Abstract
Previous data report that fibroblast growth factor-2 (FGF-2)-derived peptide FREG potently inhibits FGF-2-dependent angiogenesis in vitro and in vivo. Here, we show that FREG inhibits up to 70% in vitro growth and invasion/migration of smooth muscle and melanoma cells. Such inhibition is mediated by platelet-derived growth factor-receptor-α (PDGF-Rα); in fact, proliferation and migration were restored upon PDGF-Rα neutralization. Further experiments demonstrated that FREG interacts with PDGF-Rα both in vitro and in vivo and stimulates its phosphorylation. We have previously shown that overexpressing PDGF-Rα strongly inhibits melanoma growth in vivo; we, therefore, hypothesized that PDGF-Rα agonists may represent a novel tool to inhibit melanoma growth in vivo. To support this hypothesis, FREG was inoculated intravenously (i.v.) in a mouse melanoma model and markedly inhibited pulmonary metastases formation. Immunohistochemical analyses showed less proliferation, less angiogenesis, and more apoptosis in metastasized lungs upon FREG treatment, as compared to untreated controls. Finally, in preliminary acute toxicity studies, FREG showed no toxicity signs in healthy animals, and neither microscopic nor macroscopic toxicity at the liver, kidney, and lungs level. Altogether, these data indicate that FREG systemic treatment strongly inhibits melanoma metastases development and indicate for the first time that agonists of PDGF-Rα may control melanoma both in vitro and in vivo.
Introduction
Melanoma incidence is increasing worldwide.1 Human malignant melanoma is highly metastatic and is resistant to conventional chemo- and radiotherapies; prevention and early diagnosis are currently the most effective strategies against this tumor, as melanoma risk correlates with a complex mix of factors including skin pigmentation phenotype, sunlight exposure, and genetic predisposition. Although primary melanoma is effectively treated in most cases by surgical excision, prognosis becomes unfavorable in the metastatic phase, lowering survival to 20–40% at 10 years, in patients with clinically apparent regional lymph node metastases.1,2 Several factors control melanoma growth, including angiogenic factors such as fibroblast growth factor (FGF-2) and platelet-derived growth factors (PDGFs). PDGF family comprises dimeric growth factors expressed as five different isoforms, namely PDGF-AA, BB, CC, DD, and AB. PDGFs are known to regulate several functions including angiogenesis,3,4 tissue development and remodeling,5 stem cells recruitment,6 mesenchymal cells proliferation, and vascular wall homeostasis.7,8,9 PDGFs bind PDGF receptor α–α, β–β, and α–β with distinct specificity; namely PDGF-BB binds PDGF-R α–α, β–β, and α–β PDGF-DD interacts with PDGF-R β–β and α–β PDGF-CC and PDGF-AB bind PDGF-Rα–α and α–β more interestingly for this study, PDGF-AA has a pure PDGF-Rα-mediated effect and interacts with the receptor α–α only.5 FGF-2 belongs to a large family of growth factors; it controls proliferation/differentiation of different cell types and tissues10,11,12 and is involved in mediating tumor growth,13,14,15,16 Parkinson's and Alzheimer's diseases,17,18 and vascular diseases such as atherosclerosis and restenosis.19,20 Several evidence currently indicate growth factors and their receptors as specific targets for the novel approaches to the cancer treatment, with particular emphasis on PDGF and FGF family.21,22
We have previously shown that PDGF-BB and FGF-2 reciprocally inhibit their in vitro and in vivo actions, most likely via their high-affinity direct interaction, which may alter the binding to the corresponding receptors.23,24,25 In the presence of the respective ligands (PDGF-BB and FGF-2), PDGF-receptor-α and FGF-receptor-1 interact on endothelial and melanoma cells,26 leading to a marked antiproliferation effect. Such data strongly support the hypothesis that heterodimerization of ligands may drive heterodimerization of the corresponding receptors, giving rise to an endogenous mechanism controlling cell proliferation and angiogenesis.
Several observations presented by us and others indicate that PDGF-AA and its unique receptor PDGF-Rα may exert antiproliferation and antimigration effects on specific cell types such as smooth muscle and endothelial cells.23,24,27,28,29 More recently, we demonstrated that overexpressing PDGF-Rα markedly reduced melanoma cell proliferation both in vitro and in vivo, accompanied by a strong angiogenesis inhibition,4 suggesting for the fist time that low expression levels of PDGF-Rα may mediate melanoma progression, while high levels of PDGF-Rα induce a significant antimelanoma growth effect. According to such observation, inhibiting FGF-2 and activating PDGF-Rα may exert potent controlling effect on endothelial and melanoma growth.
An FGF-2-derived peptide named FREG has been previously reported as a potent FGF-2 inhibitor, acting as a decoy of the whole FGF-2 molecule.30 Such peptide was shown to bind FGF-2 and to interfere with the whole FGF-2 activation process, namely FGF-2 dimerization, heparin binding, receptor binding, receptor phosphorylation, and FGF-2 cell internalization, leading to a strong inhibition of all tested in vitro and in vivo actions of FGF-2. FREG peptide has been shown to mimic most of the FGF-2 actions; we thus hypothesized that such peptide may activate a PDGF-Rα-mediated action similar to the parent whole FGF-2.24 In this study, FREG is shown to strongly inhibit melanoma growth, both in vitro and in vivo, with a mechanism involving PDGF-Rα.
