Abstract
A major challenge in neurological gene therapy is delivery of the transgene to sufficient cell numbers in an atraumatic manner. This is particularly difficult for motor neuron (MN) diseases that have cells located across the entire spinal cord, brain stem, and cortex. We have used the familial mouse model of amyotrophic lateral sclerosis (ALS) to examine the feasibility of body-wide intramuscular injections of adeno-associated virus serotype 6 (AAV6), a vector capable of axonal retrograde transport, to deliver therapeutic genetic information across the lower MN axis. Neonatal muscle delivery of AAV expressing small hairpin RNAs (shRNAs) against the toxic transgene in this model, human mutant superoxide dismutase 1 (mSOD1), led to significant mSOD1 knockdown in the muscle as well as innervating MNs. This knockdown conferred neuroprotection and halted muscle atrophy in individually targeted MN pools. However, despite the vector being targeted to MNs that innervate muscle groups controlling eating, breathing, and locomotion, this approach was unable to therapeutically impact on disease progression in the ALS mouse model. These results stress the complexity of gene delivery for mSOD1 silencing and suggest that critical thresholds of protein knockdown and transduction across various cell types are required to translate local neuroprotective effects into functional improvements.
Introduction
A significant challenge facing gene therapy for the central nervous system is the delivery of the transgene to a sufficient number of cells implicated in the disease. In most cases, the blood–brain barrier limits the penetration of the vector from the periphery, and direct injections into precise regions of the brain relies on local diffusion of the vector throughout the parenchyma. Unfortunately, direct injections are not amenable to motor neuron (MN) disorders, such as spinal muscular atrophy and amyotrophic lateral sclerosis (ALS), the affected cells being located across the entire length of the spinal cord.
ALS is the most common adult paralytic disorder and is characterized by the degeneration of MNs in the spinal cord and brain. No effective treatment exists with death ensuing 3 to 5 years after diagnosis as a result of respiratory failure. One tenth of ALS cases are familial with 20% of those caused by mutations in the gene encoding for superoxide dismutase 1 (SOD1).1 Transgenic mice overexpressing mutant forms of the human SOD1 gene faithfully recapitulate the human disease and are widely used in preclinical ALS studies.
Several vectors have been used to deliver transgenes to the familial ALS (fALS) rodents to result in efficient and stable expression of a therapeutic transcript. Direct injections of adeno-associated virus (AAV) have been used to deliver trophic factors2 and antiapoptotic proteins3 directly to the affected MN environment. Lentivirus has also been used to deliver small hairpin RNAs (shRNAs) to knockdown levels of the mutant SOD1 (mSOD1) protein following spinal cord injection.4 These approaches resulted in preservation of MNs and neuronal function, however, did not extend survival in the animal.
To overcome the limited spread of transduction after intraparenchymal delivery and translate local neuroprotection into functional benefits, an alternate administration route has been essayed that takes advantage of the retrograde transport capacity endowed to MNs. Through a simple intramuscular injection, the viral vector (and/or transgenic protein) can be taken up by the nerve terminals and transported along the axon to the MN soma within the spinal cord. This allows targeting across the entire spinal cord through relatively noninvasive peripheral injections. Adenoviral, AAV and rabies-pseudotyped lentiviral vectors have been used to deliver trophic factors such as glial cell-derived neurotrophic factor,5,6 insulin-like growth factor 1,7 cardiotrophin-1,8 and vascular endothelial growth factor9 to MNs from the periphery to result in increases in lifespan of the fALS model. Most notably, neonate injections of equine infectious anemia virus (EIAV) expressing shRNA against mSOD1 resulted in a 100-day increase in animal survival, the greatest observed in the mSOD1 mouse model to date.10
The aim of the present study was to examine recombinant AAV (rAAV) vectors expressing shRNA for silencing of mSOD1 in the same paradigm. AAV vectors constitute a promising system for in vivo clinical applications, due to their neuronal tropism, stable transgene expression in quiescent cells, low pathogenicity, and poor rate of integration in the host genome.11,12 Not surprisingly, all except one of the 18 current or completed gene therapy clinical trials for the neurodegenerative diseases have chosen rAAV vectors. The serotype 6 vector was selected after our recent finding that this vector can efficiently transduce MNs following intramuscular injections in nonhuman primates.13 We have first performed restricted neonate muscle injections of the rAAV serotype 6 and characterized the effect of mSOD1 knockdown on disease pathogenesis within muscle, neuromuscular connectivity, MNs and the neighboring glial cells within a single MN pool. We have then asked whether the local neuroprotection observed within an individual pool of MNs can be translated to therapeutic benefit in the fALS mouse by scaling up injections across the musculature.
