Abstract
Despite enormous efforts by the scientific community, an effective HIV vaccine remains elusive. To further address to what degree T cells in absence of antibodies may protect against simian immunodeficiency virus (SIV) disease progression, rhesus macaques were vaccinated intramuscularly with a chimpanzee-derived Ad vector (AdC) serotype 6 and then boosted intramuscularly with a serologically distinct AdC vector of serotype 7 both expressing Gag of SIVmac239. Animals were subsequently boosted intramuscularly with a modified vaccinia Ankara (MVA) virus expressing Gag and Tat of the homologous SIV before mucosal challenge with a high dose of SIVmac239 given rectally. Whereas vaccinated animals showed only a modest reduction of viral loads, their overall survival was improved, in association with a substantial protection from the loss of CD4+ T cells. In addition, the two vaccinated Mamu-A*01+ macaques controlled viral loads to levels below detection within weeks after challenge. These data strongly suggest that T cells, while unable to affect SIV acquisition upon high-dose rectal infection, can reduce disease progression. Induction of potent T-cell responses should thus remain a component of our efforts to develop an efficacious vaccine to HIV-1.
Introduction
The recently unblinded RV-144 “Thai” trial showed reduction of HIV-1 acquisition in vaccinated individuals, thus potentially allowing for insight into correlates of protection against HIV-1 infection. In this trial, humans at moderate to high risk for infection that were immunized with a canary poxvirus vector-expressing antigens of HIV-1 followed by a recombinant gp120 protein boost generated low HIV-1-specific T-cell responses and antibodies that failed to neutralize primary HIV-1 isolates.1 In contrast, a phase IIb clinical trial, the so-called STEP trial, designed to test whether HIV-1-specific T cells may protect against HIV-1 transmission or replication failed to show efficacy, although the vaccine, a replication-defective adenovirus (Ad) vector of the human serotype 5 (AdHu5), stimulated potent HIV-1-specific T-cell responses in most recipients.2,3
Most licensed vaccines induce protection through neutralizing antibodies. The envelope protein (Env) of HIV-1, the virus sole target for neutralizing antibodies, is highly variable, heavily glycosylated and undergoes structural changes upon receptor binding,4 thus preventing the successful design of Env-based immunogens that can induce neutralizing antibodies to a broad spectrum of HIV-1 isolates. For this reason, the focus of recent HIV-1 vaccine research efforts has been on inducing HIV-specific T-cell responses, most notably those mediated by CD8+ T cells.5,6 CD8+ T cell appear to protect against progression of HIV-1 infection in elite controllers, i.e., individuals with certain major histocompatibility complex (MHC) class I alleles that, in the absence of antiretroviral therapy, suppress viral loads to very low and sometimes undetectable levels.7,8 Furthermore, CD8+ T cell-inducing vaccines have shown some efficacy in preclinical animal models based on infection of rhesus macaques with simian immunodeficiency virus (SIV) or HIV/SIV (SHIV) chimeras9,10 and depletion of CD8+ lymphocytes in SIV-infected macaques causes a rapid increase in viral loads.11,12 The negative result of the STEP trial may hence reflect specific features of the used vaccine platform and immunization regimen. Humans commonly carry neutralizing antibodies to AdHu5 that dampen the immunogenicity of AdHu5-based vaccines and, in addition, preclinical studies indicate that the repeated use of the same viral vector results in less potent transgene product-specific immune responses compared to the sequential use of heterologous vectors,13,14 which in some instances confer partial protection from virus expansion after a high-dose SIV challenge in rhesus macaques.9,15
In this study, we revisited the potential efficacy of a prime-boost immunization regimen based on the sequential use of three distinct vectors expressing SIV Gag. We chose vectors to which humans in general lack pre-existing neutralizing antibodies such as those based on chimpanzee-derived Ad (AdC) viruses, which do not circulate in humans unlike AdHu516,17,18 or AdHu2616,18 viruses and modified vaccinia Ankara (MVA) virus19 to which only those born before 1980 may have antibodies following vaccination against smallpox virus. Rhesus macaques were primed with an AdC vector derived from serotype 6 (AdC6, ref. 13), boosted with a serologically distinct AdC vector of serotype 7 (AdC7, ref. 14) and finally received a third immunization with an MVA vector. Macaques were challenged with a high dose of SIVmac239 given intrarectally. The vaccine regimen provided partial protection from virus replication and significantly reduced loss of peripheral CD4+ T cells. The two vaccinated Mamu-A*01+ macaques controlled viral loads to levels below the limit of detection within weeks after challenge. Under the assumption that SIVmac239 infection of rhesus macaques provides a valid model for HIV-1 infection of humans, these data suggest that potent T cell-inducing immunogens should remain components of future HIV-1 vaccines.