Results
FREG inhibits cell proliferation and migration in a PDGF-Rα-mediated way
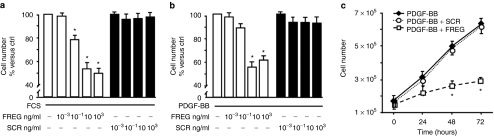
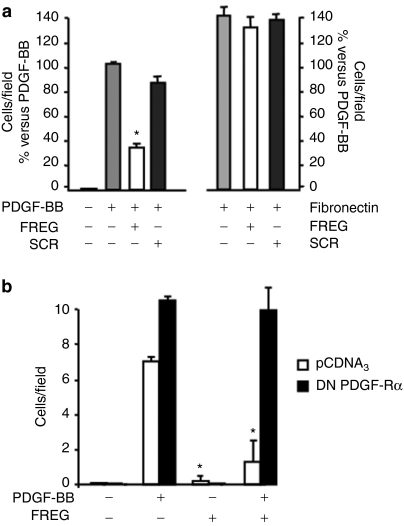
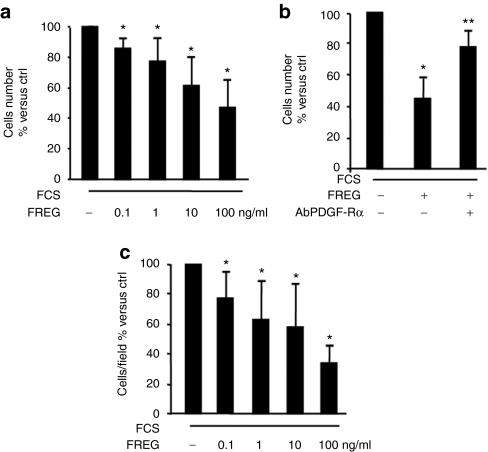
Previous evidence published from our laboratory showed that FGF-2 and FREG inhibit endothelial cells proliferation via a PDGF-Rα-mediated mechanism.24,30 Such data agree with the observation that PDGF-AA, a pure PDGF-Rα agonist, inhibits PDGF-BB-induced migration in smooth muscle cells.23,28 In this study, FREG peptide markedly inhibited rat aortic smooth muscle cell (RASMC) proliferation induced by PDGF and by fetal calf serum (FCS) in a dose- and time-dependent way (Figure 1a–c), indicating that FREG has strong inhibitory effects, similar to the inhibitory effects shown under similar experimental conditions by the parent molecule FGF-2 (ref. 23) and similar to PDGF-AA.4 The scrambled peptide (SCR) used as specificity control was ineffective in all cases. FREG, similar to PDGF-AA,23 also reduced PDGF-BB-induced RASMC migration, while it did not inhibit fibronectin-induced migration (Figure 2a). Such effect was completely abolished in RASMC overexpressing a dominant-negative form of PDGF-Rα, indicating that this inhibition is mediated by PDGF-Rα (Figure 2b). In order to evaluate the biological relevance of these results, a different cellular system was then investigated; the following experiments were carried out in a melanoma in vitro and in vivo setting, in order to investigate the role FREG may play in a pathologically relevant system. FREG strongly inhibited FCS-induced proliferation and invasion of human melanoma cells in a dose-dependent way (Figure 3a,c, respectively); inactivating the PDGF-Rα by a neutralizing antibody (0.6 µg/ml) significantly abolished the antiproliferation action (Figure 3b), further confirming that FREG inhibitory action is mediated by PDGF-Rα. Altogether, these data indicate that FREG inhibits PDGF-BB and FCS mitogenic/chemotactic effects in a PDGF-Rα-mediated way, in different cell types.
Figure 1.
FREG inhibits PDGF- induced cell proliferation. (a) FCS-induced RASMC proliferation (48 hours) was examined in the presence or in the absence of increasing doses of FREG or the negative control scrambled peptide (SCR). FREG reduced cell proliferation in a dose-dependent way reaching the plateau at 10 ng/ml, while the SCR was ineffective at all tested doses (*P < 0.05 versus FCS). (b) FREG reduced in a dose-dependent way PDGF-BB (10 ng/ml)-induced cell proliferation, reaching the plateau at 10 ng/ml. The SCR was ineffective at all tested doses (*P < 0.05 versus PDGF-BB). (c) FREG (10 ng/ml) reduced PDGF-BB mitogenic activity on RASMC in a time-dependent way, while SCR (10 ng/ml) was ineffective (*P < 0.05 versus PDGF-BB). Data are expressed as mean ± SD of three experiments. ctrl, control; PDGF, platelet-derived growth factor.
Figure 2.
FREG inhibits PDGF-induced cell migration in a PDGF-Rα-dependent way. (a) RASMC migration induced by PDGF-BB (10 ng/ml) or fibronectin (50 µg/ml) was examined in the presence of FREG or SCR (10 ng/ml) after 5 hours exposure at 37 °C. PDGF-BB chemotactic activity was markedly inhibited by FREG, while SCR was ineffective (*P < 0.05 versus PDGF-BB), while neither FREG nor SCR affected fibronectin chemoattractant activity. (b) FREG effect was investigated in RASMC transfected with the empty vector (pCDNA3) or with DN-PDGF-Rα. FREG showed strong antichemotactic action in cells transfected with pCDNA3, while in cells transfected with DN-PDGF-Rα this effect was abolished (*P < 0.05 versus ctrl). Data are expressed as mean ± SD of three experiments. PDGF-Rα, platelet-derived growth factor-receptor-α.
Figure 3.
FREG strongly inhibits melanoma growth in a PDGF-Rα-dependent way. (a) SK-MEL-110 proliferation induced by FCS 10% (48 hours) was tested in the presence of increasing FREG concentrations. FREG inhibited melanoma cell growth in a concentration-dependent manner (*P < 0.005 versus FCS). (b) In the presence of the antibody neutralizing PDGF-Rα (0.6 µg/ml), the antimitogenic action of FREG (100 ng/ml) was significantly reverted (*P < 0.05 versus FCS; **P < 0.05 versus FCS in the presence of FREG). (c) FCS-induced melanoma cell invasion through collagen IV (10 µg/ml) was inhibited by FREG in a dose-dependent way (*P < 0.05 versus FCS). Data are expressed as mean ± SD of three independent experiments. ctrl, control; FCS, fetal calf serum; PDGF-Rα, platelet-derived growth factor-receptor-α.