Results
AAV:shSOD1 transduces MNs after neonatal intramuscular delivery and silences mSOD1 in vivo
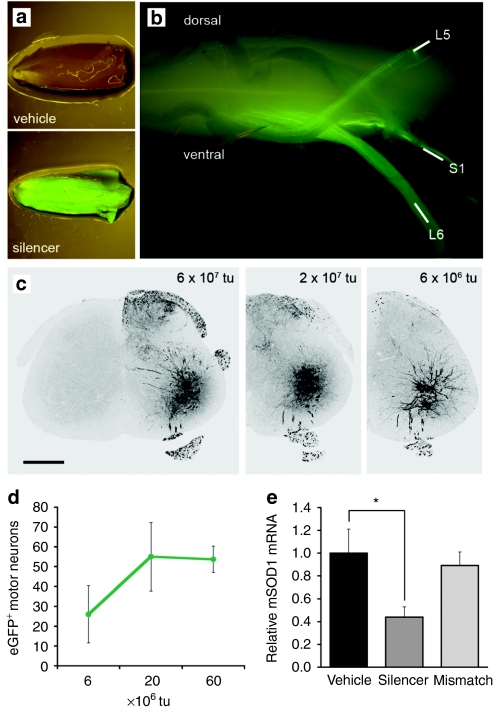
We first sought to determine whether single-stranded rAAV serotype 6 vectors were capable of retrograde transport from peripheral muscles along mouse MN axons. Vectors were generated that drive expression of enhanced green fluorescent protein (eGFP) and shRNAs directing the degradation of mSOD1 mRNA transcripts (AAV:shSOD1).14 Different doses of the vector were injected unilaterally into the triceps surae muscle (comprising the medial and lateral gastrocnemius and soleus) of neonate fALS mice and animals were killed 5 weeks after. All vector doses resulted in eGFP epifluorescence in the injected muscle (Figure 1a) and ipsilateral ventral roots of spinal cord that constitute the efferent MN fibers (Figure 1b). Intense native eGFP fluorescence was observed in the ventral horn of the spinal cord (Figure 1c) and corresponded to MNs as confirmed by vesicular acetylcholine transporter (VAChT) colabeling (Supplementary Figure S1). Neither upper MNs in the cortex nor neighboring glia and interneurons in the spinal cord expressed the reporter protein, confirming that central nervous system transduction did not occur further than the MNs extending processes to the site of injection. MN transduction was prominent between L5 and S1 of the spinal cord corresponding to transduction of the sural nerve. MN infection was dose-dependent and plateaued above 2 × 107 transducing units of vector (Figure 1d), presumably corresponding to retrograde transduction of the entire pool of MNs innervating the triceps surae. This dose was chosen for subsequent experiments.
Figure 1.
Retrograde transport of rAAV serotype 6 from the triceps surae muscle to lumbar MNs and subsequent silencing of mSOD1. (a) eGFP epifluorescence in skeletal muscle and (b) ventral roots of the spinal cord after injection of 2 × 107 tu of AAV:shSOD1 into the triceps surae. (c) Native eGFP expression in the ventral horn after injection of different viral loads. Bar = 500 µm. (d) Dose response of MN transduction across every sixth section of lumbar spinal cord after intramuscular delivery (n = 6 per group). (e) Relative levels of mSOD1 mRNA in laser-captured MNs normalized against GAPDH and actin as determined by reverse transcription qPCR (n = 4 per group). *P < 0.05 between AAV:shSOD1 and vehicle-injected animals. eGFP, enhanced green fluorescent protein; MN, motor neuron; mSOD1, mutant superoxide dismutase 1; qPCR, quantitative PCR; rAAV, recombinant adeno-associated virus; tu, transducing units.
Laser-capture microdissection on cord sections was performed to examine whether transduction resulted in mSOD1 knockdown within MNs (Figure 1e). Native eGFP expression within the neurons facilitated the laser capture of solely transduced cells (Supplementary Figure S2). Reverse transcription quantitative PCR revealed a 56 ± 8% reduction of the human SOD1 transcript in MNs transduced with the AAV:shSOD1 vector, when compared to uninfected samples (P < 0.05). In contrast, no significant reduction was observed with the mismatch vector (AAV:shSOD1mis), that contains two substituted base pairs in the shRNA sequence.
Delivery of AAV:shSOD1 into the triceps surae protects the muscle from mSOD1-mediated atrophy
To study the effects of silencing mSOD1 within an individual pool of MNs, neonatal fALS mice were bilaterally injected in the triceps surae with 2 × 107 transducing units of AAV:shSOD1 (n = 8), AAV:shSOD1mis (n = 8), or vehicle (n = 8). Animals were analyzed at 110 days of age as this time point compromises between the measurable deleterious effects at the level of skeletal muscle, neuromuscular junction (NMJ), MN viability, and spinal cord gliosis.15 Littermates not containing the mSOD1 transgene were also injected with either AAV:shSOD1 (n = 4) or AAV:shSOD1mis (n = 4) as controls.
Hematoxylin and eosin staining revealed that AAV:shSOD1-injected muscles had similar morphology to wild-type littermates whereas the vehicle and AAV:shSOD1mis groups had smaller fibers interspersed with more satellite cells (Figure 2a). The myofiber size profile of the AAV:shSOD1-injected muscles was almost identical to that for the control mice (Figure 2b), with average fiber area of 1,386 ± 140 µm2 compared to 1,401 ± 103 µm2, respectively (Figure 2c). Vehicle and AAV:shSOD1mis had significantly smaller myofibers than the AAV:shSOD1 muscles (P < 0.05). This protection from myofiber atrophy was further supported by the wet weight of the whole triceps surae with AAV:shSOD1-injected muscle weighing 140 ± 8 mg compared to vehicle and AAV:shSOD1mis-injected muscles weighing 110 ± 5 and 105 ± 8 mg, respectively (P < 0.05) (Figure 2d).
