before and after an acute attack induced with phenobarbital 3 months after the rAAV injection. (b) PBGD activity measured in the livers of female mice 3 months after the (separate) administration of two different rAAV vector serotypes. (c) Hepatic PBGD activity in male AIP animals obtained 1, 2, and 3 months after the administration of different therapeutic vectors. The Wilcoxon signed-rank test was used for comparison of porphyrin excretion before and after phenobarbital inductions. **P < 0.01 versus baseline values in each group. The nonparametric Mann–Whitney test was used for comparison of hepatic PBGD activity. ALA, δ-aminolevulinic acid; AIP, acute intermittent porphyria; cohpbgd, codon-optimized cDNA of human PBGD protein; Luc, luciferase; rAAV, recombinant adeno-associated virus; PBG, porphobilinogen; URO, uroporphyrin; WT, wild type.
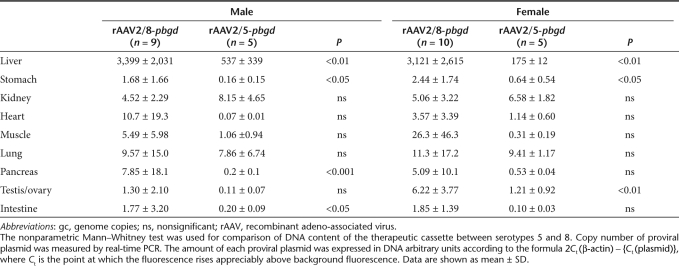
In the rAAV2/5 female cohort, hepatic PBGD activity was approximately tenfold lower when compared with females injected with rAAV2/8 vector (Figure 5b). Nevertheless, all of the females exhibited >10% of PBGD-staining hepatocytes, which fully protected against phenobarbital-induced acute attacks. In male AIP animals, the rAAV2/5 vector carrying a codon-optimized human PBGD complementary DNA (cDNA; cohPBGD) displayed a 25% increase in functionally active hepatic PBGD, compared with livers transduced with rAAV2/5-wild-type hPBGD (Figure 5c). Of note, both groups of animals injected with rAAV2/5 vectors exhibited an equivalent proportion of PBGD-stained hepatocytes (17 ± 4% in hPBGD group versus 21 ± 3% in cohPBGD group, P = 0.33, Mann–Whitney test) and hepatic DNA content [30 ± 18 DNA arbitrary units (AU), see Supplementary Materials and Methods, in hPBGD group versus 37 ± 28 AU in cohPBGD group, P = 0.82, Mann–Whitney test]. In male AIP mice injected with rAAV2/8-PBGD vector, the hepatic PBGD activity (Figure 5c) and the proportion of PBGD-stained hepatocytes (39 ± 3%) were about twofold higher compared to rAAV2/5-cohPBGD-treated mice. Liver transduction efficiency of the rAAV2/5-cohPBGD vector under specific cellular environments during an acute attack was the same in AIP mice, as measured by the ensuing PBGD activity (95 ± 15 pmolURO/mg/h without phenobarbital versus 86 ± 11 pmolURO/mg/h with phenobarbital, P = 0.7, Mann–Whitney test) and quantification of vector genome copies (11 ± 1.4 AU without phenobarbital versus 11 ± 1.3 AU with phenobarbital, P = 0.9, Mann–Whitney test). Tissue distribution analyses revealed preferential hepatocyte transduction after systemic delivery of both serotype 5 and 8 (Table 1). In the case of rAAV2/8, a low PCR signal was detected in heart, muscle, lungs, pancreas, and ovaries or testes, while in the rAAV2/5-treated AIP mice a faint band was present in lungs and kidneys.
Table 1. Tissue distribution analysis of proviral plasmid in various organs from male and female acute intermittent porphyria mice 3 months after the administration of 5 × 1012 gc/kg of rAAV vectors.