FREG interacts with PDGF-Rα
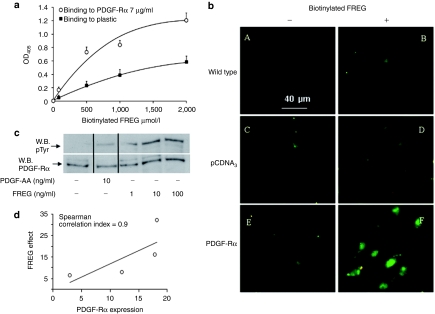
To further investigate the FREG's mechanism of action, we investigated whether FREG directly interacts with PDGF-Rα both in vitro and in live melanoma cells. Human recombinant PDGF-Rα was immobilized onto plastic and biotin-labeled FREG was incubated at increasing doses. Under such conditions, a direct saturable and concentration-dependent interaction was observed (Figure 4a). This finding suggested that FREG may directly bind PDGF-Rα to confirm such finding in an experimental set-up closer to live conditions, additional experiments were carried out using melanoma cells overexpressing PDGF-Rα and incubated with biotin-labeled FREG. Detection was achieved with Fluorescein Avidin D by a fluorescence microscopy. Figure 4b reports FREG interaction with untransfected cells (wild type), with pCDNA3 empty vector- or PDGF-Rα-transfected cells. FREG binding is similar to untransfected SK-MEL-110 and to the control pCDNA3-transfected cells (Figure 4b panels B and D, respectively), while binding is strongly increased in PDGF-Rα-transfected SK-MEL-110 (Figure 4b, panel F). Such data indicate that FREG binds in vitro PDGF-Rα immobilized onto plastic and strongly increases its binding to cells overexpressing PDGF-Rα, as compared to control cells.
Figure 4.
FREG interacts with PDGF-Rα. (a) FREG binding to plastic-immobilized PDGF-Rα (7 µg/ml) was measured in solid-phase assays. Biotinylated FREG (form 0 to 2 mmol/l) was incubated for 4 hours onto immobilized-PDGF-Rα. Unbound material was washed away and a colorimetric assay was performed. Biotinylated FREG bound PDGF-Rα in a saturable and concentration-dependent manner. (b) SK-MEL-110 untransfected or overexpressing PDGF-Rα were seeded on glass coverslips and treated with biotinylated FREG (100 ng/ml) for 10 minutes at 4 °C and stained with Fluorescein Avidin D. Bar = 40 µm. Biotinylated FREG clearly bound to PDGF-Rα-transfected cells more than pCDNA3-transfected cells or other controls. (c) Endogenous PDGF-Rα phosphorylation was evaluated in SK-MEL-110 treated for 5 minutes with PDGF-AA (10 ng/ml) or with increasing dose of FREG (1, 10, and 100 ng/ml). PDGF-AA, used as positive control, induced phosphorylation of its selective receptor; FREG induced PDGF-Rα phosphorylation with a rate comparable to PDGF-AA. (d) Correlation between peptide effect and PDGF-Rα expression in four melanoma cell lines (namely: mouse B16F10, human SK-MEL-110, human SK-MEL-28, human MEWO). FREG was used at 100 ng/ml and its inhibitory effect on cell proliferation was correlated to PDGF-Rα expression measured by western blot analysis. Spearman correlation index was computed with PRISM 5 software for Windows. PDGF-Rα, platelet-derived growth factor-receptor-α.
FREG induces PDGF-Rα phosphorylation
The above reported results strongly support the hypothesis that FREG peptide may act as a PDGF-Rα agonist. Endogenous PDGF-Rα phosphorylation was then monitored in the presence of FREG alone or in the presence of PDGF-AA, used as specific PDGF-Rα ligand.5 Cells were exposed to PDGF-AA (10 ng/ml) or to FREG (1, 10, and 100 ng/ml) for 5 minutes. Under these conditions, FREG induced endogenous PDGF-Rα phosphorylation with a rate comparable to PDGF-AA, i.e., the physiologic ligand of PDGF-Rα (Figure 4c).
Altogether, the above reported data indicate FREG as a strong inhibitor of melanoma cell proliferation and invasion, with a mechanism involving (i) PDGF-Rα binding, (ii) PDGF-Rα phosphorylation, and (iii) the presence of functional PDGF-Rα. Preliminary data collected from four melanoma cell lines (namely mouse B16F10, human SK-MEL-110, human SK-MEL-28, and human MEWO) suggest that FREG increases its antimitogenic effect as a function of PDGF-Rα expression (Figure 4d). Such data agree with previous evidence showing that PDGF-Rα overexpression strongly inhibits melanoma growth in vitro and in vivo and, likely as a consequence of that, melanoma progression may select cells with reduced expression of PDGF-Rα in fact PDGF-Rα has been found to be significantly downregulated in human melanoma biopsies.4 Such evidence suggest that PDGF-Rα agonists and FREG may represent novel approaches to the melanoma treatment. We then tested FREG in an in vivo model of murine melanoma growth.
In vivo lung metastasis assay
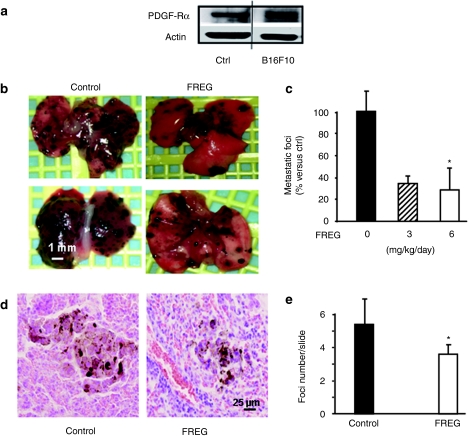
Data presented above indicate that FREG inhibits in vitro human melanoma cell proliferation via PDGF-Rα. Therefore FREG in vivo antimelanoma activity was tested in a murine model; mouse B16F10 melanoma cells were shown to express in vitro PDGF-Rα by either western blotting analysis (Figure 5a) or real time-PCR (RT-PCR; Supplementary Figure S1) as well as in vivo (Figure 6d); therefore B16F10 cells were injected intravenously (i.v.) in 20 C57BL/6 mice to develop melanoma lung metastases. Six days after cell inoculation, i.v. FREG inoculation was initiated in 10 animals according to the schedule reported in Materials and Methods section, while 10 mice were sham treated. At 15 days after cell inoculation (corresponding to 9 days after the initiation of FREG treatment) mice were killed and lung metastases were counted. FREG i.v. treatment reduced by >50% the number of macroscopic lung metastases at 3 as well as at 6 mg/kg/day dose (Figure 5b,c). Analysis of variance, followed by Dunnett's multiple comparison test as post hoc analysis, indicated that the effect induced by 6 mg/kg/day dose is significant (P < 0.05) and the post hoc test for linear trend indicated that response is significantly related to the dose (P < 0.02). Microscopic histological analyses indicated that FREG treatment significantly reduced the number of microscopic foci by 33% (P < 0.02), while size was reduced by ~50% (Figure 5d,e).