Figure 2.
Protection from mSOD1-mediated muscle atrophy after AAV:shSOD1 delivery to triceps surae. (a) Hematoxylin/eosin staining in triceps surae at 110 days after neonatal intramuscular delivery of 2 × 107 tu of AAV:shSOD1 (silencer), AAV:shSOD1mis (mismatch) or vehicle. AAV-injected wild-type littermates serve as controls (WT). Bar = 100 µm. (b) Muscle fiber area expressed as a percentage of the (b) total myofibers or (c) average for the different cohorts of animals (n = 8 per group). (d) Wet weight of triceps surae. (e) Immunoblot against mSOD1 demonstrating efficient knockdown of mSOD1 in triceps surae at 110 days. eGFP detection reveals equal levels of transduction in silencer and mismatch groups. *P < 0.05 between AAV:shSOD1 and control vehicle/mismatch-treated animals. AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; MN, motor neuron; mSOD1, mutant superoxide dismutase 1; tu, transducing units.
As muscle-specific mSOD1 expression has been shown to cause muscle atrophy in transgenic mice,16 we examined whether mSOD1 knockdown also occurred in the muscle. As expected, AAV:shSOD1 reduced levels of mSOD1 protein in the triceps surae by 83 ± 9% at 110 days compared to vehicle-injected muscles, whereas AAV:shSOD1mis did not (Figure 2e) (P < 0.05).
mSOD1 silencing within the neuromuscular unit confers neuroprotection to the transduced MN pool
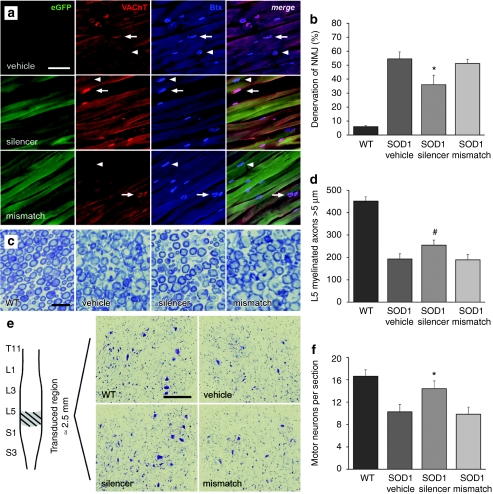
The effect of mSOD1 knockdown within the triceps surae and innervating MNs on NMJ denervation was determined by bungarotoxin (endplate) and VAChT (nerve terminal) colocalization (Figure 3a). As the triceps surae is composed of muscle subtypes that are innervated by MNs either resistant (slow twitch; soleus) or vulnerable to denervation (fast twitch; medial and lateral gastrocnemius), careful attention was made to quantify only sensitive NMJs in the fALS mice. The characteristic “zigzag” pattern of innervation as described by Pun et al.17 was used to discern the NMJs in the medial and lateral gastrocnemius from the resistant pools of the underlying soleus (Supplementary Figure S3). We observed less denervation in AAV:shSOD1-injected muscles (36 ± 7%) compared to vehicle-injected (55 ± 5%) and AAV:shSOD1mis-injected (51 ± 3%) muscles (Figure 3b) (P < 0.05). Wild-type AAV-injected littermates bared only slight denervation (6 ± 1%), suggesting that vector delivery and expression did not alter NMJ innervation.
Figure 3.
Neuroprotective effects of silencing mSOD1 on neuromuscular connections and MN survival in the triceps surae neuronal pool. (a) Immunostaining and (b) quantification of neuromuscular junctions (NMJs) in vulnerable innervation regions of the triceps surae at 110 days after neonatal intramuscular delivery of AAV:shSOD1 (silencer), AAV:shSOD1mis (mismatch) or vehicle. Arrows and arrowheads give examples of innervated and denervated NMJs, respectively. *P < 0.05 compared to vehicle-injected animals. (c) Light micrographs of toluidine blue-stained L5 ventral roots and (d) quantification of large myelinated motor fibers (≥5 µm). (e) MNs were quantified by analysis of 20 Nissl-stained cryosections taken from the transduced region of lumbar spinal cord. (f) Software-based determination of average MN numbers per section for the different cohorts of animals. *P < 0.05 between AAV:shSOD1 and control vehicle/mismatch-treated animals. #P = 0.07/0.06 between AAV:shSOD1 and vehicle/mismatch-treated SOD1 mice. Bar = (a) 200 µm, (c) 15 µm, (e) 250 µm. AAV, adeno-associated virus; Btx, bungarotoxin; eGFP, enhanced green fluorescent protein; MN, motor neuron; mSOD1, mutant superoxide dismutase 1; VAChT, vesicular acetylcholine transporter; WT, wild type.
A marginal delay in peripheral neuropathogenesis was also observed among MN axons, with L5 ventral roots of AAV:shSOD1-injected animals containing 255 ± 23 large myelinated fibers (≥5 µm) compared to 193 ± 24 in the vehicle-injected group (Figure 3c,d) (P = 0.07). This relatively mild effect is no doubt diluted by the presence of degenerating axons in other nerves that were not targeted by the vector.