Discussion
The AIP mouse model replicates the drug-precipitated biochemical abnormalities of acute porphyria in humans and develops some neuropathological features resembling those seen in patients with this inherited disease.17 The use of a hepatocyte-specific promoter in the described therapeutic rAAV vectors can restore the enzyme defect exclusively in the liver without modifying heme synthesis in nervous tissue. Histological and electrophysiological studies in AIP mice conclusively indicated that at least three components contribute to the development of porphyric neuropathy. First, in AIP mice, with 30% of normal PBGD activity, motor dysfunction and axonal neuropathy developed progressively with age, even in the absence of high levels of porphyrin precursors.8,16,17 Electrophysiological studies performed in AIP animals without previous induction of acute attack reveal progressive loss of amplitude of the compound-muscle action potential parameters in old AIP mice when compared with young AIP animals. This loss is related to the axonal degeneration that affects motor fibers in the sciatic nerve. This phenomenon increased with age in AIP mice and could be related to the lack of heme8 and/or dysfunction of the mitochondrial respiratory chain in neural tissue.18 Injection of therapeutic vector partially protected against loss of amplitude in age-matched AIP mice.
In the very rare homozygous dominant form of AIP,9 nerve structures that developed before birth were intact and patients selectively exhibit damage of oligodendrocyte structures developed postnatally. These data suggested that heme depletion in the nervous system did not impair normal neuronal development, although deficiency of heme-containing proteins can impair critical cell processes dependent on hemoproteins and earlier mitochondrial degeneration. Mitochondrial dysfunction and subsequent oxidative stress have been associated with many neurodegenerative diseases19 and specifically to axonal damage.18 Nevertheless, the activity of PBGD in homozygous patients is <10% of normal in all organs. Therefore, heme depletion must be more extensive than in the heterozygous patients, who exhibit a 50% reduction of normal PBGD activity.
Second, latency and duration values critically depend on the preservation of fast-conducting high-diameter fibers (>5 µm), while the amplitude of the compound-muscle action potentials gives an estimation of the total volume of fibers, irrespective of their diameter.20 The histological analysis showed a predominant loss of high-diameter axons in AIP animals. Electrophysiological analysis showed that latency values were only affected in AIP mice after the induction of repeated porphyric attacks. Long-term expression of the hPBGD transgene in the livers of AIP mice provided full protection against latency. These data suggest that the specific loss of the faster-conducting fibers is most likely related to the existence of a toxic compound produced in the liver during the acute attack.
Finally, the electrophysiological studies carried out in the sciatic nerve confirmed that ALA can contribute to modifying nerve conduction in wild-type mice with differences in the type of fibers affected. Toxic concentrations of ALA, but not phenobarbital itself, induced a quick and reversible increase in latency values without alteration of amplitudes in wild-type mice. As the effect was fast and reversible, these neuropathic effects of ALA could be attributable, at least in part, to its pro-oxidant properties that damage myelinating Schwann cells21 and/or reduced activity of the Na+/K+ pump resulting in a reduction of membrane depolarization.22 Accordingly, ALA administration in AIP mice induced increased latency values together with a drop in amplitude values in two-thirds of the animals. This can be interpreted in the sense that nerve cells were more vulnerable to ALA toxicity in AIP than in wild-type mice. Chronic exposure to ALA, as generated by phenobarbital administration, altered both latency and amplitude in most AIP animals. Both the pro-oxidant property of ALA itself and leakage of oxygen-free radicals from a damaged respiratory chain may contribute to a state of heightened oxidative stress that would contribute to disruption of myelin metabolism and axonal dysfunction by membrane depolarization.
These experimental data are compatible with the hypothesis of a toxic origin of the proximal motor neuropathy described in humans. This interpretation suggests that a neurotoxic compound enters the motor neuron at the neuromuscular synapses. Motor weakness occurs in relation to acute attacks7 and Hemin therapy is only effective in the early stages of neurological manifestations. Because heme does not cross the blood–brain or blood–nerve barriers,23 its therapeutic effect in peripheral neuropathy probably relates to decreases in the hepatic production of porphyrin precursors.1 Therefore, rAAV-mediated liver gene therapy can be fully protective against motor neuropathy in AIP patients, as was observed in the few reported cases of those who underwent a liver transplant.5,10
Stable expression of PBGD transgene was recently reported in male AIP mice with rAAV2/8-mediated liver transfer of murine PBGD.13 Our study extends these results demonstrating sustained enzymatic correction in female AIP mice delivering human PBGD. This is important because the majority of patients who present with severe AIP are women3 and because female mice are less amenable to liver gene transfer by different rAAV serotypes.14,15,24,25 The second specific purpose of our study was to develop a safe, efficient, and cost-effective manufacturing process of rAAV2/5-cohPBGD vector. Tissue distribution of rAAV2/5 proviral plasmid revealed preferential liver transduction after systemic delivery. This serotype displays a narrow tropism for the livers of mice, whereas significant numbers of rAAV2/8 vector genomes were identified from reproductive organs. Indeed, the use of a liver-specific promoter in our rAAV vectors offer an additional advantage as it diminishes transgene expression in extrahepatic tissues and is thus likely to reduce any immune response against the transgene. PBGD cDNA codon optimization usage improved PBGD expression levels. As codon sequences were optimized according to RNA abundance in human cells, this cohPBGD cDNA is expected to express even more PBGD in humans than in mice.