Figure 5.
FREG intravenous (i.v.) treatment inhibited melanoma lung metastases in a murine melanoma model. (a) In vitro expression of PDGF-Rα was investigated in growing B16F10 mouse melanoma cell line, by western blotting analysis. Human HUVEC cells were used as positive control, according to previously reported data.26 (b) B16F10 melanoma cells (2 × 105/200 µl PBS) were injected i.v. into C57BL/6 mice tail vein at day −6. Animals were treated i.v. with 50 µl of FREG (3 or 6 mg/kg/day) on days 0, 2, 4, 7, and 8. Macroscopic lung metastases are indicated in representative lungs. Bar = 1 mm. (c) Quantification of macroscopic lung metastases indicates a significant reduction upon FREG treatment. Asterisk (*) indicates statistical significance (P < 0.05 versus ctrl) according to one-way analysis of variance test followed by Dunnett's multiple comparison test. Linear trend post hoc analysis indicates a significant dose-related effect (P < 0.02). (d) Microscopic metastatic foci upon histological analysis. Representative image are reported, indicating representative foci-size. Bar = 25 µm. (e) Quantification of number of microscopic foci per slide indicates a significant reduction upon FREG treatment (P < 0.05 versus ctrl). ctrl, control; PDGF-Rα, platelet-derived growth factor-receptor-α.
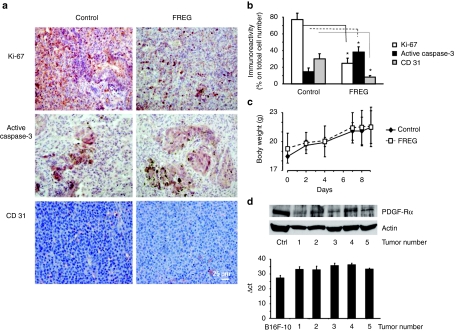
Figure 6.
Immunohistochemical analyses were carried out on mice injected intravenously with B16F10 melanoma cells and treated with FREG (6 mg/kg) at days 0, 2, 4, 7, and 8. (a) The routine hematoxylin–eosin staining and Ki-67, active caspase-3, and CD 31 labeling were performed on paraffin-embedded lungs. Bar = 25 µm. (b) Quantification of percentage of immunoreactivity expression indicates a significant reduction of cell proliferation (Ki-67 staining), a marked induction of apoptosis (caspase-3 activation), and a significant decrease of angiogenesis (CD 31 staining) upon FREG treatment (*P < 0.05 FREG versus control). (c) Total body weight was recorded in healthy C57BL/6 mice at days 0, 2, 4, 7, 8, and 9 in mice treated with 6 mg/kg/day FREG intravenously. No significant differences versus control were observed at any day indicating no gross acute toxicity upon FREG treatment. (d) PDGF-Rα expression was detected by western blotting (top) and by quantitative real-time PCR analysis (bottom) in 5 mouse primary melanomas (obtained by s.c. injection of B16F10 melanoma cells in C57BL6 male mice). The experiments were performed in triplicate; data are reported as average and SD. PDGF-Rα, platelet-derived growth factor-receptor-α.
Additionally, immunohistochemical analysis revealing Ki-67, active caspase-3, and CD 31 expression levels, chosen as proliferation, apoptosis, and angiogenesis markers, respectively, showed that proliferation is strongly reduced in the lung of FREG-treated animals versus control (25% of positive cells versus 77%). Furthermore, FREG treatment increased active caspase-3 expression versus control (38% positive cells versus 15%), indicating apoptosis induction. Finally, FREG significantly reduced angiogenesis in treated lungs versus control (8% positive cells versus 30% immunoreactivity expression of CD 31 marker; Figures 6a,b).
Preliminary toxicity studies
The in vitro and in vivo data reported above strongly support the hypothesis that FREG may exert antimelanoma activity in vivo. In order to exclude any aspecific toxic effect responsible for the observed effects, preliminary acute toxicity experiments were carried out in vivo; FREG was administered in healthy C57BL/6 mice at 6 mg/kg/day (treatment and injection schedule were identical to the highest dose used in the efficacy studies). Total body weight of each mouse was recorded at days 0, 2, 4, 7, 8, and 9. No significant differences versus controls were observed at any day (Figure 6c). Upon FREG treatment, animals showed good general conditions in all cases. No treatment-related changes such as chronic inflammation, extramedullar hematopoiesis, increased mitosis in liver, chronic inflammation and tubular basophilia in kidneys, and hemorrhage in lungs were observed microscopically in treated animals as compared to controls, according to the analysis of a professional pathologist evaluating slides in blind.
Further, data collected by hematological and clinical chemistry analysis show no sign of toxicity (e.g. no significant difference was found in red and white blood cell content, platelets, hemoglobin, total protein content, glucose, urea, and creatinine levels; Supplementary Tables S1 and S2). According to these data, we concluded that the observed antimetastatic effect of FREG is unlikely related to toxic effects.
Discussion
In this study, we present data demonstrating a strong antimelanoma effect of FREG, an FGF-2-derived peptide. FGF-2 is one of the most potent mitogenic and angiogenic factors, while its truncated forms exert antichemotactic and antiangiogenic activity.31 FGF family members are known to undergo a proteolytical maturation as a physiological mechanism to modulate their activity11,32,33 and FGF-derived fragments are reported to play specific biological activities; at least one FGF-derived fragment has been recently identified as cancer-related antigen34 and we have shown that FGF-2 loses activity and releases fragments upon thrombin-dependent proteolytic digestion.35 We have previously reported that FREG, designed from the region predicted to be at the interface of the FGF-2/FGF-2 homodimer, strongly affects in vitro and in vivo FGF-2 biological functions by inhibiting FGF-2 dimerization process and acting as a molecular decoy.30 More recently, we have shown that FGF-2 interacts with PDGF25 and that FGF-R1 heterodimerizes with PDGF-Rα26 supporting the hypothesis that FGF-2 and PDGF, as well as their receptors, may form a functional network structurally and functionally related. In agreement with this scenario, we hypothesized that FREG activity may be related to PDGF-Rα. This initial hypothesis was confirmed by the results depicted in Figures 2b and 3b, showing that indeed FREG antimitogenic and antichemotactic activity is abrogated when PDGF-Rα is neutralized, either by a dominant-negative molecule or by a neutralizing antibody, respectively. The mechanism mediating this action, at least in part, is the direct FREG/PDGF-Rα interaction and was demonstrated by different experimental approaches, depicted in Figure 4. The ability of FREG to induce PDGF-Rα phosphorylation, and therefore to activate its downstream pathway, definitely proved that this peptide inhibits smooth muscle cells and melanoma cells proliferation/migration with a mechanism mediated, at least in part, by PDGF-Rα.