Results
Study design
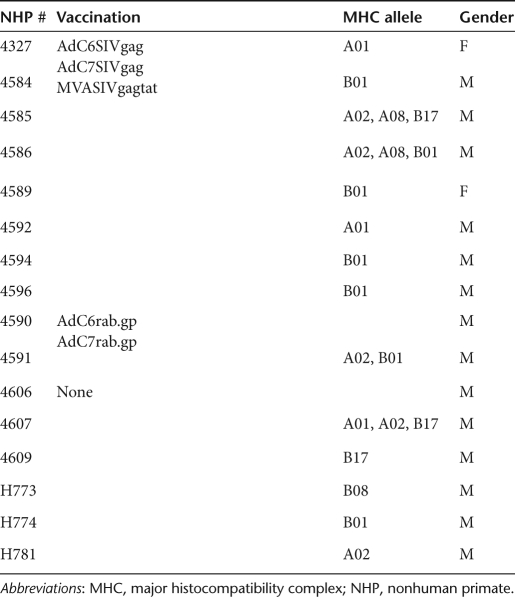
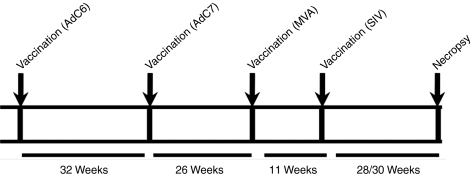
The study was designed to test the efficacy of prime-boost vaccine regimens in Indian origin rhesus macaques. Before vaccination animals were screened for neutralizing antibodies to AdC6 and AdC7; they were negative for both (data not shown). Rhesus macaques were first primed with AdC6SIVgag, then boosted ~9 months later with AdC7SIVgag. Five months later, they were boosted again with an MVA vector expressing Gag and Tat of SIV. Ad vectors were given at 1011 virus particles per animal, MVA was given at 108 plaque-forming units. All vectors were given intramuscularly as this route was shown to be highly effective in mice to stimulate not only systemic but also genital transgene product-specific CD8+ T-cell responses.20 As Ad vectors induce very sustained effector T-cell responses, the intervals between the different doses of vaccine varied to adjust for the typical delay of memory T cell development upon Ad vector vaccination.14 Specifically, the 1st boost was given 32 weeks after priming, the 2nd boost was given 26 weeks later. Animals were challenged 10 weeks after the last immunization with a high dose of 10,000 mean tissue culture-infective doses (TCID50) of SIVmac239 given intrarectally. Most of the animals were males thus precluding vaginal challenge. Eight control animals were challenged in parallel either after administration of AdC vectors expressing the rabies virus glycoprotein (two animals) or without previous vaccination (six animals). Data from all controls were combined for the analyses. Both male and female animals were enrolled in the study and we did not exclude animals based on their MHC haplotypes. The different animals, their sex, and MHC haplotypes are shown in Table 1 and the immunization schedule is shown in Figure 1.
Table 1. List of the rhesus macaques used in this study.
Figure 1.
Scheme of experimental design. Times for vaccinations, challenge, and necropsy are indicated. AdC6, AdC vector derived from serotype 6; AdC7, AdC vector derived from serotype 7; MVA, modified vaccinia Ankara; SIV, simian immunodeficiency virus.
Frequencies of circulating Gag-specific T cells after immunization and challenge
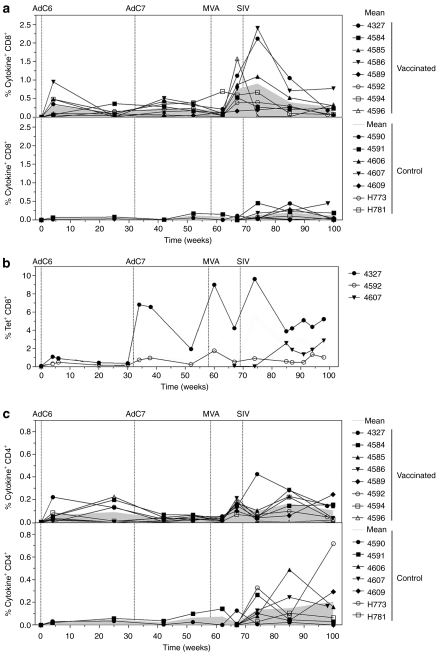
Frequencies of interferon-γ (IFN-γ), interleukin-2 (IL-2), and/or tumor necrosis factor-α (TNF-α) producing T cells were measured by intracellular cytokine staining (ICS) for SIVgag-specific CD8+ and CD4+ T cells (Figure 2a). Based on this immunological read-out, priming with the AdC6SIVgag vector induced a robust Gag-specific CD8+ T-cell response that in five out of eight macaques exceeded frequencies of 0.2% of CD8+ T cells. The booster immunization with AdC7SIVgag was relatively ineffective and, in most animals, the magnitude of secondary responses did not exceed those after priming. A third immunization with an MVA SIVgagtat vector increased frequencies of Gag-specific CD8+ T cells in all of the macaques. After SIVmac239 challenge, frequencies of Gag-specific CD8+ cells again increased in most macaques including those that had not been vaccinated even though this increase was in general modest.
Figure 2.
Kinetics of Gag-specific CD8+ T-cell responses. NHPs were vaccinated as described in Table 1 and challenged with SIVmac239. (a) PBMCs from animals from group 1 (top), and the two control groups (bottom) were stimulated with SIVgag peptide pools and tested for production of IFN-γ, IL-2, and TNF-α at various time points postimmunization. Lines show the percentage of the sum of CD8+ T cells producing the tested cytokines alone or in combinations, over all CD8+ T cells for individual animals. Gray filled lines represent means of the two groups. (b) Gag-specific CD8+ T-cell responses from blood of the two vaccinated Mamu-A01+ animals (#4327 and #4592) and the Mamu-A01+ control animal (#4607) were tested over time by staining of T cells with a Gag-specific tetramer and antibodies to CD3 and CD8. Graph shows tetramer+CD8+ T cells over all CD8+ T cells. (c) Production of IFN-γ, IL-2, and TNF-α by CD4+ T cells isolated from blood of vaccinated (upper graph) and control (lower graph) animals at various time points postimmunization was measured by ICS. Lines show the percentage of the sum of CD4+ T cells producing the tested cytokines alone or in combinations, over all CD4+ T cells for individual animals. Gray filled lines represent means of each group. AdC6, AdC vector derived from serotype 6; AdC7, AdC vector of serotype 7; IFN-γ, interferon-γ IL-2, interleukin; MVA, modified vaccinia Ankara; NHP, nonhuman primate; PBMC, peripheral blood mononuclear cell; SIV, simian immunodeficiency virus; TNF-α, tumor necrosis factor-α.