Stressful conditions during porphyric attacks do not modify rAAV gene transduction in AIP mice. Significant differences in the PBGD activity and hepatocyte transduction were observed between males and females injected with the same doses of the rAAV2/5-hPBGD vector. Hormonal differences would probably contribute to resistance in the females to hepatocyte gene transfer with some rAAV serotypes.25 Moreover, female mice still showed >10% transduced hepatocytes at the doses of 5 × 1012 gc/kg, which was the proportion calculated to provide full phenotypic protection against pharmacologically induced porphyric attacks in AIP mice. This protection could be explained not only by cross-metabolization of porphyrin precursor from noncorrected cells but also by the increased ability of these corrected hepatocytes to synthesize the amount of heme required to metabolize phenobarbital. These events are postulated to restore the metabolic balance and repress the upregulated ALAS1. This bystander effect is considered critical for the success in the clinic.
In conclusion, in AIP mice multiple mechanisms cause neurologic manifestations, including heme depletion in nerve cells and a high accumulation of neurotoxic porphyrin precursors of hepatic origin. Sustained expression of the hPBGD transgene in the livers of AIP mice prevents accumulation of porphyrin precursors of hepatic origin and provides full protection against changes in latency, which is most closely related to the preservation of fast-conducting high-diameter axons. We have successfully produced rAAV vectors that confer sustained protection against induced acute attacks in AIP mice, both in males and females using the insect cell platform. rAAV2/5-cohPBGD vector can be produced in sufficient quantity for intended phase I/II studies. This prototype vector (patents P6021400EP and P6021400US) meets the rigorous regulations of the European Medicines Agency administration and has received Orphan Drug Designation for its use in preclinical and clinical studies for patients with recurrent porphyria attacks and/or at risk of developing irreversible nerve damage.
Materials and Methods
Plasmids and production of rAAV. All rAAV plasmids contain an expression cassette flanked by two inverted terminal repeats from the AAV2. The expression cassette of rAAV2 serotype 8 (rAAV2/8) contains the liver-specific human α-1-antitrypsin promoter with regulatory sequences from the human albumin enhancer (EalbAAT),12 the human cDNA encoding the housekeeping PBGD enzyme (GenBank accession no. X04808) or Luciferase reporter gene (GenBank accession no. M15077), and the bovine growth hormone polyadenylation sequence [bGH poly(A)] (bases 2,326–2,533: GenBank accession no. M57764). The woodchuck hepatitis virus post-transcriptional regulatory element (bases 1,021–1,750: GenBank accession no. J04514) was added to enhance transcription.26 rAAV2/8 vectors were produced by calcium phosphate–mediated transfection of HEK-293 cells as reported.27,28 The virus was harvested and purified by ion exchange column chromatography and iodixanol-gradient centrifugation followed by filtration and further concentration against phosphate-buffered saline–5% sucrose.