In vivo experiments carried out in a murine metastatic melanoma model demonstrated that systemic FREG inoculation is able to strongly reduce number and size of lung metastases as compared to untreated melanoma. Doses used were chosen according to previous data indicating that low molecular weight peptides with short circulation time are quickly metabolized in vivo, requiring high doses and repeated inoculations, to obtain acceptable antimetastatic effects in vivo.20,36,37 Upon FREG treatment immunohistochemical analyses showed a strong inhibition of proliferation, measured by Ki-67 staining of lung sections, and a marked antiangiogenic effect, by CD 31 staining in lung sections, as compared to untreated melanoma, suggesting that FREG antimetastatic action in vivo may derive from the combined antiangiogenic effect targeting endothelial cells24 and the antimitogenic effect targeting melanoma cells. Tissue starvation deriving from reduced angiogenesis may also explain, at least in part, the observed strong increase of active caspase-3 staining, marker of increased apoptosis.
Finally, it should be highlighted that no toxicity signs were evidenced in healthy animals treated with FREG at the same dose and inoculation schedule used in the melanoma model, suggesting that FREG has no aspecific toxic action.
Although indicating for the first time that a synthetic PDGF-Rα agonist may exert potent antimelanoma effects, data presented in this study agree with previous data published by our lab and by others indicating FGF-2 and PDGF-Rα as negative modulators of cell proliferation and angiogenesis, under particular experimental conditions.4,23,24,26 Interestingly PDGF-Rα has been shown to mediate inhibitory effects on vascular smooth muscle cell migration,23,27 while it can have opposing effects on the stromal tissues.38,39
In conclusion, this study demonstrates that PDGF-Rα may represent a relevant target for novel antimelanoma approaches and indicates FREG as potent PDGF-Rα agonist with significant in vitro and in vivo antimelanoma growth activity.
Materials and Methods
Peptides synthesis. FREG peptide has the sequence DPHIKLQLQAE, corresponding to region 112–122 of human FGF-2 (accession number P09038). (Previously, FREG was referred to as FREG-38-48 (ref. 30) according to accession number XP_055784, currently removed from NCBI protein database at http://www.ncbi.nlm.nih.gov/protein); scrambled FREG (KHIAQLDEPLQ, here referred to as SCR) was used as a sequence specificity control. Both peptides were custom synthesized by Polypeptide Laboratories (Strasbourg, France). Peptides' purity, assessed by reverse-phase high-performance liquid chromatography and mass spectrometry, was higher than 95% in all cases. Different batches of FREG and SCR were tested and gave similar results in all biological assays.
Cell isolation and cell cultures. Human metastatic melanoma cells (SK-MEL-110)4 were cultured at 37 °C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT) supplemented with 2 mmol/l -glutamine (Gibco, Invitrogen, Carlsbad, CA), 100 IU/ml penicillin–streptomycin (Gibco, Invitrogen, Carlsbad, CA), and 10% heat-inactivated FCS (Hyclone). Primary RASMCs were obtained as previously reported23 and grown in the same medium. Three different preparations were carried out and the purity, evaluated by α-actin immunostaining, was consistently higher than 95%. Proliferating RASMCs (passages 3–6) at 70% confluence were used in all assays.
Migration assays. Migration assays were carried out in modified Boyden chambers (Corning Life Sciences, Lowel, MA) as described.40 RASMC or SK-MEL-110 (2 × 105) were seeded in the upper portion of the Boyden apparatus, onto 8-µm pores polycarbonate filters (Corning Life Sciences) previously coated with porcine skin gelatin (5 mg/l; Sigma-Aldrich, St Louis, MO) or with murine collagen type IV (10 µg/ml; Becton Dickinson, Bedford, MA). Human recombinant factors used as chemoattractants were PDGF-BB (10 ng/ml; R&D Systems, Minneapolis, MN) or fibronectin (50 µg/ml; Gibco, Invitrogen, Carlsbad, CA) for RASMC, and FCS 10% for SK-MEL-110. FREG or SCR were dissolved in DMEM 0.1% bovine serum albumin (BSA; Sigma-Aldrich) and were dispensed in the upper portion of the Boyden chamber.
Assays were then carried out at 37 °C in 5% CO2 for 4 hours; filters were then removed, fixed with absolute ethanol, and stained with Giemsa (Merck, Darmstadt, Germany). Cells migrated across the filters were counted at ×400 magnification; 10 fields/filter were evaluated and the average number of cells/field was reported. All experiments were performed at least three times in duplicate.
Cell transfection with wild-type and dominant-negative PDGF receptor. Plasmid coding for wild-type PDGF-Rα, dominant-negative form of PDGF-Rα (DN-PDGF-Rα generous gifts of C.H. Heldin, Ludwig Institute for Cancer Research, Uppsala, Sweden) or pCDNA3 (Gibco, Invitrogen) empty vectors were transfected in RASMC or in SK-MEL-110 as previously reported.23 Cells were cotransfected with pEGFP-N1 (Clontech, Mountain View, CA) reporter vector (molar ratio 3:1). Transfection was carried out with Lipofectamine plus reagent (Gibco, Invitrogen, Carlsbad, CA) diluted in DMEM, for 3 hours at 37 °C in a 5% CO2 atmosphere. Medium was then replaced with DMEM–10% FCS and then cells were used for assays.