Three of the macaques included in this study were Mamu-A*01+: two were vaccinated, one served as a control. Frequencies of Gag-specific CD8+ T cells were measured in these animals by staining with a Mamu-A*01-SIVgag-specific tetramer (Tet) and the obtained results markedly differed from those obtained by ICS (Figure 2b). After priming, both vaccinated Mamu-A*01+ animals developed a robust Tet+CD8+ T-cell response to Gag. By the time of the first boost, frequencies had contracted but then after boosting they increased in both animals with peak frequencies exceeding those observed after priming. There was a further increase in Gag-specific CD8+ T cells after the third immunization. After challenge, Gag-specific tetramer responses increased in one of the vaccinated Mamu-A*01+ macaques.
AdC6 priming induced Gag-specific CD4+ T-cell responses at or above frequencies of 0.2% over all CD4+ T cells in only two of the vaccinated animals. There was no increase in frequencies after the boost with the AdC7 vectors. Increases in frequencies were seen after the MVA boost and in most animals following challenge. Responses of comparable magnitude were observed in the unvaccinated control macaques after their challenge (Figure 2c).
Differentiation status of Gag-specific cytokine-producing CD8+ and CD4+ T cells
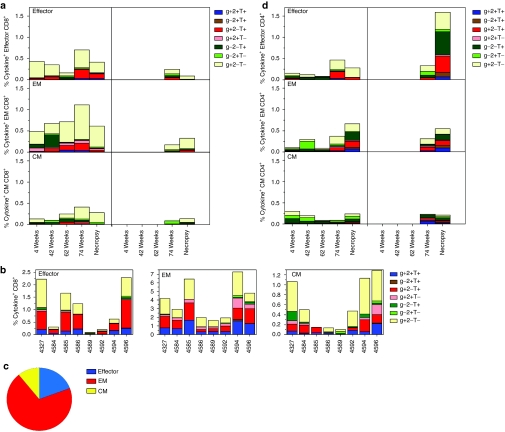
We stained for CD95, CD28, and CD62L or CCR7 to determine the differentiation status of T cells and simultaneously characterized the pattern of cytokine secretion by different T-cell sub-populations. After priming with AdC6SIVgag, we observed mainly effector (Teff, CD95+CD28−CD62Llow or CCR7low) and effector memory (EM, CD95+CD28+CD62Llow or CCR7low) CD8+ T cells that predominantly secreted IFN-γ, whereas central memory (CM, CD95+CD28+CD62Lhigh or CCR7high) CD8+ T cells were detectable at lower frequencies (Figure 3a). Booster immunization with AdC7SIVgag shifted the cytokine profiles of EM and CM cells toward production of TNF-α or IL-2. A further boost with MVA decreased frequencies of CD8+ Teff cells and slightly increased frequencies of CM cells. After MVA immunization, the pattern of cytokine secretion showed predominantly IFN-γ production by the EM population, which comprised the major subtype of responding CD8+ T cells. After challenge, we observed an overall increase in frequencies of cytokine-producing CD8+ T cells with average frequencies of Gag-specific CD8+ Teff, EM and CM in vaccinated macaques exceeding those of control animals. Analysis of the pattern of cytokine production revealed a predominance of CD8+ T cells producing IFN-γ or IFN-γ together with TNF-α in all sub-populations. Frequencies and cytokine profiles of Gag-specific CD8+ T cell populations harvested just before challenge (Figure 3b,c) showed that Gag-specific CD8+ T cells belonged mainly to the effector/EM subset and Teff and EM cells with high proportions of cells exhibiting multiple functions. Similar results for subset distribution were obtained using tetramer staining of the Mamu A*01+ macaques (data not shown).
Figure 3.
Cytokines profiles of T cell subsets. Percentages of all possible combinations of IFN-γ (g), IL-2 (2), and TNF-α (T) production by effector (Effector), effector memory (EM), and central memory (CM) T cells isolated from blood shortly after each immunization and at necropsy were determined. (a) Graphs show average CD8+ T-cell responses over time for vaccinated animals on the left and for control animals on the right. (b) Graphs show responses for individual vaccinated animals tested 2 weeks before challenge. (c) Graph shows average relative distribution of the different CD8+ T cell subsets in blood tested just 2 weeks before challenge. (d) Graphs show average CD4+ T-cell responses over time for vaccinated animals on the left and for control animals on the right. AdC6, AdC vector derived from serotype 6; AdC7, AdC vector of serotype 7; IFN-γ, interferon-γ IL-2, interleukin; MVA, modified vaccinia Ankara; SIV, simian immunodeficiency virus; TNF-α, tumor necrosis factor-α.
Gag-specific CD4+ T cells after priming belonged largely to the CM subset and produced IL-2 or TNF-α (Figure 3d). Overall frequencies of CD4+ T cells were low and did not increase upon booster immunizations although they increased upon SIV challenge. Gag-specific CD4+ T cells detected early after SIV infection included mainly Teff and EM cells showing a broad spectrum of cytokine production. Surprisingly, at that time only the control animals showed robust frequencies of Gag-specific CD4+ CM cells potentially suggesting more pronounced viral mutations.
