Abstract
AAV2-sFLT01 is a vector that expresses a modified soluble Flt1 receptor designed to neutralize the proangiogenic activities of vascular endothelial growth factor (VEGF) for treatment of age-related macular degeneration (AMD) via an intravitreal injection. Owing to minimal data available for the intravitreal route of administration for adeno-associated virus (AAV), we initiated a 12-month safety study of AAV2-sFLT01 administered intravitreally at doses of 2.4 × 109 vector genomes (vg) and 2.4 × 1010 vg to cynomolgus monkeys. Expression of sFlt01 protein peaked at ~1-month postadministration and remained relatively constant for the remainder of the study. Electroretinograms, fluorescein angiograms, and tonometry were assessed every 3 months, with no test article-related findings observed in any group. Indirect ophthalmoscopy and slit lamp exams performed monthly revealed a mild to moderate but self-resolving vitreal inflammation in the high-dose group only, which follow-up studies suggest was directed against the AAV2 capsid. Histological evaluation revealed no structural changes in any part of the eye and occasional inflammatory cells in the trabecular meshwork, vitreous and retina in the high-dose group. Biodistribution analysis in rats and monkeys found only trace amounts of vector outside the injected eye. In summary, these studies found AAV2-sFLT01 to be well-tolerated, localized, and capable of long-term expression.
Introduction
Age-related macular degeneration (AMD) is the leading cause of central vision loss in individuals 65 years of age and older in industrialized countries.1
The neovascular form of the disease is characterized by choroidal neovascularization which is marked by proliferation of blood vessels and cells including those of the retinal pigment epithelium (RPE).2 Ultimately, photoreceptor death and fibrous disciform scar formation result in a severe loss of central vision and the inability to read, write, and recognize faces or drive. Many patients can no longer maintain gainful employment, carry out daily activities and consequently report a diminished quality of life.3
Preventative therapies have demonstrated little effect and therapeutic strategies have focused primarily on treating the neovascular lesion. Whereas some currently used therapies (laser thermal photocoagulation, photodynamic therapy with verteporfin, Macugen, Avastin, or Lucentis) may slow the progression of vision loss or in some cases improve vision, none of these treatments prevent neovascularization from recurring,4,5,6 and each has to be readministered to prevent the disease from worsening. The need for repeat treatments, can incur additional risk to patients, and is inconvenient for both patients and treating physicians. Thus, there is significant need for a long acting therapeutic. An effective gene therapy product with a long duration of action could fulfill this need, and could have a profound impact on the treatment of this disease.
Gene therapy approaches have been described for AMD treatment using an adenoviral vector expressing pigment epithelium-derived factor, a factor that inhibits angiogenesis. Preclinical studies in monkeys with Ad5-pigment epithelium-derived factor have demonstrated dose-limiting inflammation,7 however, a phase I clinical trial only displayed mild inflammation in 25% of the patients,8 a promising sign for use of gene therapy vectors in macular degeneration.
Adeno-associated viruses (AAV) have gained increased favor in the gene therapy field owing to their lack of human pathogenicity, low toxicity and, particularly, their long-term expression compared to adenoviruses while remaining episomal.9 AAV2-based gene therapy vectors have been employed as a candidate therapy for several disorders including hemophilia B, neurodegenerative disorders such as Parkinson's disease, lysosomal storage diseases, and others.10,11,12,13 Diseases of the eye have recently been targeted using these vectors with encouraging results. Groups targeting the RPE65 variant of Leber's congenital amaurosis have used AAV2 vectors dosed subretinally in dog models of the disease, resulting in reversal of blindness lasting several years (new14). Studies with AAV2-RPE65 vectors have recently advanced into humans resulting in increased responsiveness of the study eyes to light, with little to no side effects of the treatment out to 1-year postinjection.15,16,17,18 While the administration of AAV vectors to the subretinal region of the eye has been well described through these studies, we sought to determine if dosing AAV intravitreally would suffice for our purposes.
We have developed a recombinant, replication defective AAV2 vector for the treatment of AMD that expresses a portion of the sFlt1 vascular endothelial growth factor (VEGF) receptor termed sFlt01.19 sFLT01 encodes domain 2 of sFlt1 linked to a human Fc domain and is expressed from the chicken β-actin promoter. Previous results have shown that expression of sFLT01 in mouse models can exceed 1 year. In addition, administration of AAV2-sFLT01 is efficacious in mouse and primate models of choroidal neovascularization (ref. 19 and M. Lukason, E. DuFresne, H. Rubin, P. Pechan, Q. Li, I. Kim et al., unpublished results). The safety of AAV vectors administered intravitreally has not been described, nor has the long term (>6 months) neutralization of VEGF in primates. Here, we describe results from studies that aim to establish the safety, expression, and biodistribution of the AAV2-sFLT01 vector over the period of one year in primates following intravitreal administration.
Results
Twelve-month nonhuman primate toxicology study
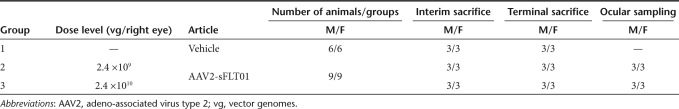
A toxicology study to evaluate the long-term effects of AAV2-sFLT01 was initiated in the cynomolgus monkey. Animals were injected once into the right eye with vehicle or AAV2-sFLT01 at doses of 2.4 × 109 vector genomes (vg) or 2.4 × 1010 vg per eye (Table 1). Several analyses were conducted over the course of the study and are described below.
Table 1. Study design of 12-month toxicology study.