Proliferation assays. SK-MEL-110 proliferation was induced for 48 hours with FCS 10% and cells were treated with FREG in the presence or in the absence of neutralizing PDGF-Rα antibody (0.6 µg/ml; R&D Systems). PDGF-BB- or FCS-induced RASMC proliferation was assayed in time course or at 48 hours in dose-dependent experiments as described23 in the presence or in the absence of FREG or SCR. Cells were then harvested and counted with a hemacytometer. All experiments were carried out at least three times in duplicate.
PDGF-Rα phosphorylation and expression. SK-MEL-110 grown to 70% confluence were starved for 24 hours in serum-free DMEM. Five-minute exposure to PDGF-AA (10 ng/ml) or FREG (1, 10, and 100 ng/ml) was then carried out. Cells were then rinsed with ice-cold phosphate-buffered saline (PBS), scraped, and lysed for 5 minutes with 1% Triton X-100, 100 mmol/l NaCl, 10% glycerol, 20 mmol/l HEPES, pH 7.4, 5 mmol/l EDTA, 10 µmol/l Na3VO4, 10 mmol/l sodium fluoride, 2 mmol/l phenylmethylsulfonyl fluoride and proteases inhibitors cocktail (Sigma-Aldrich). Lysates were centrifuged at 14,000 r.p.m. for 10 minutes and 50 µg total proteins were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were electrotransferred to a nitrocellulose membrane and blocked with 0.1% Tris–PBS and 5% nonfat milk. Ponceau S staining (Sigma-Aldrich) was performed to verify the equal loading. Western blot analysis was carried out by probing the nitrocellulose membrane with the antiphosphotyrosine antibody (1 µg/ml, sc-7020; Santa Cruz Biotechnology, Alexa, CA) overnight and with anti-PDGF-Rα (1:200, sc-431; Santa Cruz Biotechnology), followed by washing and incubation with horseradish peroxidase–conjugated secondary antibody (Pierce Endogen, Rockford, IL). Bands were then revealed by ECL detection system (Amersham, Buckinghamshire, UK). All experiments were performed at least three times. For PDGF-Rα expression studies, western blot analysis of PDGF-Rα was carried out onto four melanoma cell lines (namely mouse B16F10, human SK-MEL-110, human SK-MEL-28, and human MEWO) and onto five mouse primary melanomas (excised 2 weeks after subcutaneous injection of B16F10 melanoma cells in C57BL6 male mice).
FREG–PDGF-Rα interaction analyzed by solid-phase assay. Biotinylated FREG interaction to recombinant PDGF-Rα was evaluated by solid-phase assay according to a procedure previously described26 with modifications. Briefly, microtiter plates (Corning Life Sciences) were coated with recombinant PDGF-Rα (0.7 µg; R&D Systems) diluted in AC7.5 buffer (50 mmol/l Tris–HCl, pH 7.5, 100 mmol/l KCl, 3 mmol/l MgCl2, 1 mmol/l CaCl2) for 4 hours at room temperature (RT). After blocking in 30 mg/ml BSA (300 µl/well) AC7.5 buffer overnight at 4 °C and washing three times with AC7.5T/BSA buffer (AC7.5 buffer containing 0.1% Tween-20 and 0.15 mg/ml BSA), wells were incubated for 4 hours at RT with increasing doses of biotinylated FREG. They were then washed four times with AC7.5T/BSA buffer, incubated for 1 hour at RT with 100 µl/well Vectastain ABC Reagent (Vector Laboratories, Burlingame, CA) and stained with the ELISA Amplification System (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Absorption at 495 nm (A495) was determined.
Fluorescence microscopy. Wild-type—SK-MEL-110, or pCDNA3 empty vector—transfected cells, or PDGF-Rα-transfected cells were amplified on glass coverslips and treated with biotinylated FREG (100 ng/ml), for 10 minutes at 4 °C. Cells were then washed three times with PBS and fixed with fresh 2% paraformaldehyde for 10 minutes at RT. They were then washed three times with PBS, incubated with PBS–BSA 2% for 30 minutes at RT. Binding of biotinylated FREG to the cells was analyzed by Fluorescein Avidin D staining (Vector Laboratories) for 1 hour at RT. The analysis was then carried out on a Zeiss Axioplan 2 microscope (bar = 40 µm).
Quantitative real-time PCR of PDGF-Rα mRNA expression. Total RNA (2 mg/sample) extracted from HUVEC cells, murine B16F10, and primary mouse fibroblasts was prepared using TRIzol (Invitrogen) according to the manufacturer's instructions. mRNAs levels were analyzed using the SYBR-GREEN qPCR method Qiagen. Quantitative RT-PCR was performed on complementary DNAs obtained following retrotranscription of mRNAs. Complementary DNAs were used as templates for Taqman qRT-PCR with ABI Assays-on Demand on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primers for murine and human PDGF-Rα were designed with the Primer Express software, version 2.0 (Applied Biosystems, Foster City, CA). Glyceraldehyde-3-phosphate dehydrogenase was amplified on the same plate for each sample for normalization purposes.
In vivo melanoma growth: lung metastasis assay. According to an accepted animal study protocol, murine B16F10 melanoma cells (2 × 105/200 µl PBS) were injected i.v. at day −6 into 12-week-old male C57BL/6 mice (10 mice/group) for experimental metastasis studies, according to a procedure previously described.41 Mice were treated with i.v. inoculations of 50 µl of FREG (two doses, 3 or 6 mg/kg/day) on days 0, 2, 4, 7, and 8. Mice were killed 9 days after starting FREG treatment and macroscopic lung metastases were counted at a surgical microscope (×20). The routine hematoxylin–eosin staining as well as Ki-67 (Abcam, Cambridge, MA), active caspase-3 (Abcam), and CD 31 (Serotec, Oxford, UK) labeling were performed on paraffin-embedded lungs.