The entire lumbar cord was sectioned and the zone of MN transduction was ascertained by native fluorescence (Supplementary Figure S4). MN transduction extended across >3 mm of the spinal cord, targeting up to 40% of MNs in some sections. Twenty sections from the zone of high transduction (≈2.5 mm) from each animal were stained with cresyl violet and Metamorph was used to quantify MN numbers (Figure 3e). AAV:shSOD1-injected animals contained 14.4 ± 1.4 MNs/section in the transduced region compared to vehicle and AAV:shSOD1mis animals that had 10.3 ± 1.3 and 9.8 ± 1.3 MNs/section, respectively (Figure 3f) (P < 0.05). Interestingly, the AAV:shSOD1 animals had only a few less MNs per section compared to wild-type littermates (16.6 ± 1.1 MNs/section). This MN loss compared to the wild-type littermates can be explained by the presence of adjacent MNs innervating hindlimb muscles that were not injected with the vector.
Neuroprotection was further exemplified by quantifying cell loss only within the transduced MN pool. This was achieved by comparing the number of eGFP+ MNs remaining at 110 days in the fALS mice with the control littermates for the AAV:shSOD1 and AAV:shSOD1mis vectors (Supplementary Figure S5). While only 33 ± 3 eGFP+ MNs remained in the fALS mice compared to 55 ± 3 eGFP+ MNs in the control littermates injected with the mismatch vector, the same number of eGFP+ MNs were present in both the fALS mice and control littermates for the silencer vector (60 ± 3 and 61 ± 3, respectively). This suggests that there was near complete protection from MN loss at the 110-day time point.
mSOD1 silencing within the neuromuscular unit does not alter the toxic spinal cord microenvironment
As evidence suggests there exists a complex interplay between different cell populations within fALS spinal cord, we examined whether selective knockdown of mSOD1 within MNs altered the activation of neighboring glial cells. Metamorph was used to measure the fluorescent integrated intensity of eGFP+ MN-containing spinal cord sections interrogated with antibodies against the astrocyte marker, glial fibrillary acidic protein (Figure 4a,b) or the microglial marker, ionized calcium-binding adaptor molecule 1 (Figure 4c,d). No significant difference was observed between the groups of fALS mice suggesting that the mSOD1 knockdown within MNs did not alter local inflammation. Likewise, cord sections from AAV:shSOD1 had similar levels of fluorescent staining against ubiquitin compared to vehicle and AAV:shSOD1mis mice, suggesting that the gene therapy strategy did not alter local ubiquitin deposition (Figure 4e,f).
Figure 4.
No effect of silencing mSOD1 on inflammation or ubiquitin deposition in the vicinity of mSOD1-silenced MNs. Immunofluorescence (left panel) and integrated intensity (right panel) in the L5 spinal cord ventral horn using antibodies against astrocyte activation, (a,b) glial fibrillary acidic protein (GFAP), activated microglia, (c,d) ionized calcium-binding adaptor molecule 1 (Iba1), and (e,f) ubiquitin. No significant difference was observed between AAV:shSOD1 and control fALS groups. *P < 0.05 between wild-type animals and all SOD1 cohorts of mice. Bar = 80 µm. AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; fALS, familial amyotrophic lateral sclerosis; MN, motor neuron; mSOD1, mutant superoxide dismutase 1; WT, wild type.
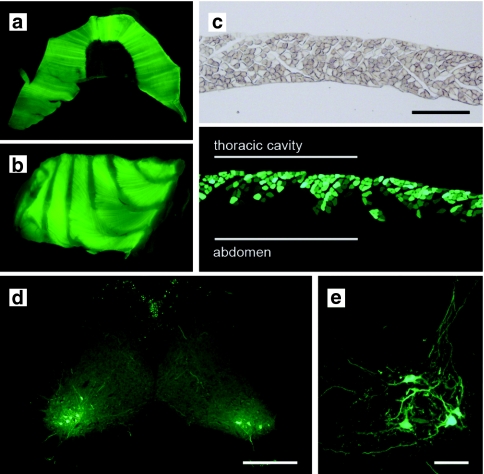
AAV:shSOD1 transduces phrenic nerve MNs after injection into the thoracic cavity
We speculated that knockdown of mSOD1 in MNs controlling breathing would be required to translate the positive effects in the triceps surae neuron pool to therapy for the fALS mice. As targeting the diaphragm by direct injection would be difficult considering the poor accessibility and membranous nature of the muscle, AAV:shSOD1 (8 × 107 transducing units) was delivered into the thoracic cavity as described by Mah et al.18 This resulted in whole tissue eGFP epifluorescence of both diaphragm and intercostals muscles (Figure 5a,b). Cryosections of the diaphragm revealed that this eGFP expression resulted from transduction of myofibers within the most superficial layers (Figure 5c). We next asked whether this passive mode of vector administration facilitated MN transduction. eGFP+ cells were observed in the cervical spinal cord ventral horn (Figure 5d,e) and corresponded to MNs of the phrenic nerve. Transduced cells were also present in the thoracic spinal cord and may have arisen through infection of neurons innervating the intercostal muscles.
Figure 5.
Transduction of the diaphragm and cervical MNs after intrathoracic delivery of AAV:shSOD1. eGFP epifluorescence in the (a) diaphragm and (b) intercostal muscles after injection of 8 × 107 tu of AAV:shSOD1 into the thoracic cavity. (c) Transverse cryosections of diaphragm revealing transduction of the thoracic surface of the muscle. (d,e) eGFP expression in the ventral horn of cervical spinal cord corresponding to MNs of the phrenic nerve. Bar = (c) 200 µm, (d) 400 µm, (e) 50 µm. AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; MN, motor neuron; tu, transducing units.