Expression of sFLT01
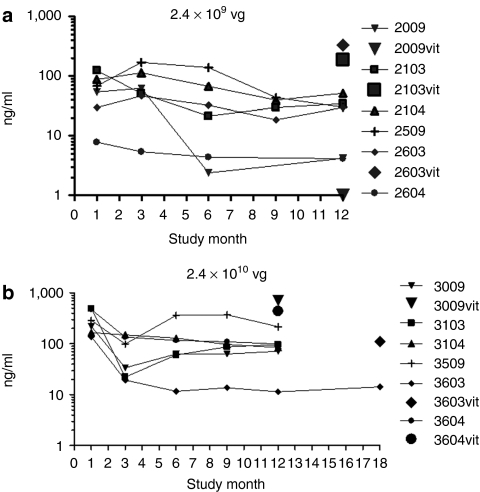
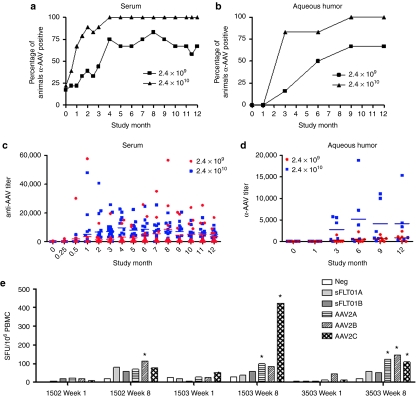
There was a dose-dependent increase in the amount of sFlt01 protein observed in the aqueous humor obtained from treated animals, with a range of 27–299 ng/ml in the 2.4 × 109 vg dosing group and 59–528 ng/ml in the 2.4 × 1010 vg dosing group over the course of the study. In the 2.4 × 109 vg dosing group, expression progressively but mildly decreased over the course of 9 months, but then stabilized (Figure 1a). Expression was present at the 1-month time point in the 2.4 × 1010 vg dosing group, was reduced at month 3 then stabilized over the course of the remaining months (Figure 1b). In the vitreous humor, which was harvested at terminal sacrifice from half of the “Ocular Sampling” group animals, four of five animals (two of three from the 2.4 × 109 vg dosing group and two of two from the 2.4 × 1010 vg dosing group) had detectable sFlt01 levels between 187 and 333 ng/ml for the 2.4 × 109 vg dosing group and 716 and 438 ng/ml for the 2.4 × 1010 vg dosing group (Figure 1a,b). One “Ocular Sampling” group animal in the 2.4 × 1010 vg dosing group was carried out to 18 months for purposes of evaluating ocular inflammation (see below), where sFlt01 expression had dipped below the limit of quantitation at 3 months postinjection. sFlt01 levels in the uninjected (left) eye for all samples were below the limit of quantitation. It is noted that a wide range of expression levels were observed in this study even within each dosing group. At this time it is unknown exactly what factors contribute to this variation, however, it is likely this may reflect the inherent variability of viral-based gene therapy combined with the injection of the vector into the highly viscous vitreous which might impede diffusion to transducing sites within the eye.
Figure 1.
Expression of sFlt01 protein in the aqueous humor of the eye. Aqueous humor was sampled from the injected (right) eye of nonhuman primates at 1, 3, 6, 9, and 12 months postinjection of AAV2-sFLT01 from (a) group 2 (2.4 × 109 vg) or from (b) group 3 (2.4 × 1010 vg). One animal in group 3 (#3603) was kept on study for an additional 6 months. At the end of the study, the eyes from animals 2009, 2103, 2603, 3009, 3603, and 3604 were dissected for vitreous collection (“vit”) and were assayed for sFlt01 expression. AAV2, adeno-associated virus type 2; vg, vector genomes.
No consistent and reproducible findings of sFlt01 protein were seen in the serum throughout the study.
Ophthalmic exams
Ophthalmic examinations with slit lamp biomicroscopy and indirect ophthalmoscopy were performed on all animals before dosing, on days 3 and 14 postdose, and monthly thereafter. Vitreal inflammation (“haze and cells”) in the anterior and posterior portion of the eye was scored using a system adapted by McDonald and Nussenblatt, respectively.20,21
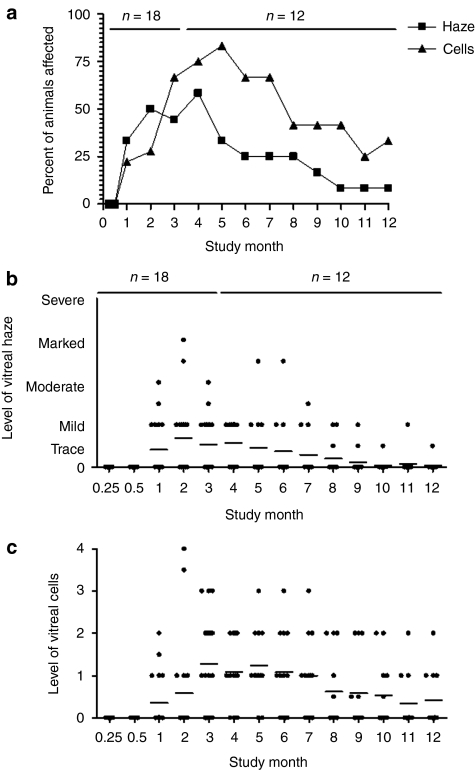
No abnormalities were noted in the vitreous or fundus of either eye at any time point in the vehicle dosing group and the 2.4 × 109 vg dosing group or in the 2.4 × 1010 vg dosing group during the day 3 and 14 examinations. However, after this point, varying degrees of vitreal haze and cells were noted in the right eyes of 78% (14/18) of the animals in the 2.4 × 1010 vg dosing group (Figure 2). Beginning at the month 1 examinations, vitreal haze and/or cells were noted in the injected eyes of 6/18 animals (43% of the 14 that would develop inflammation); at the month 2 examinations the injected eyes of 9/18 animals were affected (64% of the 14 that would develop inflammation), and by the month 3 examination, the injected eyes of 13/18 animals were involved (93% of the 14 that would develop inflammation). After the month 3 examination, one additional animal developed minimal vitreal haze and cells at month 5. After this, there was no increase in the number of eyes affected. With the exception of one animal, the degree of vitreal haze and cells did not worsen with time and tended to become less severe at successive examinations. As noted, one animal exhibited a stabilization of vitreal haze and cells in the right eye at the month 4 examination, but the vitreal haze and cells were dramatically increased (moderate to marked vitreal haze and 3+ vitreal cells) at the month 5 examination. This increase persisted at the month 6 examinations before showing evidence of decreasing again at month 7. The modulation of the vitreal haze and cells then continued through the month 12 examination. The prevalence and severity of these findings through month 12 are displayed graphically in Figure 2a–c. There did not appear to be a correlation between levels of expression of the sFlt01 protein and inflammation (Supplementary Figure S1)
Figure 2.