The therapeutic expression cassette of rAAV2 serotype 5 (rAAV2/5) contains the human cDNA of the housekeeping PBGD isoform under the control of the liver-specific EalbAAT promoter and the human PBGD polyadenylation sequence (bases 9,550–9,655: GenBank accession no. M95623). We developed a new sequence of the human cDNA sequence by (i) introducing a Kozak sequence to increase translational initiation, (ii) increasing the GC content to prolong RNA half-life, and (iii) changing rarely used codons with those that are used with high frequency in humans. That codon choice was related to the transfer RNA abundance in human cells to increase mammalian expression.29 Finally, two stop codons were added to ensure termination. Amino acid sequence of the human PBGD codon-optimized cDNA (cohPBGD) is identical to that obtained from the human housekeeping PBGD isoform. rAAV2/5 vectors were generated in Sf9 insect cells30 using the Baculovirus Expression Vector System (Protein Sciences, Meriden, CT) in a wave bioreactor. Purification was performed using AVB Sepharose high-performance affinity medium (GE Healthcare, Piscataway, NJ). Eluate was diafiltrated with 5 eluate volumes of phosphate-buffered saline–5% sucrose.
rAAV titers, in terms of gc/ml, were determined by real-time quantitative polymerase chain reaction TaqMan (Applied Biosystems, Foster City, CA) analysis using primers: pr300fw: 5′-CCCTGTTTGCTCCTCCGATAA-3′ pr301rv: 5′-GTCCGTATTTAAGCAGTGGATCCA-3′ amplifying a 95-bp fragment from the EalbAAT promoter region. Composition and purity of viral production was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Animal model. AIP mice are compound heterozygotes of two different disruptions of the PBGD gene:17 T1 strain [C57BL/6-pbgdtm1(neo)Uam and T2 strain (C57BL/6-pbgdtm2(neo)Uam]. Transgenic strains were kindly provided by Urs A. Meyer (The University of Basel). Experimental protocols were approved by the Ethics Committee of the University of Navarra (CEEA022-06), according to European Council Guidelines.
Safety and therapeutic efficacy of rAAV2/8-hPBGD administration. The therapeutic efficacy of rAAV2/8-mediated liver gene transfer was evaluated in adult AIP mice (3–4 months of age). Five males and five females were injected via the tail vein with 5 × 1012 gc/kg of rAAV2/8-hPBGD or rAAV2/8-luciferase control vector. Two additional groups of five AIP male mice were injected with 5 × 1011 or 5 × 1010 gc/kg of AAV2/8-hPBGD. Extra groups of wild-type and AIP mice were also included. Previously, the ability of rAAV to induce an acute inflammatory response and/or an acute attack was excluded in AIP male mice injected with 5 × 1012 gc/kg of rAAV2/8. To biochemically imitate a human porphyria attack, AIP mice were injected intraperitoneally with increasing doses of phenobarbital (75, 80, 85, and 90 mg/kg body weight) for four consecutive days. At 15, 28, and 90 days after rAAV injection, motor disturbance and porphyrin precursor excretion were determined in mice (Supplementary Materials and Methods and ref. 12) before and after the acute attack. Upon killing, PBGD expression was analyzed by enzymatic activity, immunoblot, quantitative PCR, and immunohistochemistry (Supplementary Materials and Methods). The number and caliber of axons in the sciatic nerve were measured in light micrographs of semiplastic sections (Supplementary Materials and Methods). The protective effect of rAAV2/8-hPBGD against motor dysfunction regarding sciatic nerve conduction velocity was assayed before and after seven consecutive acute attacks in AIP male mice. Phenobarbital protocol was administered biweekly. Six-month-old AIP mice were injected with 5 × 1011 gc/kg of rAAV2/8-hPBGD and nerve conduction assays were performed when animals reached the age of 14 months. As negative controls, cohorts of young AIP mice (5–6 months of age) and old (11–14 months of age) wild-type and AIP animals were also included. The role of the porphyrin precursors in nerve conduction dysfunction was assayed in 1-year-old wild-type and AIP mice by the i.v. administration of ALA (3 mmol/kg/day for 4 consecutive days). Nerve conduction velocity was measured 2 hours after the last ALA injection. As a positive control for an acute attack, mice received increasing doses of phenobarbital on 4 consecutive days, starting 1 week after administration of ALA. As a negative control, the same animals received phosphate-buffered saline administration (150 µl/day, IV) on the same schedule as phenobarbital-treated mice, 1 week before ALA administration.