Multiple injections of AAV:shSOD1 transduce MN across the entire spinal cord
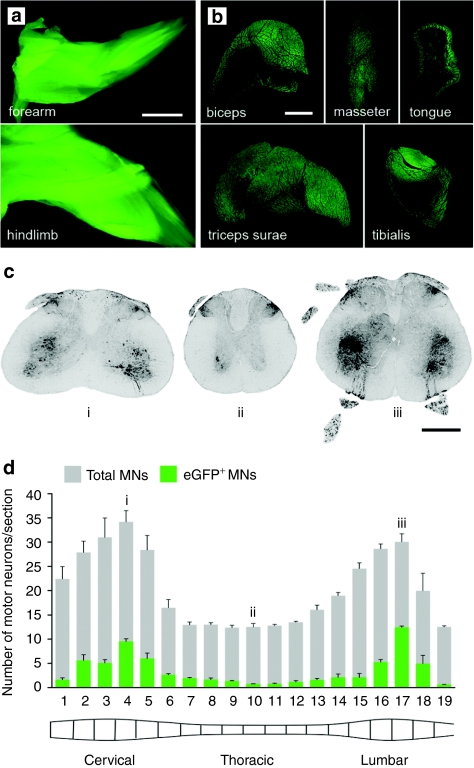
As an attempt to translate intramuscular delivery of AAV:shSOD1 vectors into functional improvements, multiple injections were performed to scale up the MN transduction to the whole spinal cord and brain stem. Neonate mice were injected in hindlimb, forelimb, intercostal and facial muscles and within the thoracic cavity. The tongue was injected at P15 to avoid impaired breathing that can result from neonatal injections. A dose of vector was used for each muscle relative by approximate muscle size to the optimal triceps surae dose and animals were killed 6 weeks later (n = 6) (Supplementary Table S1). Epifluorescence of the entire forearm and hindlimb revealed ubiquitous transgene expression across the muscles (Figure 6a). Transverse sections of muscle had between ~50% and near total transduction of myofibers, varying randomly between muscle groups and animals (Figure 6b).
Figure 6.
MN transduction profile after AAV:shSOD1 delivery to multiple muscle groups and the thoracic cavity. Efficient transduction of skeletal muscles controlling movement and feeding after multiple muscle injections as determined by (a) whole tissue epifluorescence and (b) cryosections. (c) Native eGFP signal in representative (i) cervical, (ii) thoracic, and (iii) lumbar spinal cord sections 6 weeks after intramuscular deliveries. (d) Longitudinal transduction profile across entire spinal cord expressed as eGFP+ MNs against the total number of MNs per section as determined by VAChT staining. Bar = (a) 3 mm; (b) 1 mm; (c) 150 mm. AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; MN, motor neuron.
MNs positive for eGFP were prominent in the cervical and lumbar enlargements, and to a lesser extent in the thoracic cord (Figure 6c). Every sixth cryosection from the entire spinal cord was labeled with VAChT to facilitate a systemic quantification of transduced MNs (Figure 6d). Bilateral counts revealed infection rates between 5 and 40%, with peak percentage of transduced MNs occurring in cervical and lumbar sections.
Multiple injections of AAV:shSOD1 fails to improve motor performance or increase survival in the fALS mouse model
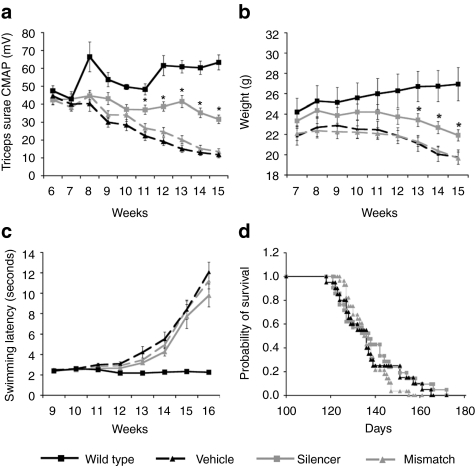
AAV:shSOD1 was delivered to multiple muscle groups as described above to determine whether the local neuroprotection afforded by mSOD1 knockdown in the triceps surae motor unit could be translated to functional improvements in the fALS mice. Cohorts were large (n ≥ 20), litter-matched and gender-balanced to ensure that apparent effects were not due to experimental noise.19 AAV:shSOD1 therapy considerably delayed the loss of compound muscle action potential (CMAP) within the triceps surae compared to AAV:shSOD1mis and vehicle-treated animals (Figure 7a) (P < 0.001). Mice injected with the vehicle or control vector had ~80% loss of normal CMAP (21 ± 3 and 19 ± 3% relative to control animals, respectively) whereas AAV:shSOD1 animals had only a 50 ± 4% reduction. AAV:shSOD1 mice were also significantly heavier than AAV:shSOD1mis and vehicle-treated animals (P < 0.01) although all groups had similar weight profiles in terms of onset and rate of decline (Figure 7b).
Figure 7.