Vitreal inflammation observed in 2 × 1010 vg dosing group. Animals on study were examined by indirect ophthalmoscopy. 2 × 1010 vg dosed animals were found to develop inflammation over the course of the study, with onset in the majority of animals by 3 months (a). Severity averaged between trace and mild haze (b) and a grade 1–2 cells (c) with some animals displaying marked haze and/or higher grades of cells at brief points during the study. vg, vector genomes.
Two animals were kept on study beyond the 12-month time point as they still retained low levels of vitreal inflammation and there was a desire to understand further the kinetics of resolution of this long-term inflammation. Vitreal haze cleared from the eyes of the two recovery animals by months 13 and 16; residual cells were observed for the duration of the recovery period. One of these animals was also noted to the have the lowest levels of expression throughout the study (#3603, Figure 1b), thus there is a possible correlation between the length of time the eye is in an inflammatory state and the ability to continue expressing the transgene.
Throughout the study, the mean intraocular pressures and electroretinographic responses (Supplementary Figure S2a–c) remained normal, were comparable for all groups, and had no test article-related changes. With the exception of vitreal haze interfering with visualization of the fundus in some animals in the 2.4 × 1010 vg dosing group at month 3, fluorescein angiography results were judged to be normal and comparable to prestudy results for all animals (Supplementary Figure S3a–b). The vitreal haze caused the vitreous to appear hyperfluorescent, suggesting some diffusion of fluorescein into the vitreous, but that appearance did not persist in the later stages of the angiography sessions, and diffusion of light by the vitreous haze may have been the cause for the apparent early hyperfluorescence of the vitreous. No obvious and persistent leakage of fluorescein from the vessels was detected.
Ocular histology
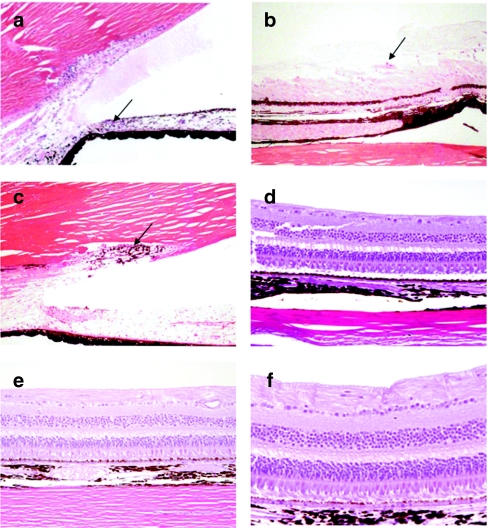
At the 3-month interim sacrifice, histological lesions were observed in group 3 (high-dose group) but not in the vehicle or the 2.4 × 109 vg dosing group. Two of three males and two of three females in the 2.4 × 1010 vg dosing group had inflammatory changes consisting of lymphocytes or plasma cells in the trabecular meshwork and the iridocorneal angle, inflammatory cells in the vitreous, or perivascular lymphocytes in the retina (Figure 3a). The cell type in the vitreous was not always identifiable, but some appear to be macrophages and preliminary data suggests that some are B-cells. All of these changes were scored as minimal to moderate depending on the number of cells seen but none of the lesions seen had evidence of tissue destruction or reorganization. Because no tissue destruction was seen, the inflammatory changes at 3 months were judged to be likely reversible. Importantly, these four animals from the 2.4 × 1010 vg dosing group that displayed these findings of inflammation also displayed variable levels of vitreal haze and cells upon indirect ophthalmoscope evaluation—the two animals that had no histopathological findings also did not have any findings of inflammation in life.
Figure 3.
Inflammatory cells in the eye. At both the 3- and 12-month sacrifice, inflammatory cells were seen in the eye, most often commensurate with ophthalmologic exams performed on the same animal. At the 3-month sacrifice, lymphocytes were seen in the (a) trabecular meshwork. At the 12-month sacrifice, lymphocytes and macrophages were observed within and adjacent to the pars plana of the (b) ciliary body and pigmented cells within the (c) trabecular meshwork. Retinal integrity was also evaluated in (d) vehicle and (e,f) high-dose animals. At no point was any damage noted to ocular structures.
At the 12- and 18-month sacrifice, all test article-related lesions recorded were again in the high-dose group. Two of three males and one of three females had inflammatory changes characterized by small numbers of lymphocytes, macrophages, and plasma cells in the trabecular meshwork, widely dispersed in the anterior uvea, and in the inner aspect of the ciliary body epithelium (Figure 3b–c). One of these animals also had pigmented and nonpigmented macrophage cells in the vitreous near the pars plana. The retinal was also evaluated for structural abnormalities, with no alterations observed in any retinal layer (Figure 3d–f).
Identification of intraocular inflammation source
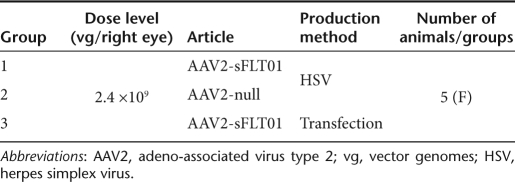
An additional study was conducted to attempt to determine what component of the AAV2-sFLT01 vector was the source of the intravitreal inflammation observed in the study described above (Table 2). Three vectors were utilized—the AAV2-sFLT01 vector used in the study above and an AAV2-null vector without a transgene, with both vectors having been produced using the recombinant herpes simplex virus (rHSV) production system. A third vector, AAV2-sFLT01 that was produced using a transfection method rather than the HSV-based viral method was used for comparison. All were dosed at the level that resulted in inflammation in the 12-month study described above—2.4 × 1010 vg/eye. The one common component among all three vectors was the presence of AAV2 capsid.
Table 2. Study design of 3-month NHP study.
All three groups of animals displayed varying levels of vitreal inflammation at some point in this study (Figure 4a–b). The presence of inflammation in the AAV2-null group, suggests the exclusion of that element as a contributing component to the inflammation. It is therefore most probable that the viral vector capsid is the causative agent in the development of the inflammation. It is not possible from these results to conclusively explain the reason for the differences in severity of the uveitis in the different groups, especially with respect to the marked inflammation noted in one animal in each of two groups—these events are possibly linked to the injection procedure itself.
Figure 4.