Therapeutic efficacy of rAAV2/5-hPBGD in AIP mice. The therapeutic efficacy of 5 × 1012gc/kg i.v. of rAAV2/5-hPBGD was compared with the same dose of rAAV2/8-hPBGD in male and female AIP mice (3 to 4 months of age). Control AIP animals received the same dose of rAAV2/5 vector carrying the luciferase gene. An additional group of male AIP mice was injected with rAAV2/5-cohPBGD to evaluate every month the efficacy of codon-optimized hPBGD cDNA sequence to express the human PBGD protein. At 15, 28, and 90 days after the rAAV administration, an acute attack was induced in these animals with phenobarbital. Mice were killed 3 months after rAAV vector administration to analyze hepatic PBGD content. Finally, we checked rAAV2/5-cohPBGD efficiency to transduce the liver under conditions of cellular stress during acute attack induced by phenobarbital in four wild-type and five AIP male mice. As a negative control for the acute porphyric attack, four wild-type and five AIP male animals not injected with phenobarbital were administered with the same dose of vector (5 × 1012gc/kg, i.v.). Mice were killed 1 month after the viral administration.
SUPPLEMENTARY MATERIAL Materials and Methods.
Acknowledgments
We are grateful to Juan Percaz and Elena Ciordia for animal care and vivarium management. We also thank Laura Guembe (CIMA, Pamplona) for help in preparing and staining the tissue sections, and Carlos Ortiz de Solorzano, Miguel Galarraga, and José Antonio Ayala (Morphology and Imaging Unit, CIMA, Pamplona) for help with the axon image processing. Jesus Javier Sola Gallego (University of Navarra, Pamplona), Ignacio Melero, and Rubén Hernández-Alcoceba (CIMA, Pamplona) are acknowledged for scientific discussion and support. This work was supported in part by grants from UTE project of Centro de Investigación Médica Aplicada, University of Navarra, Spanish Fondo de Investigación Sanitaria (PI061475 and PS09/02639) and Spanish Fundación Mutua Madrileña de Investigación Médica.
Supplementary Material
REFERENCES
- Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142:439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- Kauppinen R. Porphyrias. Lancet. 2005;365:241–252. doi: 10.1016/S0140-6736(05)17744-7. [DOI] [PubMed] [Google Scholar]
- Anderson K, Sassa S, Bishop D, Desnick R.2001Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyriasIn: Scriver, CR, Beaudet, AL, Sly, WS, and Valle, E (eds.). The Metabolic and Molecular Bases of Inherited Disease, 8th edn., vol. 1. McGraw Hill: New York. pp. 2991–3062.
- Innala E, Bäckström T, Bixo M., and, Andersson C. Evaluation of gonadotropin-releasing hormone agonist treatment for prevention of menstrual-related attacks in acute porphyria. Acta Obstet Gynecol Scand. 2010;89:95–100. doi: 10.3109/00016340903390729. [DOI] [PubMed] [Google Scholar]
- Seth AK, Badminton MN, Mirza D, Russell S., and, Elias E. Liver transplantation for porphyria: who, when, and how. Liver Transpl. 2007;13:1219–1227. doi: 10.1002/lt.21261. [DOI] [PubMed] [Google Scholar]
- Pischik E., and, Kauppinen R. Neurological manifestations of acute intermittent porphyria. Cell Mol Biol (Noisy-le-grand) 2009;55:72–83. [PubMed] [Google Scholar]
- Meyer UA, Schuurmans MM., and, Lindberg RL. Acute porphyrias: pathogenesis of neurological manifestations. Semin Liver Dis. 1998;18:43–52. doi: 10.1055/s-2007-1007139. [DOI] [PubMed] [Google Scholar]
- Meyer RP, Lindberg RL, Hoffmann F., and, Meyer UA. Cytosolic persistence of mouse brain CYP1A1 in chronic heme deficiency. Biol Chem. 2005;386:1157–1164. doi: 10.1515/BC.2005.132. [DOI] [PubMed] [Google Scholar]
- Solis C, Martinez-Bermejo A, Naidich TP, Kaufmann WE, Astrin KH, Bishop DF, et al. Acute intermittent porphyria: studies of the severe homozygous dominant disease provides insights into the neurologic attacks in acute porphyrias. Arch Neurol. 2004;61:1764–1770. doi: 10.1001/archneur.61.11.1764. [DOI] [PubMed] [Google Scholar]
- Soonawalla ZF, Orug T, Badminton MN, Elder GH, Rhodes JM, Bramhall SR, et al. Liver transplantation as a cure for acute intermittent porphyria. Lancet. 2004;363:705–706. doi: 10.1016/S0140-6736(04)15646-8. [DOI] [PubMed] [Google Scholar]