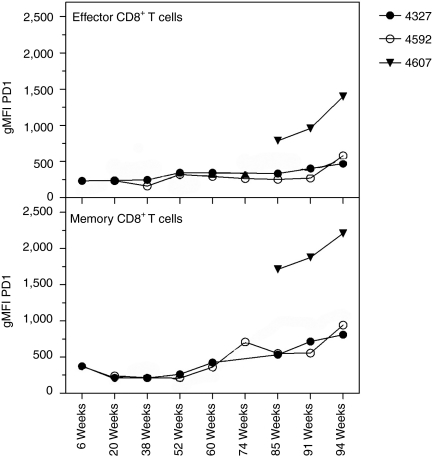
Levels of programmed death-1 (PD-1) expression, a marker for T cell activation and exhaustion that has been reported to be upregulated on CD8+ T cells upon HIV-1 or SIV infection,21,22 were analyzed on Gag-specific memory (Tet+CD95+CD28+) and effector (Tet+CD95+CD28−) CD8+ T cells from Mamu-A*01+ macaques (Figure 4). We found that PD-1 levels in both cell populations remained low after vaccination, except for a slight increase on memory CD8+ T cells following the MVA boost. After SIV challenge, PD-1 expression remained low on effector CD8+ T cells from the vaccinated animals until week 25, when levels slightly increased. PD-1 expression on Tet+CD8+ T cells progressively increased markedly after SIV challenge in the naive macaque.
Figure 4.
Levels of PD-1 expression by Mamu-A*01 Tet+CD8+ T cells. Geometric mean fluorescence intensity (gMFI) of PD-1 expression by effector and memory (CD28+CD95+) Mamu-A*01 Tet+CD8+ T cells isolated from blood of NHP # 4327, 4592, and 4607 were analyzed at various time points after immunization. PD-1, programmed death-1.
Breadth of the SIVgag-specific T-cell response
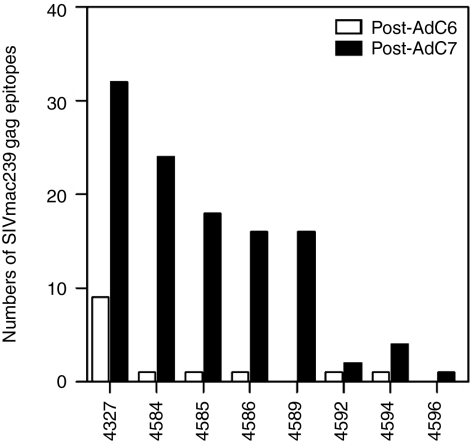
In the STEP trial, AdHu5 vaccination was reported to induce only a narrow T-cell response and although the vaccine expressed several antigens of HIV-1, most humans only developed T-cell responses to 1 or 2 epitopes,3 which may have allowed for rapid escape of the virus. To determine the number of epitopes that were being recognized by SIV Gag-specific T cells in the immunized macaques, we used peptides arranged in matrices in an ELISpot assay for IFN-γ (Figure 5). After priming, most of the responding animals recognized only a single epitope. However, after the 2nd immunization five out of eight animals recognized over 15 unique epitopes. In general, SIVgag-specific T cells from nonhuman primates (NHPs) with high responses recognized more epitopes compared to low responders.
Figure 5.
Prime-boost vaccine regiment increases breadth of Gag-specific IFN-γ T-cell responses. Breadths of specific IFN-γ responses were analyzed with PBMCs isolated from animals after AdC6 (20 weeks, open bars) and after AdC7 (10 weeks, black bars) immunizations by ELISpot assays using peptide matrices. AdC6, AdC vector derived from serotype 6; AdC7, AdC vector of serotype 7; IFN-γ, interferon-γ PBMC, peripheral blood mononuclear cell.
Viral loads, CD4+ T cell counts, and mortality after SIV challenge
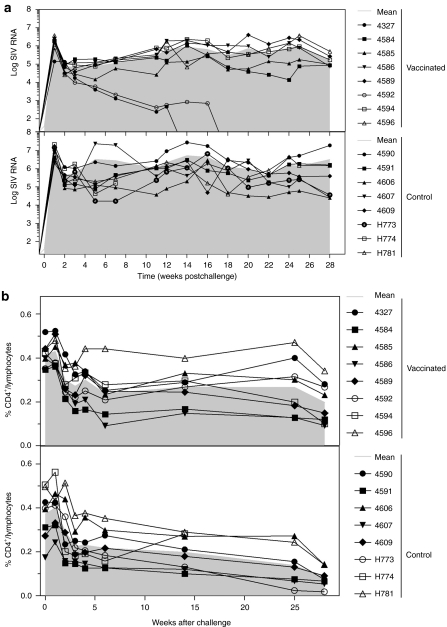
To determine the effect of the immunization regimens in protecting from SIV, viral loads were measured biweekly in the plasma of challenged macaques (Figure 6a). All animals became infected following a high-dose rectal challenge with SIVmac239. Vaccinated and control animals showed the same pattern of viral loads over time (P = 0.275, comparison conducted using Tukey adjustment). However, over time there were significant differences in viral loads between the groups; titers in vaccinated animals were 6.6 times lower than those of control (P < 0.0005). It should be noted that the two vaccinated Mamu-A*01+ animals completely controlled plasma viral loads by weeks 14 (#4327) and 18 (#4592). The Mamu-A*01+ control macaque (#4607) had viral titers that, at most time points, were comparable to those of the other control animals. If we excluded all Mamu-A*01+ NHPs from the analyses, the difference in viral titers between vaccinated and unvaccinated animals remained significant (P = 0.0028).