Source of intravitreal inflammation. Fifteen nonhuman primates were injected with 2.4 × 1010 vg of AAV2-sFLT01 into the right eye. Five animals were dosed with AAV2 vector with no transgene (AAV2-null), five dosed with AAV2-sFLT01 prepared by the HSV-based production system, identical to the preparation used in the 12-month toxicology study (AAV2-sFLT01-HSV) and five were dosed with a preparation of AAV2-sFLT01 made by transfection methods lacking any HSV component (AAV2-sFLT01-tfxn). Varying degrees of inflammation as noted by (a) vitreal haze and cells were observed in all groups. AAV2, adeno-associated virus type 2; HSV, herpes simplex virus; vg, vector genomes.
Immunogenicity
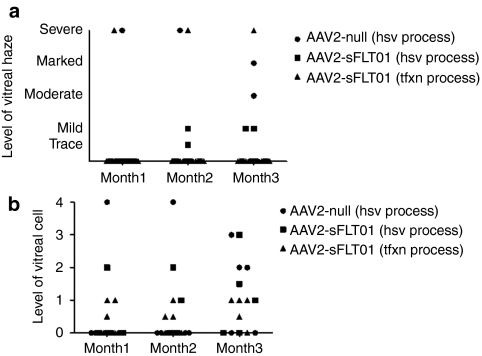
Serum and aqueous humor were evaluated in the 12-month toxicology study for the presence of antibodies against sFlt01 and the vector, AAV2. Serum was analyzed from all animals, while three males and three females in the 2.4 × 109 vg and 2.4 × 1010 dosing groups (“Ocular Sampling” group) had aqueous humor analyzed as well. Serum was sampled every 2 weeks for the first 3 months and monthly thereafter, whereas aqueous humor was sampled at months 1, 3, 6, 9, and 12.
There were no findings of antibodies against sFlt01 in the serum or aqueous humor at any time point. There was a dose- and time-dependent increase in animals that possessed a titer against AAV2 in the serum (Figure 5a,c). There was one animal in the 2.4 × 109 vg and one animal in the 2.4 × 1010 dosing groups that showed predose titers against AAV2 (1:2,129 and 1:314, respectively). There were no correlations between this pre-existing AAV2 titer and vitreal inflammation or subsequent increase in AAV2 titer. The animal in the 2.4 × 109 dosing group was also in the “Ocular Sampling” group (#2604) and displayed expression levels of sFlt01 lower than the other five animals in that group (see Figure 1a). Thus, it is possible that pre-existing antibody titers against AAV2 may impact successful transduction. As in serum, titers against AAV2 were seen in a dose- and time-dependent manner in the aqueous (Figure 5b,d). No predose titers against AAV2 were seen in the aqueous humor.
Figure 5.
Immunogenicity against AAV2 capsid protein. Serum and aqueous humor were sampled at various intervals throughout the course of the 12-month toxicology study and analyzed for total IgG titer against AAV2 capsid. There was a dose- and time-dependent response and the majority of animals that would go on to develop anti-AAV2 antibodies seroconverted in both the serum and aqueous by (a,b) 3 months postinjection. Varying levels of anti-AAV2 antibodies were observed, with higher levels seen in the (c) serum compared to the (d) aqueous. In the source of inflammation study, T-cell responses (indicated by IFN-γ production) against AAV2 and sFlt01 were examined at various timepoints (e). Here, results from week-1 and week 8 postinjection samples, where responses were maximal, are shown. Pools of peptides representing overlapping sequence of sFlt01 (sFlt01A, sFlt01B) and AAV2 capsid protein (AAV2A, AAV2B, AAV2C) were incubated with PBMCs and were analyzed for intracellular expression of interferon-γ by ELISpot analysis. Bars marked with an *indicate a significant induction wells where more the amount of γ-interferon was greater than three times that measured in the “negative control” cells and the value, in SFU/106 PBMCs, was >55. Animals 1502 and 1503 were in group 1 in this study, and received 2.4 × 1010 vg/eye of HSV-method produced AAV2-sFLT01. Animal 3503 was in group 3 in this study, and received 2.4 × 1010 vg/eye of transfection-method produced AAV2-sFLT01. AAV2, adeno-associated virus type 2; ELISpot, enzyme-linked immunosorbent spot; HSV, herpes simplex virus; IgG, immunoglobulin G; IFN, interferon; PBMC, peripheral blood mononuclear cell; SFU, spot-forming units; vg, vector genomes.
T-cell responses against the AAV2 capsid and the sFlt01 protein were assessed from the 15 animals used for determining the source of inflammation (see Figure 4). Peripheral blood mononuclear cells (PBMCs) were prepared from blood harvested from all animals on study at weeks −3, −1, 2, 4, 8, and 12 postdose and exposed to peptide libraries containing overlapping sequences of either AAV2 capsid protein or sFLT01 transgene product. To determine whether T-cells were activated by the peptides, the amount of γ-interferon was measured by enzyme-linked immunosorbent spot assay. There was no T-cell response directed toward the sFLT01 protein in any of the animals at any time point. Three animals exhibited detectable T-cell responses against the AAV2 capsid at various time points during the study (Figure 5e, only those animals that yielded a response are shown). While all three of these animals displayed inflammation in this study, it was at a severity similar to several other animals that did not possess a T-cell response. Additionally, there was no correlation between these cellular responses and the antibody titers against AAV2 in these animals (data not shown).
Biodistribution of AAV2-sFLT01
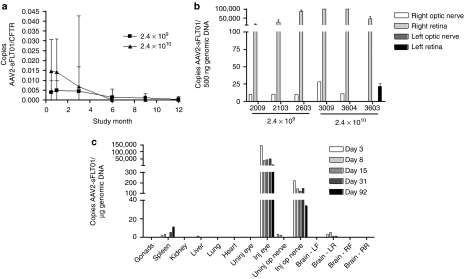
In the 12-month toxicology study, serum samples were assayed for the presence of vector DNA by real-time quantitative PCR at selected time points. Also, at the 12- and 18-month sacrifice, the left and right eyes of three “Ocular Sampling” group animals from the 2.4 × 109 vg and 2.4 × 1010 dosing groups were analyzed for the presence of vector DNA in the retina and optic nerve (the 18-month time point for the one “Ocular Sampling” group animal left on study for an additional 6 months—see “Ophthalmic Exams”).