No therapeutic effect on motor performance or survival after multiple injections of AAV:shSOD1. Multiple muscle deliveries and thoracic injection of either AAV:shSOD1 (silencer, n = 21), AAV:shSOD1mis (mismatch, n = 20), or vehicle (n = 28) were administered to neonatal fALS mice. Animals were evaluated weekly from adulthood for (a) evoked compound muscle action potential (CMAP) in the triceps surae, (b) weight change, and (c) swimming ability. (a) *P < 0.001; (b) *P < 0.05. (d) No effect on survival was observed between the groups. AAV, adeno-associated virus; fALS, familial amyotrophic lateral sclerosis; SOD1, mutant superoxide dismutase 1.
The appearance and progression of motor deficits was determined by measuring swimming velocity. Despite increased CMAP and weight, AAV:shSOD1-injected animals did not perform better than the other fALS cohorts in this test (Figure 7c). Survival was also unaltered between the different groups of mice (Figure 7d) (silencer, 138 ± 3 days; mismatch, 137 ± 3 days; vehicle, 136 ± 2 days; P = 0.8; χ2 = 0.5) demonstrating that the gene therapy strategy did not improve behavioral decline in the fALS mice.
Discussion
The present study examined the ability of rAAV serotype 6 to deliver mSOD1 silencing instructions to vulnerable MN pools from the periphery in a manner amenable to the clinic. We found that the AAV:shSOD1 vector reduced mSOD1 levels in skeletal muscle and MNs, conferring neuroprotection to the transduced vulnerable neurons as well as halting mSOD1-mediated muscle atrophy. Despite this favorable outcome within an individual MN pool, our attempt to scale up the gene therapy across the lower motor neuraxis did not alter the onset or the course of disease in the fALS mice.
Neonate muscle injections of rAAV serotype 6 resulted in complete protection from muscle wasting. AAV:shSOD1 transduced myotubes were larger and had less satellite cells than control fALS mice and were almost indistinguishable from wild-type littermates in terms of morphology. Whether this protection arose through reduction of mSOD1 levels in the muscle or the innervating neuron pool remains uncertain. Evidence suggests that mSOD1 action within skeletal muscle does not contribute directly to MN degeneration in fALS rodent models.14,20 However, this does not exclude a role for mSOD1 in damage to the muscle itself. A number of studies have elucidated a role of oxidative stress within myofibers leading to muscle atrophy.21,22 Indeed, transgenic mice with muscle-restricted mSOD1 expression develop muscle weakness and atrophy through oxidative stress-induced autophagy, independent of MN degeneration.16 This suggests that knockdown of mSOD1 in muscle may have partially contributed to the protection from muscle atrophy observed here.
Knockdown of mSOD1 in the triceps surae muscle and innervating MN pool also conferred neuroprotection at the 110-day time point. Whole MN counts revealed an ~40% loss in the control fALS mice versus only 14% loss in the AAV:shSOD1 group. This was further demonstrated by the presence of equal numbers of eGFP+ MNs in the fALS and wild-type mice injected with the AAV:shSOD1, alluding to an almost complete protection from MN loss. As excision of the mSOD1 gene selectively in MNs of mSOD1 Cre/Lox mice delayed disease onset in fALS pathogenesis23 it is likely that the neuroprotection observed here was the result of the mSOD1 knockdown within MNs.
Considering the impressive protection from MN loss, there was a relatively mild benefit on neuromuscular connectivity, with less than two thirds of intact NMJ remaining in the AAV:shSOD1 animals. This is not surprising considering that NMJ loss is an early event in disease pathogenesis. Indeed, mSOD1 gene copy numbers correlate with disease outcome in fALS mice24 demonstrating that mSOD1 protein elicits disease in a dose-dependent manner. Perhaps the partial knockdown of mSOD1 merely delayed neurodegeneration in the transduced MN pool, where MN loss was not yet apparent at the selected 110-day time point.
The second aim of this work was to determine whether the observed AAV-mediated neuroprotection within one MN pool could be scaled up to deliver therapeutic benefit for the fALS mice. Multiple muscle injections across the neonate mice resulted in transduction of MNs at all spinal cord levels and within the MN nuclei of the brain stem. MNs controlling breathing were also targeted by intrathoracic delivery of the AAV:shSOD1. The finding that multiple AAV:shSOD1 injections increased the weight of the fALS mice foremost confirms the effect of mSOD1 knockdown in this second cohort of animals. The increase in body mass likely reflects the protection from atrophy in the targeted muscles. Hindlimb CMAP values were also substantially preserved in the AAV:shSOD1 group and may result from the combination of increased NMJ connectivity and, considering that CMAP amplitude is directly proportional to myofiber area, reduced muscle atrophy. Despite these favorable outcomes, and although achieving up to 28 and 41% transduction of MNs in cervical and lumbar spinal cord regions, respectively, onset and progression of motor deficits and survival were not ameliorated in the AAV:shSOD1-treated animals.