- Johansson A, Nowak G, Möller C, Blomberg P., and, Harper P. Adenoviral-mediated expression of porphobilinogen deaminase in liver restores the metabolic defect in a mouse model of acute intermittent porphyria. Mol Ther. 2004;10:337–343. doi: 10.1016/j.ymthe.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Unzu C, Sampedro A, Mauleón I, Vanrell L, Dubrot J, de Salamanca RE, et al. Porphobilinogen deaminase over-expression in hepatocytes, but not in erythrocytes, prevents accumulation of toxic porphyrin precursors in a mouse model of acute intermittent porphyria. J Hepatol. 2010;52:417–424. doi: 10.1016/j.jhep.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Bishop DF, Fowkes M, Cheng SH, Gan L., and, Desnick RJ. AAV8-mediated gene therapy prevents induced biochemical attacks of acute intermittent porphyria and improves neuromotor function. Mol Ther. 2010;18:17–22. doi: 10.1038/mt.2009.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pañeda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ, et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther. 2009;20:908–917. doi: 10.1089/hum.2009.031. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang H, Bell P, McCarter RJ, He J, Calcedo R, et al. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg RL, Martini R, Baumgartner M, Erne B, Borg J, Zielasek J, et al. Motor neuropathy in porphobilinogen deaminase-deficient mice imitates the peripheral neuropathy of human acute porphyria. J Clin Invest. 1999;103:1127–1134. doi: 10.1172/JCI5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg RL, Porcher C, Grandchamp B, Ledermann B, Bürki K, Brandner S, et al. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat Genet. 1996;12:195–199. doi: 10.1038/ng0296-195. [DOI] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Fatokun AA, Stone TW., and, Smith RA. Oxidative stress in neurodegeneration and available means of protection. Front Biosci. 2008;13:3288–3311. doi: 10.2741/2926. [DOI] [PubMed] [Google Scholar]
- Kimura J.ed2001Principles and variations of nerve conduction studiesIn: Electrodiagnosis in diseases of nerve and muscle: principles and practice, 3rd edn. Oxford University Press: New York. pp. 91–129.
- Felitsyn N, McLeod C, Shroads AL, Stacpoole PW., and, Notterpek L. The heme precursor delta-aminolevulinate blocks peripheral myelin formation. J Neurochem. 2008;106:2068–2079. doi: 10.1111/j.1471-4159.2008.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Krishnan AV, Lee MJ, Zagami AS, You HL, Yang CC, et al. Nerve function and dysfunction in acute intermittent porphyria. Brain. 2008;131:2510–2519. doi: 10.1093/brain/awn152. [DOI] [PubMed] [Google Scholar]
- De Matteis F, Zetterlund P., and, Wetterberg L. Brain 5-aminolaevulinate synthase. Developmental aspects and evidence for regulatory role. Biochem J. 1981;196:811–817. doi: 10.1042/bj1960811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraondo P, Crettaz J, Ochoa L, Pañeda A, Prieto J, Trocóniz IF, et al. Intrahepatic injection of recombinant adeno-associated virus serotype 2 overcomes gender-related differences in liver transduction. Hum Gene Ther. 2006;17:601–610. doi: 10.1089/hum.2006.17.601. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Ng CY, Zhou J, Spence Y., and, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- Donello JE, Loeb JE., and, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens WT, ter Brake O, Dijkhuizen PA, Sonnemans MA, Grimm D, Kleinschmidt JA, et al. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum Gene Ther. 1999;10:1885–1891. doi: 10.1089/10430349950017563. [DOI] [PubMed] [Google Scholar]
- Gao GP, Lu F, Sanmiguel JC, Tran PT, Abbas Z, Lynd KS, et al. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol Ther. 2002;5:644–649. doi: 10.1006/mthe.2001.0591. [DOI] [PubMed] [Google Scholar]
- Kotlar D., and, Lavner Y. The action of selection on codon bias in the human genome is related to frequency, complexity, and chronology of amino acids. BMC Genomics. 2006;7:67. doi: 10.1186/1471-2164-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe M, Ding C., and, Kotin RM. Insect cells as a factory to produce adeno associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.