In the serum, dose-dependent levels of vector DNA were observed beginning on day 13 (first serum sample taken) (Figure 6a). The amounts of DNA extracted from serum were very low, thus the amount of vector DNA amplified was normalized to an endogenous gene, the cystic fibrosis transmembrane conductance regulator. Levels of vector DNA ranged from 0.39% of cystic fibrosis transmembrane conductance regulator at day 13 to 0.45% at day 98 in group 2 and 1.47% at day 13 to 0.68% at day 98 in group 3. By day 175, all levels were below the limit of quantitation. These values indicate that AAV2-sFLT01 vector was present at very low levels compared to an endogenous gene throughout the study.
Figure 6.
Biodistribution of AAV2-sFLT01. (a) Animals from the 12-month toxicology study were sampled for serum every 3 months and processed for total DNA. Vector-specific AAV2-sFLT01 sequences were amplified by PCR. The total copy number of AAV2-sFLT01 was normalized to copy number of the endogenous gene CFTR. (b) At the terminal sacrifice, the retina and optic nerve of three animals each from groups 2 and 3 of the “Ocular Sampling” group were harvested, processed for DNA, and samples were amplified by PCR for unique AAV2-sFLT01 sequences. (c) An additional study was performed in rat to more comprehensively evaluate the spread of AAV2-sFLT01. In this one hundred animal study rats were dosed with 6.4 × 107 vg AAV2-sFLT01 or vehicle into the right eye. Ten animals from each group were sacrificed and necropsied for various organs and blood at 3, 8, 15, 31, and 92 days postinjection. Total DNA was prepared from each tissue and unique AAV2-sFLT01 sequences were amplified by PCR. Copies detected of AAV2-sFLT01 are reported as copies per µg genomic DNA. AAV2, adeno-associated virus type 2.
In the retina and optic nerve, amounts of vector DNA were expressed as copy number/500 ng DNA. Vector DNA was detected in the retina in all injected eyes, ranging from 427 to >100,000 copies/500 ng DNA in the 2.4 × 109 dosing group and 4,631 to > 100,000 copies/500 ng DNA in the 2.4 × 1010 dosing group (Figure 6b). The retina of the uninjected eye was negative in all animals with the exception of one animal in the 2.4 × 1010 dosing group that may represent contamination as both the injected and uninjected optic nerves in this animal, the route by which the vector would travel to the uninjected retina, tested negative. Also, aqueous and vitreous humor from the fellow eye in this animal were negative for anti-AAV2 antibodies (data not shown). In the optic nerve, vector DNA was detectable, but not quantifiable (level of quantitation—10 copies/500 ng DNA) in the 2.4 × 109 vg dosing group and was present in two out of three animals in the 2.4 × 1010 dosing group at levels of 11 and 28 copies/500 ng DNA. All optic nerve samples from the noninjected eye were negative for vector DNA.
An additional biodistribution study was performed in rats that more comprehensively evaluated the spread of AAV2-sFLT01. The data showed that the vast majority of vector DNA remains in the injected right eye (Figure 6c). Vector DNA was also found in the right optic nerve at levels <1% of that detected in the injected eye. Also, sporadic low level (tens of copies) positive samples were observed in the left hindbrain, spleen and liver tissues. At day 92, all of the left hindbrain samples were negative for vector DNA, indicating that vector presence in that tissue is transient. In those left hindbrain, spleen and liver samples positive for vector DNA, adjacent tissue samples were analyzed for transgene message expression by reverse transcriptase-PCR and all were negative (data not shown). Due to sample size and processing constraints, reverse transcriptase-PCR expression analysis was not performed on eye and optic nerve tissue samples. These results indicate that while AAV2-sFLT01 vector was detected in nontarget tissues, it did not result in detectable sFLT01 transgene expression.
Discussion
We present here thorough and extensive safety evaluation of an intravitreally administered AAV gene therapy vector. Several aspects of this therapeutic approach were under close scrutiny during these studies, and principal among them were (i) the effect of an AAV vector on the biology of the eye, (ii) the consequences of long-term VEGF suppression in the eye, and (iii) expression of transgene and biodistribution of the vector after intravitreal administration.
The primary finding in our safety studies evaluating AAV2-sFLT01 was induction of mild to moderate inflammation that, on average, is induced within 2 months of vector administration and resolves by 5 months but which can exist at very low levels for up to 15 months. This inflammation appears to be the consequence of injection of the AAV2 capsid and not expression of the sFlt01 protein, as a follow-up study found similar levels of inflammation in eyes that had been dosed with an AAV2-null vector. The finding of inflammation was not unprecedented, as several studies utilizing gene therapy vectors and/or protein administration to the eye have also observed inflammation to varying degrees.7,22 Similarly, preclinical and clinical studies investigating the anti-VEGF antibody ranibizumab that has been approved for treatment of AMD have observed a short-lived moderate-intensity inflammatory process in both the anterior and posterior portions of the eye.4,5,22 What was unexpected with AAV2-sFLT01 was the timing of the inflammation observed—the relatively late onset (1–3 months postinjection) and the length of time the inflammation persisted. AAV2 capsid appears to be the culprit for this inflammation, as vectors not expressing sFlt01 also caused similar levels of inflammation, and no humoral or cellular immune response directed against sFlt01 was detected throughout the 12-month toxicology study. On the other hand, antibodies were generated against the AAV2 capsid both in the serum and the aqueous humor in the 12-month toxicology study as well as a detectable cellular immune response observed in some animals in the follow-up study. It is possible that injection of AAV particles into the viscous vitreous delayed inflammation and also acted as a depot for persistent inflammation to occur.
Expression of sFlt01 protein in the eye proved to be quite variable, ranging sometimes as much as tenfold in the aqueous humor at any given time point (Figure 1). On average, there did appear to be a dose-dependent level of expression of sFlt01 (see Supplementary Figure S4). As with the possible “depot” effect of AAV2 capsid in viscous vitreous, diffusion of the vector to transducible cells may happen slowly and in a variable fashion, leading to a wide range of expression levels.