Figure 6.
Vaccine efficacy against SIV challenge. NHPs were challenged rectally with SIVmac239 and SIV RNA levels and CD4+ T counts were monitored. (a) Data show log of SIV RNA copies/ml of plasma of individual animals from vaccinated (top), and control (bottom) animals. (b) Percentages of CD4+ T lymphocytes in peripheral blood were analyzed from vaccinated (top), and control (bottom) animals. Gray filled lines represent means of each group. NHP, nonhuman primate; SIV, simian immunodeficiency virus.
At 24 weeks after challenge, viral loads were also determined from splenic naive CD4+ T cells, EM and CM CD4+ T cells. The two animals that controlled plasma viral loads also showed lack of cell-associated virus, whereas virus could be detected in all CD4+ T cell sub-populations with no significant differences between the groups (data not shown).
Counts of circulating CD4+ T cells were determined every 2 weeks following challenge (Figure 6b). There was a significant difference in rate of decline between vaccinated and control animals (P = 0.033). There was also a significant difference in absolute counts between the two cohorts as of week 25 (P = 0.011 for week 25 and 0.017 for week 28), indicating that vaccination induced protection against peripheral CD4+ T cell loss.
The health status of animals was checked routinely and two control animals had to be euthanized at 8 and 28 weeks after challenge, respectively, after they developed AIDS-related symptoms. None of the vaccinated animals developed overt symptoms of AIDS or died during the observation period.
Frequencies and differentiation status of Gag-specific CD8+ T cells harvested from different compartments
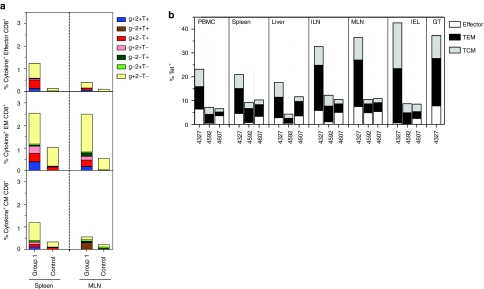
At necropsy frequencies, differentiation status and cytokine production profiles of Gag-specific CD8+ T cells from spleens and mesenteric lymph nodes were tested to assess if data obtained with blood-derived lymphocytes were predictable for those obtained from tissues. The overall pattern between different Gag-specific CD8+ T cell subsets from blood and spleen but not mesenteric lymph nodes matched although overall frequencies were higher in spleens (Figure 7a). To assess biodistribution of Gag-specific CD8+ T cells subsets in more detail, lymphocytes isolated from blood, spleen, liver, ileocolical lymph nodes, mesenteric lymph nodes, and the intestinal epithelium of the Mamu-A01+ NHPs were stained with the Gag tetramer and antibodies to differentiation marker. All of the macaques, including the control animal, had high frequencies of Gag-specific CD8+ T cells in tissues, i.e., spleen, liver, ileocolical lymph nodes, mesenteric lymph nodes, intestinal epithelium and the genital tract, with the latter tested from the one female Mamu-A*01+ macaque (Figure 7b). There was a subtle trend toward higher proportions of resting CD8+ T cells, i.e., CM cells in the two vaccinated animals, which considering that they had controlled viral loads was to be expected.
Figure 7.
T-cell responses in tissues. (a) At necropsy, frequencies of all combined cytokine-producing CD8+ T cells were determined in lymphocytes isolated from spleen and MLN of vaccinated and control animals. Graphs show average frequencies of CD8+ T cells producing the different cytokine combinations in response to Gag peptides. (b) At necropsy Gag-specific CD8+ T cell subsets were identified on lymphocytes from the indicated tissues by staining of lymphocytes with the Gag-specific tetramer and antibodies to various T cell markers. CM, central memory; EM, effector memory; GT, genital tract; IEL, intestinal epithelium; ILN, ileocolical lymph nodes; MLN, mesenteric lymph nodes.
Discussion
In this study, we used two SIVgag-expressing AdC vectors administered sequentially and followed by a boost with a SIVgagtat-expressing MVA vector to address if such a regimen induces immune responses that protect rhesus macaques from a high-dose rectal challenge with SIVmac239. Vaccinated macaques developed lower viral loads, showed a slower decline of circulating CD4+ T cells, and had reduced mortality compared to control animals.
We did not exclude macaques based on their MHC haplotype and the trial contained three Mamu-A*01+ animals, which had been previously described to achieve better control over SIV infections upon vaccination,23 as well as animals with so-called elite controller haplotypes (B*08 and B*17, ref. 24,25). Three vaccinated and three control macaques had so-called elite controller haplotypes, which did not appear to affect virus progression, with the exception of the Mamu-A*01 haplotype. The two vaccinated Mamu-A*01+ macaques controlled viral loads down to levels below detection by weeks 14 and 18, respectively. One of those, animal #4327, had a broad T-cell response after the 1st boost by recognizing over 30 unique epitopes of Gag whereas animal #4592 had a narrow response to only two epitopes. While animals #4327 had a robust CD8+ T-cell response to Gag animal #4592 developed only modest responses upon vaccination as was especially noticeable for frequencies of Gag-specific effector and EM CD8+ T cells. Although #4327 controlled viral loads slightly earlier than #4592, these results nevertheless cast doubt on the simple explanation that control of viral load in Mamu-A*01+ animals depended on superior frequencies, breadth or differentiation status of circulating Gag-specific CD8+ T cells.