The failure of the present gene transfer to increase lifespan suggests that either (i) lower MN protection is not directly coupled to survival in the fALS mouse model and that intervention is required on other cell types or (ii) that the transduction was not sufficiently scaled up to the whole animal and that greater numbers of lower MNs are required for a therapeutic effect. While it is clear that astrocytes and microglia are involved in disease pathogenesis, standard histological examination, and gene excision studies reveal that glial cell activation occurs after disease onset and acts to exacerbate MN degeneration and disease progression.23,25 Indeed, excision of the mSOD1 gene selectively in MNs of mSOD1 Cre/Lox mice delays disease onset23 demonstrating that knockdown of mSOD1 in MNs is sufficient to impact on animal survival. This suggests that scale up of the neuroprotective gene therapy approach from the triceps surae MN pool to the whole animal may have been an issue.
Central to the question of scale up is the discrepancy between the present work and Ralph et al.10 that doubled the lifespan of fALS mice following intramuscular delivery of EIAV. Although EIAV has led to poor retrograde transduction of MNs in our experience, an increased level of transduction with EIAV compared to rAAV serotype 6 could account for the observed difference in therapeutic efficacy between the two studies. The EIAV study reported >50% transduction of lumbar spinal cord MNs which is slightly more than observed here (up to 40% lumbar MNs). A thorough characterization throughout the lower MN axis following EIAV delivery (as in Figure 6d) would clarify this matter and determine if there exists a threshold percentage of MN transduction required for therapeutic benefit. In light of these findings it now seems imperative that future studies are performed in parallel to compare EIAV and AAV in terms of (i) MN retrograde transduction capability and (ii) the ability to knockdown mSOD1 and extend survival in fALS mice. These studies will resolve once and for all whether mSOD1 knockdown within MNs alone is enough to impact on survival in the fALS animal model, and indeed, in human disease.
In conclusion, we have demonstrated that rAAV serotype 6 is capable of delivering shRNA to vulnerable MN pools of fALS mice after peripheral administration. Knockdown of mSOD1 in the triceps surae and innervating MNs led to significant protection from muscle atrophy, NMJ denervation, and MN loss. This serves as proof-of-principle for future studies using the rAAV serotype 6 vector as a tool to knockdown or overexpress proteins with putative roles in ALS pathogenesis. This also demonstrates that ALS pathogenesis can be examined within an individual pool of MNs without having to rely on animal survival as a therapeutic readout, and conversely, that successful neuroprotective strategies may be overlooked if animal survival is the sole therapeutic criterion. Indeed, multiple injections of the vector did not translate the neuroprotection within the triceps surae MN pool to therapeutic benefit in the fALS mice, stressing the complexity of gene delivery to sufficient numbers of MNs and perhaps other cell types in the context of the human disease.
Materials and Methods
rAAV vectors. The engineering and production of the vectors were as described previously.14 Briefly, H1:shRNA and CMV:eGFP expression cassettes were cloned in the pAAV-MCS (Stratagene, La Jolla, CA) and then co-transfected with the pDF6 helper plasmid into 293AAV cells.26 Cell lysates were purified 48 hours later on a heparin affinity column using high-pressure liquid chromatography. Vector transducing units were determined by FACS analysis 72 hours after 293T cell infection.
Animals and vector administration. Mixed background (B6SJL) transgenic mice carrying the human SOD1 gene with the G93A mutation (The Jackson Laboratory, Bar Harbor, ME) were mated with B6SJL/F1 females (Charles River Laboratories, Wilmington, MA). Progenies were genotyped at birth by PCR against human SOD1 and the mouse α-globin gene.4 Injections were performed between P1 and P5 in hindlimb, forelimb, intercostal and facial muscles, and in the thoracic cavity except the tongue that was injected at P15 (Supplementary Table S1). Newborn mice were anesthetized through intraperitoneal injection of 4 µl medetomidine (100 µg/150 µl) and midazolam (250 µg/150 µl) mix. Anesthesia was reversed using 5 µl of antisedan (1 mg/ml). Intramuscular delivery was achieved using a 1 cm × 30G needle from an insulin syringe (Becton Dickinson, Frankin, NJ) attached to a 10-µl Hamilton syringe (Hamilton, Reno, NV) with polyethylene tubing (A-M Systems, Carlsborg, WA).27 Intrathoracic injections were achieved using the same apparatus that was inserted 4-mm deep into left side of the chest between the two lower ribs. A drop of paraffin oil was placed on the chest to aid visualization through the skin. No mortality was observed for the ~50 intrathoracic injections performed in this study. All animal protocols were conducted in accordance with the Swiss legislation on animal experimentation.
Quantification of mSOD1 knockdown in transduced MNs. Mice were deeply anesthetized using sodium pentobarbital and decapitated. Lumbar spinal cord was dissected and frozen in cryoembedding fluid on dry ice. Cryosections were taken at 14 µm, collected on membrane slides and dehydrated in ethanol. Approximately 100 transduced MN sections were microdissected per animal using an MMI CellCut laser-dissection microscope (MMI, Glattbrugg, Switzerland). Captured cells were covered with extraction buffer (Arcturus, Mountain View, CA) and total cellular RNA isolated with the PicoPure RNA isolation kit (Arcturus). Complementary DNA was generated from the isolated RNA using StrataScript First-Strand Synthesis System (Stratagene). Relative amounts of mSOD1 transcripts were determined by SYBR Green (Stratagene) quantitative PCR using QuantiTect Primer Assays (Qiagen, Valencia, CA) against human SOD1 (QT01671551) and normalized with mouse β-actin (QT00095242), and mouse glyceraldehyde-3-phosphate dehydrogenase (QT01658692). Amplifications were performed using the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Dissociation curves confirmed amplification of solely one PCR product for all quantitative PCR.