Another concern upon initiation of this safety evaluation was the effect of long-term VEGF suppression in the eye. VEGF suppression can be thought of as a delicate balancing act, as those normal vessels in the choroid that support the retinal pigment epithelia and photoreceptors as well as the retinal vessels supporting the ganglion cell layer are quite necessary for the proper light sensing and information transport from the eye. While VEGF is thought to be a factor that mostly affects naive growing vessels and neutralization unlikely to affect the viability of mature vessels,23 this has nevertheless led several groups to investigate by a variety of methods the consequences of repressing VEGF for long periods of time. Saint-Geniez et al.24 has utilized an adenovirus expressing the full-length soluble VEGF receptor sFlt1 systemically, and found an increase in apoptosis of inner and outer nuclear layer cells coinciding with a decrease in light sensing in the eye as measured by electroretinography; this research has also led to the use of a mouse expressing only the VEGF188 isoform leading to choriocapilaris degeneration.25 On the other hand, several studies involving VEGF neutralization have been performed that have observed no adverse effects on the health of the eye. Doxycycline-induced expression of a soluble VEGF receptor to the eye for 7 months caused no effects on the fenestrations of the choriocapillaris, retinal, and choroidal ultrastructure, and electroretinography,26 while retaining its ability to reduced choroidal neovascularization upon laser-induced rupture of Bruch's membrane. Also, a study evaluating the safety and efficacy of an AAV2 vector expressing the full-length sFlt protein administered subretinally in monkeys found no defects in photoreceptors or on electroretinographic analysis after 8 months of expression.27 Although some of the published reports noted above question the safety of VEGF neutralization in the eye, the data pertaining to long-term effects appear to suggest that while there may or may not be some subtle effects early, these do not appear later and certainly do not worsen. This is also the case from published data in AMD patients from the ranibizumab ANCHOR and MARINA trials4,5 that had been treated for up to 2 years with minimal safety events. Finally, the results presented here show long-term safety of a constitutively expressed anti-VEGF molecule in the eye, with no histological evidence of degeneration in any part of the eye or any perturbation in electrophysiological evidence of sight as provided by electroretinography.
In summary, the data here outlines the safety assessment of an AAV2-based gene therapy expressing a potent anti-VEGF molecule that has been shown to reduce neovascularization in the eye. Given the mild nature of the side effects described in this report and the prospect of a single intravitreal, as opposed to the more complicated subretinal, injection to treat the complications of AMD, these data warrant investigation of this treatment in neovascular diseases of the eye. Human clinical trials are underway and will be helpful in determining the impact of the inflammatory nature of the vector and the variability of sFLT01 expression on safety and efficacy of this investigative therapy.
Materials and Methods
Animals and study designs. Cynomolgus macaques (Macaca fascicularis) of Chinese or Indonesian origin, aged 2–5 years were used for both nonhuman primate studies. For the biodistribution study, Sprague–Dawley rats were used. All in-life procedures were conducted in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals and the Office of Laboratory Animal Welfare.
For the 12-month toxicology study, 48 experimentally naive cynomolgus monkeys (24 males and 24 females), were assigned to dose groups—vehicle (group 1), 2.4 × 109 vg (group 2), and 2.4 × 1010 vg (group 3)—as shown in Table 1.
Interim sacrifice animals were scheduled to be euthanized at 3 months postinjection for histological analysis and terminal sacrifice animals were scheduled to be euthanized at 12 months postinjection for histological analysis. “Ocular Sampling” animals had aqueous humor harvested at 1, 3, 6, 9, and 12 months postinjection and were kept on study until 12 months postinjection, at which time the animals were euthanized. The eyes from half the animals in this group were analyzed for vector presence by PCR. The right eye of each animal was dosed via a single intravitreal injection on day 1 and the left eye was uninjected. The animals were evaluated for changes in clinical signs daily and body weights monthly. Ophthalmic exams were performed every other week for the first 3 months and then monthly thereafter. Fluorescein angiography, electroretinography, tonometry, and clinical pathology were performed every three months. Serum samples were obtained every other week to test for the presence of sFlt01 protein, AAV2-sFLT01 vector and antibodies to AAV2 and sFlt01. Urinalysis and urine chemistry samples were obtained by bladder puncture during necropsy. At termination, a full necropsy was conducted on all animals, and tissues were collected and evaluated histologically. At the 12-month time point, four animals in group 3 retained some level of intraocular inflammation—two of these animals were kept on study for an additional 6 months to evaluate inflammation resolution.
An additional study was performed to identify the source of intraocular inflammation (Table 2). AAV2-sFLT01 (group 1) and AAV2-null (group 2), both produced by an HSV-based manufacturing process, and another AAV2-sFLT01 (group 3) preparation produced by transfection methods were used to isolate potential causes of intraocular inflammation. All animals were given a dose of 2.4 × 1010 vg in order to replicate the findings of the 12-month toxicology study. Five male cynomolgus monkeys per group were given a single intravitreal administration of vector into the right eye and ophthalmic exams were performed on all monkeys before dosing, on months 1, 2, and 3 postdose.
A comprehensive biodistribution study was performed in rats that evaluated the spread of AAV2-sFLT01. Animals were dosed intravitreally in the right eye either with vehicle or 6.4 × 107 vg AAV2-sFLT01 (equivalent to a dose of 2.4 × 1010 vg in cynomolgus monkeys based on vitreal volume) and 10 animals/group were sacrificed at days 3, 8, 15, 31, and 92 days postinjection for a full tissue necropsy and subsequent PCR evaluation for the presence of AAV2-sFLT01.
Intravitreal injection. An intravitreal injection of test article or vehicle was performed in the right eye. For the nonhuman primate studies, a lid speculum was inserted to keep the lid open during the procedure and the globe was retracted. The needle of the dose syringe was inserted through the sclera and pars plana ~4 mm posterior to the limbus. The needle was directed posterior to the lens into the mid-vitreous. The test article or vehicle was slowly injected into the vitreous. Forceps were used to grasp the conjunctiva surrounding the syringe prior to needle withdrawal. Photographs and diagrams were used at the time of injection to document the site of injection. This information was utilized at the time of necropsy so that a suture could be placed at the approximate site of injection. For the rat study, the animals were positioned with the corneoscleral junction horizontal. Under direct observation through an operating microscope, a 10.0-µl Hamilton syringe fitted with a 30-gauge needle was introduced bevel-up into the vitreous cavity through the pars plana (~1 mm from the limbus), taking care to not violate the lenticular bag, and the test material was delivered.
sFlt01 measurement. Biochemical assessment of sFlt01 in serum and aqueous humor was performed by enzyme-linked immunosorbent assay using recombinant human VEGF (rhVEGF165) as capture and goat antihuman immunoglobulin G (Fc) peroxidase conjugate as a detection reagent. Samples that had <0.09 ng of sFlt01 were considered below the limit of detection. Final results are reported as ng sFlt01/ml serum or aqueous humor. In this assay, the lower limit of quantitation for sFlt01 in monkey serum is 50.0 ng/ml. For aqueous humor, the lower limit of quantitation is 25.0 ng/ml.