Other previously described vaccine regimens have achieved partial protection against SIV transmission. Rhesus cytomegalovirus vectors expressing Gag, Rev, Nef, and Env induced high frequencies of effector-like CD8+ and CD4+ T cells and protected some macaques against viral acquisition upon repeated low-dose rectal challenge without reducing viral loads in animals that became infected.26 In a different study, DNA priming followed by an AdHu5 boost with vectors that expressed all SIV proteins, with the exception of Env,27 acquisition rates were not reduced upon low-dose SIVsmE660 challenge, but several of the vaccinated animals controlled viral loads in the acute phase of the infection. Similarly, animals immunized with a single high dose of a chimeric Ad vector-expressing several antigens of SIV including Env showed reduction in peak and set point viral loads and improved survival upon an intravenous SIVmac239 challenge.28
In our study based on high-dose rectal challenge, protection was most pronounced in Mamu-A*01+ animals, a finding that recapitulates that of previous studies. For example, repeated immunization with Gag-expressing DNA vaccines followed by a single dose of an AdHu5 vector in Mamu-A*01+ and Mamu-A*01− macaques showed that only in Mamu-A*01+ animals vaccination resulted in reduced peak viral titers and in a transient reduction of set point viral loads upon high dose challenge with SIVmac239 given intrarectally.15 Our vaccine regimen outperformed DNA priming followed by an AdHu5 boost as not only Mamu-A*01+ but also Mamu-A*01− animals showed a reduction in viral loads. Although vaccination of Mamu-A*01+ macaques with MVA vectors-expressing Gag and Tat resulted in reduced peak and early set point viral loads following an intravenous challenge with SIVmac239, vaccination failed to affect disease progression.19 Again, in our study vaccinated Mamu-A*01+ rhesus macaques controlled viral loads to levels below detection, which was not achieved in either of these studies indicating that an aggressive T cell-inducing vaccine regimen based on three distinct vectors outperforms the previous regimens.
An AdHu26 vector followed by a boost with an AdHu5 vector both expressing only Gag was shown to provide protection against an intravenous challenge with SIVmac251 by lowering peak and set point viral loads, preserving CD4 counts and improving survival.9 The same study showed that two immunizations with an AdHu5 vector failed to induce protection. Our study, based on a high-dose rectal challenge with a different albeit related SIV strain, mirrors in some aspects the AdHu26/AdHu5 arm of this previously published study9 confirming that vaccines designed to only induce T-cell responses to Gag can provide some protection against SIV challenge. Although our vaccine regimen showed a less pronounced reduction in viral loads, we would like to point out a pertinent difference in addition to technical differences in route of challenge and the strain and dose of SIV used for challenge. Neutralizing antibodies to AdHu5 are very common and can be found in up to 70–90% of humans from Sub-Saharan Africa,18 which are most direly affected by the AIDS pandemic. Two recent studies showed that prevalence of neutralizing antibodies to AdHu26 is also very high in developing countries; adults from Sub-Saharan Africa showed prevalence rates of AdHu26 neutralizing antibodies well above 50%.16,19 This contrasts a previous report with smaller sized cohorts, which reported prevalence rates below 20%.29 It is unclear why the initial report, which claimed that AdHu26 was globally a rare serotype could not be reproduced in subsequent studies using larger cohorts. In contrast, most humans including those residing in developing countries lack neutralizing antibodies to AdC viruses and those that carry antibodies in general have very low titers.16,18 Neutralizing antibodies to Ad-based vaccines reduce induction of transgene product-specific T-cell responses as was shown in mouse30 and nonhuman primate models14 as well as in human vaccine recipients.3 Furthermore, pre-existing neutralizing antibodies to Ad vectors at least in animals severely reduce transgene product-specific B cell responses,14,31 which as the results from the Thai RV-144 trial suggest1 may be paramount for protection against HIV-1 infection. One would thus expect that a vaccine regimen based on AdC vectors to which the vaccine recipient has no or only low titers of pre-existing neutralizing antibodies would outperform a vaccine regimen based on common human serotypes of Ad virus.
In summary, this study shows that a triple immunization regimen based on the sequential use of heterologous vaccine vectors expressing only SIV Gag can provide partial protection against a high-dose rectal SIVmac239 challenge.
Materials and Methods
Vaccine vectors. Ad vectors were derived from the chimpanzee serotypes 6 (AdC6) or 7 (AdC7). The Ad vectors expressed Gag of SIVmac239 (AdC6SIVgag and AdC7SIVgag) or as a control the rabies virus glycoprotein (rab.gp). Vectors were E1-deleted and generated from viral molecular clones by viral rescue on HEK 293 cells. They were grown, purified, titrated, and quality controlled as described.14 Expression of Gag from recombinant viruses was confirmed by western blot analyses of lysates of infected cells (data not shown). Recombinant MVA virus expressing SIV Gag and Tat was produced and quality controlled as described previously.19
NHPs. Two to three-year-old, healthy, and SIV-uninfected Indian origin Macaca mulatta were purchased and housed at Bioqual (Rockville, MD). Animals were typed for Mamu-A*01, A*02, A*08, A*11, B*01, B*03, B*04, B*08, and B*17 alleles (UW AIDS Vaccine Research Lab, Madison, WI) and results are shown in Table 1. All NHPs were screened before enrollment for neutralizing antibodies to AdC6 and AdC7 vectors and were found to be seronegative. All procedures involving handling and sacrifice of animals were performed according to approved protocols and upon approval by the relevant Institutional Animal Care and Usage Committees.