In vivo toxicity studies. Preliminary acute toxicity studies were carried out on a total of 12 healthy animals, by injecting FREG i.v. at 6 mg/kg/day (treatment and injection schedule were identical to the highest dose used in the efficacy studies) in healthy C57BL/6 male mice, in a volume of 10 ml/kg. The control group received vehicle alone (0.5% wt/vol dextrose solution). All animals in the toxicity study were killed on day 9 as in the efficacy study. Mortality, behavior, and general conditions were monitored daily. Body weight was recorded on days 0, 2, 4, 7, 8, and 9. Hematological and clinical chemistry examinations were performed on day 9 from abdominal aorta blood. Postmortem macroscopic and microscopic examination were performed on day 9. For the microscopic examination, liver, kidneys, and lungs collected at necropsy and fixed in 10% buffered formalin were trimmed and embedded in paraffin. Five-µm histological sections were stained with hematoxylin and erythrosine and examined by a board-certified pathologist with a Zeiss Axioskop 2 plus microscope (Carl Zeiss, Chester, VA).
Statistical analysis. All in vitro and in vivo efficacy experiments were performed at least three times in duplicates. Student's t-test and one-way analysis of variance test (followed by Dunnett's multiple comparison test as post hoc analysis and by the test for linear trend to investigate the role of the dose–response) were carried out with PRISM software (Graph Pad Software, La Jolla, CA); P < 0.05 was considered to be significant.
SUPPLEMENTARY MATERIAL Figure S1. Quantitative real-time polymerase chain reaction of PDGF-Rα mRNA expression in B16F10 mouse melanoma cell line. Table S1. Within the acute toxicity studies hematological analysis was carried out in healthy mice upon FREG treatment. Non significant toxicity signs were observed. Table S2. Within the acute toxicity studies clinical-chemistry analyses ware performed in healthy mice upon FREG treatment. Non significant toxicity signs were observed.
Acknowledgments
We thank Bioinformatics/Proteomics Facility at CNR (Avellino) and Facility for Complex Protein Mixture Analysis at the Dipartimento di Ematologia, Oncologia e Medicina Molecolare, ISS (Rome), Italy, for the support in collecting and analyzing data. The authors declared no conflict of interest. This study was supported in part by grant from Italian Ministry of Health Contract (RF07 Onc-25/3) and by Progetto Oncoproteomica Italia–USA 527B/2A/5 to A.F.
Supplementary Material
Quantitative real-time polymerase chain reaction of PDGF-Rα mRNA expression in B16F10 mouse melanoma cell line.
Within the acute toxicity studies hematological analysis was carried out in healthy mice upon FREG treatment. Non significant toxicity signs were observed.
Within the acute toxicity studies clinical-chemistry analyses ware performed in healthy mice upon FREG treatment. Non significant toxicity signs were observed.
REFERENCES
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Spatz A, et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2010;46:270–283. doi: 10.1016/j.ejca.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ouyang J, Wang N, Zhang Y, Bie J., and, Zhang Y. Promotion of PDGF-induced endothelial cell migration by phosphorylated VASP depends on PKA anchoring via AKAP. Mol Cell Biochem. 2009;335:1–11. doi: 10.1007/s11010-009-0234-y. [DOI] [PubMed] [Google Scholar]
- Faraone D, Aguzzi MS, Toietta G, Facchiano AM, Facchiano F, Magenta A, et al. Platelet-derived growth factor-receptor α strongly inhibits melanoma growth in vitro and in vivo. Neoplasia. 2009;11:732–742. doi: 10.1593/neo.09408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch RV., and, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- Long JS, Yokoyama K, Tigyi G, Pyne NJ., and, Pyne S. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid- and platelet-derived-growth-factor-induced cell migration. Biochem J. 2006;394 Pt 2:495–500. doi: 10.1042/BJ20051674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH., and, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Ostman A., and, Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R., and, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM., and, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperinde GV., and, Nugent MA. Mechanisms of fibroblast growth factor 2 intracellular processing: a kinetic analysis of the role of heparan sulfate proteoglycans. Biochemistry. 2000;39:3788–3796. doi: 10.1021/bi992243d. [DOI] [PubMed] [Google Scholar]
- Beenken A., and, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillari G, Sgadari C, Fiorelli V, Samaniego F, Colombini S, Manzari V, et al. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the α5β1 and αvβ3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood. 1999;94:663–672. [PubMed] [Google Scholar]
- Wang H, Rubin M, Fenig E, DeBlasio A, Mendelsohn J, Yahalom J, et al. Basic fibroblast growth factor causes growth arrest in MCF-7 human breast cancer cells while inducing both mitogenic and inhibitory G1 events. Cancer Res. 1997;57:1750–1757. [PubMed] [Google Scholar]
- Chandler LA, Sosnowski BA, Greenlees L, Aukerman SL, Baird A., and, Pierce GF. Prevalent expression of fibroblast growth factor (FGF) receptors and FGF2 in human tumor cell lines. Int J Cancer. 1999;81:451–458. doi: 10.1002/(sici)1097-0215(19990505)81:3<451::aid-ijc20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Fibroblast growth factor-1 stimulates branching and survival of myocardial arteries: a goal for therapeutic angiogenesis. Circ Res. 2000;87:176–178. doi: 10.1161/01.res.87.3.176. [DOI] [PubMed] [Google Scholar]