Anti-AAV2 antibody measurement. Biochemical assessment of total immunoglobulin G antibodies in the serum and aqueous humor against AAV2 in was performed by enzyme-linked immunosorbent assay using heat-inactivated AAV2 as capture and goat anti-monkey immunoglobulin G γ-peroxidase conjugate as a detection reagent. AAV2 particles at a concentration of 1 µg/ml was used to coat plates. For serum, samples with titers <1:200 were considered below the limit of detection, and titer was not able to be determined. For aqueous humor, samples with titers <1:100 were considered below the limit of detection, and titer was not able to be determined. Final results are reported as AAV2 antibody dilution (titer).
Measurement of T-cell reactivity toward AAV2 and sFlt01. Ten milliliters of whole blood was collected from each animal in a heparin tube. Blood samples were shipped at ambient temperature to the University of Pennsylvania by overnight carrier. PBMCs were isolated from the whole blood by density gradient separation with Percoll and frozen in liquid nitrogen for storage. PBMCs from each animal were thawed and exposed to peptide libraries containing overlapping sequences of either AAV2 capsid protein or sFLT01 transgene product. Both peptide libraries were synthesized as 15-mers with a 10 amino acid overlap with the preceding peptides (Mimotopes, Victoria, Australia). The resulting peptides were dissolved in dimethyl sulfoxide at a concentration of 100 mg/ml. Peptide pools were aliquoted and used at a final concentration of 2 µg/ml for all experiments. AAV2cap was grouped in three pools: pool A: from peptide 1 to 50, pool B from peptide 51 to 100 and pool C from peptide 101 to 145. sFLT01 was grouped in two pools: pool A from peptide 1 to 35 and pool B from peptide 36 to 70. To determine whether the T-cells were activated by the peptides, the amount of γ-interferon was measured by enzyme-linked immunosorbent spot assay. If the amount of γ-interferon was greater than three times that measured in the “negative control” cells and the value, in spot-forming units /106 PBMCs, was >55, the PBMCs were said to contain activated peripheral T-cells. The assay was performed on blood collected before vector administration on weeks −3, −1 and following vector administration on weeks 2, 4, 8, and 12.
Vector PCR. To detect vector transgene sequence, DNA was extracted from serum samples (QIAmp DNA Blood Mini Kit; Qiagen, Valencia, CA). These serum DNA samples were analyzed for AAV2-sFLT01 vector DNA by PCR. The amplicon of this real-time quantitative PCR assay spans the junction of transgene and polyA regions and is vector specific. The yield of DNA obtained from these serum samples was below the level required to accurately quantify by optical densities. For this reason, the number of vector copies detected in each sample were normalized to the number of cystic fibrosis transmembrane conductance regulator gene copies, also determined by a real-time quantitative PCR. This study contained a subset of animals from which retina sections and optic nerve samples were collected for analysis to detect vector DNA. A protocol employing proteinase K digestion followed by phenol/chloroform /IAA extraction was used to extract DNA from tissue samples. Sample DNA concentrations were determined by optical density measurement.
This vector specific PCR assay was also used for analysis of organ tissue and blood samples from a comprehensive rat vector biodistribution study. Samples from each tissue type were analyzed in chronological order until all samples for two consecutive time points were negative for the detection of vector DNA. In those samples that tested positive for vector DNA, RNA was extracted from adjacent tissue collected at sacrifice for analysis in a reverse transcriptase-PCR assay specific for transgene expression. Due to sample size and processing constraints, only PCR-based vector DNA analysis was performed on eye, optic nerve, and blood samples. All custom real-time PCR volumes were 50 µl, performed in 96-well plates on an ABI 7900 Sequence Detection System instrument (Applied Biosystems, Foster City, CA).
AAV vector production. AAV2-sFLT01 was produced using two methods. Material used in the 12-month toxicology study and biodistribution studies was prepared using a HSV mediated method.28 Briefly, two ICP27-deficient rHSV constructs, one bearing the rep2 and cap genes of rAAV, and the second bearing an AAV2 ITR-sFLT01 cassette are used to infect in human embryonic kidney 293 cells. Cells are detergent lysed in situ and virus is column purified.28 Alternatively, the vector can be produced using a triple-transfection method. This material was used to compare to HSV-produced material. Briefly, human embryonic kidney 293 cells are transfected with three plasmids encoding for AAV ITR-sFLT01, AAV-rep, and AAV-cap. Cells are lysed and virus is column purified.
SUPPLEMENTARY MATERIAL Figure S1. Correlation between levels of sFLT01 expression and inflammation. At three months post-injection, most animals that would go on to develop inflammation have at this point. At varying levels of sFLT01 protein levels in the aqueous humor, there did not appear to be a correlation between this and inflammation. One animal in the 2e10 dosing group (#3603) that had inflammation at this time point continued to show varying levels of inflammation throughout the course of the 12 month study, and this animal also had the lowest level of expression at the end of the study. Thus, it there is a possible correlation between the length of time in an inflammatory state and the ability to continue expression. Figure S2. AAV2-sFLT01 effects on electroretinographic responses. At three month intervals, animals were evaluated for a variety of electroretinographic responses. A representative tracing from the photopic response of a group 3 (2.4x1010 vg) animal at 6 months (A) and results over the course of the study for the photopic response of that animal compared to the average responses from the vehicle control group (B and C) are shown. Figure S3. AAV2-sFLT01 effects on fluorescein angiography. At three month intervals, animals were evaluated for ocular blood vessel integrity by dosing with fluorescein and monitoring filling of vessels. Representative fundus and angiographic photographs from a group 3 (2.4x1010 vg) animal are shown at the 3 month (A) and 6 month (B) timepoints. This animal possessed vitreal inflammation at the 3 month time point. Fluorescein photos were taken in the 1-2 minute post-fluorescein dose time range. Figure S4. Average expression of sFlt01 between dose groups. Data that is presented in figure 1 is presented here averaging sFlt01 expression levels between dose group. On average, the higher dose group possessed increased levels of sFlt01 expression.