Immunization regimen. The 16 animals were divided into three groups (Table 1). Animals were injected with 1 × 1011 virus particles of AdC6SIVgag (group 1) or AdC6rab.gp (group 2). Thirty-two weeks later, animals were boosted with AdC7SIVgag (group 1), or AdC7rab.gp (group 2). Twenty-six weeks after boost, animals of group 1 were immunized with 108 plaque-forming units of MVA-SIVgagtat. All vaccinations were performed by injecting animals with vector diluted in 1 ml of saline into the quadriceps muscles.
Viral challenge. Eleven weeks after the last immunization with the MVA-based vaccine, experimental and control groups were challenged rectally with 10,000 TCID50 SIVmac239 prepared from COS-1 cell supernatant after transfection with DNA from a pBR239 full-length SIVmac239 clone most kindly provided by Louis Picker (Oregon Health Sciences University and Oregon National Primate Research Center, Beaverton, OR). An additional control group of six unvaccinated animals was also challenged.
Isolation and preservation of lymphocytes. Peripheral blood mononuclear cells and lymphocytes from tissues were isolated as described.14 They were tested immediately after isolation or frozen in 90% fetal bovine serum and 10% dimethyl sulfoxide (Sigma, St Louis, MO) at −80 °C till testing.
Synthetic peptides. Peptide pools of 15-mers (overlapping by 11 amino acids) spanning the SIVmac239 Gag protein were reconstituted in dimethyl sulfoxide and pools were prepared from individual peptide stocks obtained from the National Institutes of Health AIDS Research and Reference Reagent Program.
ELISpot assays. The ELISpot assays for IFN-γ were conducted as described.14 To determine the breadth of the T-cell response, the SIVmac239 Gag peptides were arranged in a matrix. The matrix was created with 125 15-mer peptides, overlapping by 11 amino acids for sequential peptides, in an 11 × 12 arrangement resulting in 23 peptide pools. For stimulation, peptide pools were used at a concentration of 2 µg/ml of each peptide. Candidate peptides were further confirmed by individual retesting in IFN-γ ELISpot assays from selected animals using individual peptides at a concentration of 5 µg/ml. Responses to adjacent peptides were scored as recognition of one epitope. Spots were counted using the C.T.L. Series 3A Analyzer and ImmunoSpot 3.2 (Cellular Technology, Cleveland, OH). The minimum spot size was set to 0.0016 mm2, and the maximum spot size was set to 0.0878 mm2. The criteria for determining positive samples included that for every 106 cells stimulated with peptides, at least 55 spots after subtraction of background spots (spots without antigenic stimulation) had to be detected. Additionally, the number of spots upon peptide stimulation had to be at least three times higher than that seen in control wells. Data shown on graphs represent values of peptide-stimulated wells from which background values had been subtracted.
Intracellular cytokine staining. The function of SIV-specific CD8+T cells was assessed by ICS after stimulation with the SIV Gag peptide pool. All peptides were used at a final concentration of 2 µg of each peptide per ml. Frozen cells were thawed and immediately washed with Hank's-buffered salt solution supplemented with 2 units/ml DNase I, resuspended with RPMI media and stimulated for 6 hours with anti-CD28 (clone CD28.2), anti-CD49d (clone 9F10), and Brefeldin A.14 Cells were stained with violet-fluorescent reactive dye-Pacific Blue (Invitrogen, Carlsbad, CA), anti-CD14-Pacific Blue (clone M5E2), anti-CD16-Pacific Blue (clone 3G8), anti-CD8-APC-H7 (clone SK1), anti-CD4-Alexa700 (clone OKT4), anti-CD95-PE-Cy5 (clone DX2), and anti-CD28-Texas Red (clone CD28.2; Beckman Coulter, Fullerton, CA) for 30 minutes at 4 °C. Additionally, cells were stained with anti-CD62L-PE (clone SK11) (fresh cells) or anti-CCR7-PE (clone 150503) (frozen cells). After fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) for 30 minutes at 4 °C, cells were stained with anti-IFN-γ-APC (clone B27), anti-IL-2-FITC (clone MQ1-17H12), anti-TNF-α-PE-Cy7 (clone MAb11; R&D System) and anti-CD3-PerCp-Cy5.5 (clone SP34-2) for 30 minutes at 4 °C. Cells were washed twice, fixed with BD Stabilizing Fixative (BD Biosciences), and then analyzed by FACS using LSRII (BD Biosciences) and DiVa software. Flow cytometric acquisition and analysis of samples was performed on at least 400,000 events. Postacquisition analyses were performed with FlowJo (TreeStar, Ashland, OR). Data shown on graphs represent values of Gag peptide-stimulated wells from which background values have been subtracted. Polyfunctionality pie-chart graphs were generated using SPICE software (National Institutes of Health, Bethesda MD). Single color controls used CompBeads Anti-Mouse Igκ (BD Biosciences) were used for compensation. Unless otherwise noted, antibodies were purchased from BD (BD Biosciences).
Tetramer staining. Mamu-A*01 MHC class I tetramer specific for SIVmac239 immunodominant epitope Gag181–189 CM9 (CTPYDINQM) conjugated with APC was provided by National Institutes of Health Tetramer Core Facility (Emory University, Atlanta, GA). Lymphocytes were stained with tetramer-Mamu-A01-APC along with violet-fluorescent reactive dye-Pacific Blue (Invitrogen), anti-CD14-Pacific Blue, anti-CD16-Pacific Blue, anti-CD8-APC-H7, anti-CD4-Alexa700, anti-CD95-PE-Cy5, anti-CD28-Texas Red and anti-CD62L-PE or anti-CCR7-PE for 30 minutes at 4 °C. Cells were fixated using BD Stabilizing Fixative, then analyzed as described for ICS.