- Jensen P, Pedersen EG, Zimmer J, Widmer HR., and, Meyer M. Functional effect of FGF2- and FGF8-expanded ventral mesencephalic precursor cells in a rat model of Parkinson's disease. Brain Res. 2008;1218:13–20. doi: 10.1016/j.brainres.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Bellucci C, Lilli C, Baroni T, Parnetti L, Sorbi S, Emiliani C, et al. Differences in extracellular matrix production and basic fibroblast growth factor response in skin fibroblasts from sporadic and familial Alzheimer's disease. Mol Med. 2007;13:542–550. doi: 10.2119/2007-00034.Bellucci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S, Murao K, Imachi H, Cao WM, Yu X, Li J, et al. Platelet derived growth factor regulates ABCA1 expression in vascular smooth muscle cells. FEBS Lett. 2006;580:4371–4376. doi: 10.1016/j.febslet.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Chen PY, Simons M., and, Friesel R. FRS2 via fibroblast growth factor receptor 1 is required for platelet-derived growth factor receptor β-mediated regulation of vascular smooth muscle marker gene expression. J Biol Chem. 2009;284:15980–15992. doi: 10.1074/jbc.M809399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kong D, Li Y., and, Sarkar FH. PDGF-D signaling: a novel target in cancer therapy. Curr Drug Targets. 2009;10:38–41. doi: 10.2174/138945009787122914. [DOI] [PubMed] [Google Scholar]
- Schwertfeger KL. Fibroblast growth factors in development and cancer: insights from the mammary and prostate glands. Curr Drug Targets. 2009;10:632–644. doi: 10.2174/138945009788680419. [DOI] [PubMed] [Google Scholar]
- Facchiano A, De Marchis F, Turchetti E, Facchiano F, Guglielmi M, Denaro A, et al. The chemotactic and mitogenic effects of platelet-derived growth factor-BB on rat aorta smooth muscle cells are inhibited by basic fibroblast growth factor. J Cell Sci. 2000;113 (Pt 16):2855–2863. doi: 10.1242/jcs.113.16.2855. [DOI] [PubMed] [Google Scholar]
- De Marchis F, Ribatti D, Giampietri C, Lentini A, Faraone D, Scoccianti M, et al. Platelet-derived growth factor inhibits basic fibroblast growth factor angiogenic properties in vitro and in vivo through its α receptor. Blood. 2002;99:2045–2053. doi: 10.1182/blood.v99.6.2045. [DOI] [PubMed] [Google Scholar]
- Russo K, Ragone R, Facchiano AM, Capogrossi MC., and, Facchiano A. Platelet-derived growth factor-BB and basic fibroblast growth factor directly interact in vitro with high affinity. J Biol Chem. 2002;277:1284–1291. doi: 10.1074/jbc.M108858200. [DOI] [PubMed] [Google Scholar]
- Faraone D, Aguzzi MS, Ragone G, Russo K, Capogrossi MC., and, Facchiano A. Heterodimerization of FGF-receptor 1 and PDGF-receptor-α: a novel mechanism underlying the inhibitory effect of PDGF-BB on FGF-2 in human cells. Blood. 2006;107:1896–1902. doi: 10.1182/blood-2005-04-1524. [DOI] [PubMed] [Google Scholar]
- Palumbo R, Gaetano C, Antonini A, Pompilio G, Bracco E, Rönnstrand L, et al. Different effects of high and low shear stress on platelet-derived growth factor isoform release by endothelial cells: consequences for smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2002;22:405–411. doi: 10.1161/hq0302.104528. [DOI] [PubMed] [Google Scholar]
- Koyama N, Hart CE., and, Clowes AW. Different functions of the platelet-derived growth factor-α and -β receptors for the migration and proliferation of cultured baboon smooth muscle cells. Circ Res. 1994;75:682–691. doi: 10.1161/01.res.75.4.682. [DOI] [PubMed] [Google Scholar]
- Heldin CH. Simultaneous induction of stimulatory and inhibitory signals by PDGF. FEBS Lett. 1997;410:17–21. doi: 10.1016/s0014-5793(97)00318-9. [DOI] [PubMed] [Google Scholar]
- Facchiano A, Russo K, Facchiano AM, De Marchis F, Facchiano F, Ribatti D, et al. Identification of a novel domain of fibroblast growth factor 2 controlling its angiogenic properties. J Biol Chem. 2003;278:8751–8760. doi: 10.1074/jbc.M209936200. [DOI] [PubMed] [Google Scholar]
- Levin EG, Sikora L, Ding L, Rao SP., and, Sriramarao P. Suppression of tumor growth and angiogenesis in vivo by a truncated form of 24-kd fibroblast growth factor (FGF)-2. Am J Pathol. 2004;164:1183–1190. doi: 10.1016/S0002-9440(10)63206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddahi A, Lemdjabar H, Caruelle JP, Barritault D., and, Hornebeck W. Inhibition by dextran derivatives of FGF-2 plasmin-mediated degradation. Biochimie. 1995;77:703–706. doi: 10.1016/0300-9084(96)88185-5. [DOI] [PubMed] [Google Scholar]
- Grieb TA., and, Burgess WH. The mitogenic activity of fibroblast growth factor-1 correlates with its internalization and limited proteolytic processing. J Cell Physiol. 2000;184:171–182. doi: 10.1002/1097-4652(200008)184:2<171::AID-JCP4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hanada K, Yewdell JW., and, Yang JC. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427:252–256. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- Totta P, De Cristofaro R, Giampietri C, Aguzzi MS, Faraone D, Capogrossi MC, et al. Thrombin-mediated impairment of fibroblast growth factor-2 activity. FEBS J. 2009;276:3277–3289. doi: 10.1111/j.1742-4658.2009.07042.x. [DOI] [PubMed] [Google Scholar]
- Strieth S, Eichhorn ME, Sutter A, Jonczyk A, Berghaus A., and, Dellian M. Antiangiogenic combination tumor therapy blocking α(v)-integrins and VEGF-receptor-2 increases therapeutic effects in vivo. Int J Cancer. 2006;119:423–431. doi: 10.1002/ijc.21838. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Cheng JK, Zeng Q, Xu ZZ, Decosterd I, Xu X, et al. Selective inhibition of JNK with a peptide inhibitor attenuates pain hypersensitivity and tumor growth in a mouse skin cancer pain model. Exp Neurol. 2009;219:146–155. doi: 10.1016/j.expneurol.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, et al. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69:369–378. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kawamura T, Furuhashi M, Tsukamoto K., and, Shimada S. Improving chemotherapeutic drug penetration in melanoma by imatinib mesylate. J Dermatol Sci. 2008;51:190–199. doi: 10.1016/j.jdermsci.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Aguzzi MS, Giampietri C, De Marchis F, Padula F, Gaeta R, Ragone G, et al. RGDS peptide induces caspase 8 and caspase 9 activation in human endothelial cells. Blood. 2004;103:4180–4187. doi: 10.1182/blood-2003-06-2144. [DOI] [PubMed] [Google Scholar]
- Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, et al. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitative real-time polymerase chain reaction of PDGF-Rα mRNA expression in B16F10 mouse melanoma cell line.
Within the acute toxicity studies hematological analysis was carried out in healthy mice upon FREG treatment. Non significant toxicity signs were observed.
Within the acute toxicity studies clinical-chemistry analyses ware performed in healthy mice upon FREG treatment. Non significant toxicity signs were observed.