Supplementary Material
Correlation between levels of sFLT01 expression and inflammation. At three months post-injection, most animals that would go on to develop inflammation have at this point. At varying levels of sFLT01 protein levels in the aqueous humor, there did not appear to be a correlation between this and inflammation. One animal in the 2e10 dosing group (#3603) that had inflammation at this time point continued to show varying levels of inflammation throughout the course of the 12 month study, and this animal also had the lowest level of expression at the end of the study. Thus, it there is a possible correlation between the length of time in an inflammatory state and the ability to continue expression.
AAV2-sFLT01 effects on electroretinographic responses. At three month intervals, animals were evaluated for a variety of electroretinographic responses. A representative tracing from the photopic response of a group 3 (2.4x1010 vg) animal at 6 months (A) and results over the course of the study for the photopic response of that animal compared to the average responses from the vehicle control group (B and C) are shown.
AAV2-sFLT01 effects on fluorescein angiography. At three month intervals, animals were evaluated for ocular blood vessel integrity by dosing with fluorescein and monitoring filling of vessels. Representative fundus and angiographic photographs from a group 3 (2.4x1010 vg) animal are shown at the 3 month (A) and 6 month (B) timepoints. This animal possessed vitreal inflammation at the 3 month time point. Fluorescein photos were taken in the 1-2 minute post-fluorescein dose time range.
Average expression of sFlt01 between dose groups. Data that is presented in figure 1 is presented here averaging sFlt01 expression levels between dose group. On average, the higher dose group possessed increased levels of sFlt01 expression.
REFERENCES
- Gottlieb JL. Age-related macular degeneration. JAMA. 2002;288:2233–2236. doi: 10.1001/jama.288.18.2233. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Mitchell J., and, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4:97. doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, ANCHOR Study Group et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, MARINA Study Group et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- D'Amico DJ, Masonson HN, Patel M, Adamis AP, Cunningham ET, Jr, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group et al. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology. 2006;113:992–1001.e6. doi: 10.1016/j.ophtha.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Rasmussen HS, Smith AC, Durham RJ, Rasmussen CS, Bee WH, Wills MC, et al. Safety of intravitreal adenoviral gene therapy containing human pigment epithelium-derived factor transgene in monkeys. Preclinica. 2003. pp. 77–85..
- Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- Van Vliet KM, Blouin V, Brument N, Agbandje-McKenna M., and, Snyder RO. The role of the adeno-associated virus capsid in gene transfer. Methods Mol Biol. 2008;437:51–91. doi: 10.1007/978-1-59745-210-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., and, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth W, Aleman TS, Kaushal S, Schwartz SB, Boye SL.et al. (2009Human RPE65 gene therapy for leber congenital amaurosis: persistence of early visual improvements and safety at one year Hum Gene Ther 20999–1004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechan P, Rubin H, Lukason M, Ardinger J, DuFresne E, Hauswirth WW, et al. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther. 2009;16:10–16. doi: 10.1038/gt.2008.115. [DOI] [PubMed] [Google Scholar]
- Hackett RB., and, McDonald TO.1996Assessing ocular irritation Marzuli FB., and, Maibach HI.eds). Dermatotoxicology, 5th edn. Hemisphere Publishing: Washington, DC [Google Scholar]
- Nussenblatt RB, Palestine AG, Chan CC., and, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- Husain D, Kim I, Gauthier D, Lane AM, Tsilimbaris MK, Ezra E, et al. Safety and efficacy of intravitreal injection of ranibizumab in combination with verteporfin PDT on experimental choroidal neovascularization in the monkey. Arch Ophthalmol. 2005;123:509–516. doi: 10.1001/archopht.123.4.509. [DOI] [PubMed] [Google Scholar]
- Suburo AM., and, D'Amore PA.2006Development of the endothelium Handb Exp Pharmacol176 Pt 1): 71–105. [DOI] [PubMed]
- Saint-Geniez M, Maharaj ASR, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3:1–13. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE., and, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Pease ME, Wersinger DM, Masuda T, Vinores SA, Licht T, et al. Prolonged blockade of VEGF family members does not cause identifiable damage to retinal neurons or vessels. J Cell Physiol. 2008;217:13–22. doi: 10.1002/jcp.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CM, Shen WY, Brankov M, Lai YK, Barnett NL, Lee SY, et al. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol Ther. 2005;12:659–668. doi: 10.1016/j.ymthe.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Kang W, Wang L, Harrell H, Liu J, Thomas DL, Mayfield TL, et al. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009;16:229–239. doi: 10.1038/gt.2008.158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between levels of sFLT01 expression and inflammation. At three months post-injection, most animals that would go on to develop inflammation have at this point. At varying levels of sFLT01 protein levels in the aqueous humor, there did not appear to be a correlation between this and inflammation. One animal in the 2e10 dosing group (#3603) that had inflammation at this time point continued to show varying levels of inflammation throughout the course of the 12 month study, and this animal also had the lowest level of expression at the end of the study. Thus, it there is a possible correlation between the length of time in an inflammatory state and the ability to continue expression.
AAV2-sFLT01 effects on electroretinographic responses. At three month intervals, animals were evaluated for a variety of electroretinographic responses. A representative tracing from the photopic response of a group 3 (2.4x1010 vg) animal at 6 months (A) and results over the course of the study for the photopic response of that animal compared to the average responses from the vehicle control group (B and C) are shown.
AAV2-sFLT01 effects on fluorescein angiography. At three month intervals, animals were evaluated for ocular blood vessel integrity by dosing with fluorescein and monitoring filling of vessels. Representative fundus and angiographic photographs from a group 3 (2.4x1010 vg) animal are shown at the 3 month (A) and 6 month (B) timepoints. This animal possessed vitreal inflammation at the 3 month time point. Fluorescein photos were taken in the 1-2 minute post-fluorescein dose time range.
Average expression of sFlt01 between dose groups. Data that is presented in figure 1 is presented here averaging sFlt01 expression levels between dose group. On average, the higher dose group possessed increased levels of sFlt01 expression.