Plasma viral load. Plasma SIV viral load was determined by quantitative real-time reverse transcriptase-PCR as previously described.32
Quantitative PCR for SIV gag DNA. Quantitative DNA-PCR was used to measure cell-associated SIV-DNA. Quantification of SIVmac gag DNA was performed in sorted CD4+ CM and EM cells by quantitative PCR by the 5′ nuclease (TaqMan) assay with an ABI7700 system (PerkinElmer Life and Analytical Sciences, Waltham, MA) as previously described.22 For cell number quantification, quantitative PCR was performed simultaneously for monkey albumin gene copy number. The sequence of the forward primer for SIVmac is 5′-GTCTGCGTCAT(T/C)TGGTGCATTC-3′. The reverse primer sequence is 5′-CACTAG(C/T)TGTCTCTGCACTAT(A/G)TGTTTTG-3′. The probe sequence is 5′-CTTC(A/G)TCAGT(C/T)TGTTTCACTTTCTCTTCTGCG-3′.
Cell sorting and CD4+ counts. Sorting of CD4+ EM and CM cells was performed on a FACSAria III Cell Sorter (Becton Dickinson, San Jose, CA). Cells were initially gated based upon characteristic light scatter properties, followed by positive staining for CD3 and CD4. CD4+ EM and CM cell subsets were gated based upon characteristic expression patterns of CD28, CD95, and CCR7.
Statistical analysis. Standard descriptive statistics were used to summarize the efficacy of prime-boost vaccine regimens. Mixed effects models, with nonlinear responses over time, were used to model variables measured repeated over the course of the study; such as viral loads and CD4+ counts. These models are similar to repeated analysis of variance models, but allow for incomplete data due to a missing time point for a particular animal. Tukey adjustment for multiple comparisons was used when conducting pairwise comparisons between the groups overall and at each time point. Associations between variables measured at a single time point were assessed using Spearman correlation coefficients. Analyses were conducted using GraphPad Prism 5 and SAS 9.2.
Acknowledgments
This work was funded by grants from NIH/DAIDS and institutional grants to the Wistar Institute including a US National Cancer Institute Cancer Core Grant (CA10815) and the Commonwealth Universal Research Enhancement Program from the Pennsylvania Department of Health. We thank Christina Cole for help in preparation of the manuscript. The authors declared no conflict of interest.
REFERENCES
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Stanfield RL., and, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci USA. 2005;102:14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut LH., and, Ertl HC. Obstacles to the successful development of an efficacious T cell-inducing HIV-1 vaccine. J Leukoc Biol. 2009;86:779–793. doi: 10.1189/jlb.0209094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber BT, Letvin NL., and, Haynes BF. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J Virol. 2009;83:8300–8314. doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Block BL, Rothchild AC., and, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opin Biol Ther. 2009;9:55–69. doi: 10.1517/14712590802571928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Johnson RP, McClure HM, Manson KH, Wyand MS, Kuroda MJ, et al. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J Virol. 2005;79:8131–8141. doi: 10.1128/JVI.79.13.8131-8141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM., and, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol. 2003;171:6774–6779. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- Tatsis N, Lasaro MO, Lin SW, Haut LH, Xiang ZQ, Zhou D, et al. Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens. J Immunol. 2009;182:6587–6599. doi: 10.4049/jimmunol.0900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79:15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A.et al. (2010Adenovirus-based vaccines: comparison of vectors from three families of adenovirideae J Virol 8410522–10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina SF, Gao GP, Xiang ZQ, Rux JJ, Burnett RM, Alvira MR, et al. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerging Infect Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engram JC, Dunham RM, Makedonas G, Vanderford TH, Sumpter B, Klatt NR, et al. Vaccine-induced, simian immunodeficiency virus-specific CD8+ T cells reduce virus replication but do not protect from simian immunodeficiency virus disease progression. J Immunol. 2009;183:706–717. doi: 10.4049/jimmunol.0803746. [DOI] [PubMed] [Google Scholar]
- Haut L, Lin S, Tatsis N, DiMenna L, Giles-Davis W, Pinto A.et al. (in press). Robust genital gag-specific CD8+ T-cell responses in mice upon intramuscular immunization with simian adenoviral vectors expressing HIV-1-gag Eur J Immunol [DOI] [PMC free article] [PubMed]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR, et al. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76:292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Friedrich TC, León EJ, Stephany JJ, Rodrigues DS, Spencer SP, et al. CD8+ T cells from SIV elite controller macaques recognize Mamu B*08 bound epitopes and select for widespread viral variation. PLoS ONE. 2007;2:e1152. doi: 10.1371/journal.pone.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2006;80:5074–5077. doi: 10.1128/JVI.80.10.5074-5077.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, Bett AJ, et al. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol. 2009;83:6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Liu J, Lynch DM, O'Brien KL, La Porte A, Simmons NL, et al. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J Virol. 2009;83:9584–9590. doi: 10.1128/JVI.00821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CL, Gaines BA., and, Ildstad ST. Xenotransplantation. Annu Rev Immunol. 1995;13:339–367. doi: 10.1146/annurev.iy.13.040195.002011. [DOI] [PubMed] [Google Scholar]