Histological analysis. Mice were anesthetized and transcardially perfused with 4% paraformaldehyde. Tissues were dissected and postfixed for 4 hours at room temperature after which they were transferred to 30% sucrose in phosphate-buffered solution and placed at 4 °C overnight. Whole tissue epifluorescence was observed using a Leica MZFLIII fluorescent stereomicroscope (Leica Microsystems, Wetzlar, Germany) attached to a Leica DFC 300 FX camera before cryoembedding. For the analysis of muscle atrophy at disease onset, mice were processed as above except that one triceps surae was dissected, weighed on a fine balance, covered in cryoembedding fluid and snap frozen in liquid-nitrogen-cooled isopentane before the transcardial perfusion of the animal.
Skeletal muscle atrophy was examined by hematoxylin and eosin staining of transverse 7 µm cryosections from the unfixed triceps surae. Three sections were photographed from each animal and the area of 100 individual myofibers was measured using ImageJ. The remaining portion of muscle was then thawed in lysis buffer and processed for immunoblot against mSOD1 as described previously.14
NMJs were quantified by immunolabeling longitudinal 25 µm cryosections from the fixed triceps surae. Sections were blocked with 10% normal goat serum and 0.1% Triton X-100 in phosphate-buffered solution for 1 hour at 37 °C and then incubated overnight at 4 °C with rabbit anti-VAChT (1:1,000; Sigma-Aldrich, St Louis, MO) in blocking solution. Samples were washed with phosphate-buffered solution and interrogated with Cy3-conjugated goat anti-rabbit IgG Ab (1:1,000; Jackson ImmunoResearch Laboratories, West Grove, PA) and Alexa Fluor 647-conjugated bungarotoxin (1:100; Invitrogen; Molecular Probes, Carlsbad, CA) in phosphate-buffered solution. Samples were then rinsed and mounted in Mowiol. Sections were viewed using a Leica DM5500 upright fluorescent microscope (Leica Microsystems). More than 100 endplates (bungarotoxin) were scored for each animal as either innervated (VAChT+) or denervated (VAChT–).
L5 ventral roots were postfixed in 2.5% glutaraldehyde overnight and incubated for 4 hours in 2% osmium tetroxide (Sigma-Aldrich), 50 mmol/l sodium cacodylate before embedding in resin. 2 µm transverse cross-sections were stained with toluidine-blue, photographed at high magnification and the number of myelinated fibers with diameter ≥5 µm determined using ImageJ.
MN protection was assessed using MetaMorph (Universal Imaging, Downingtown, PA). The entire lumbar spinal cord was cryosectioned free-floating at 25 µm and every eighth section was mounted to identify the region of transduction via native eGFP fluorescence. Every fourth section from the transduced zone (20 sections in total) were then Nissl stained and mounted for each animal. The regions mounted from vehicle-injected animals were identified by matching the spinal cord morphology from the vector-injected animals. Single bright field images of entire glass slide containing eight sets of spinal cords were taken using the tile function of the Leica DM5500. The images were then opened in Metamorph and ventral horns selected as regions. Following application of a threshold to remove background staining, the software was used to quantify MNs by counting all objects within the region that were ≥250 µm2 and with a circularity ≥0.2. MetaMorph was also used to determine integrated intensity from glia and ubiquitin staining on spinal cord sections. Six spinal cord sections were taken at random from the transduction zone and were fluorescently stained with rabbit anti-glial fibrillary acidic protein (1:1,000; DakoCytomation, Glostrup, Denmark), rabbit anti-ionized calcium-binding adaptor molecule 1 (1:100; Wako Pure Chemical Industries, Osaka, Japan) or rabbit anti-ubiquitin (1:1,000; DakoCytomation) using the Cy3-conjugated goat anti-rabbit IgG Ab as described above. All sections from each antibody configuration were mounted on the same slide which were then photographed in the TRITC channel using the tile function of the Leica DM5500. The images were then opened in Metamorph, ventral horns selected and integrated intensity quantified. Integrated intensity from sections without the primary antibody was used to normalize signal intensities.
MN transduction after multiple muscle and thoracic delivery was quantified by counting the total number of VAChT+ cells and the total number of eGFP+ and VAChT+ cells from every sixth section throughout the spinal cord from six animals.
Behavioral testing and electromyography. Cohorts used for behavioral experiments were gender-balanced and litter-matched. Mice were evaluated weekly from 6 weeks of age for weight, swimming ability, and CMAP. The swimming tank test consists of measuring the time for a mouse to transverse a water-filled tank across a distance of 1 m.4 Evoked CMAP amplitude in the triceps surae was evaluated using the Keypoint electromyogram apparatus (Dantec Dynamis, Bristol, UK) as described previously.3 Death was determined at the time where mice could no longer rear themselves within 20 seconds of being placed on their sides. All animal experiments were performed in a blinded manner.
Statistical analysis. The relative level of mSOD1 mRNA within MNs, average myofiber area, wet muscle mass, NMJ denervation, myelinated axons, number of MNs, and integrated intensity measurements between the different groups of mice were subject to a one-way analysis of variance followed by a Newman–Keuls post hoc test. CMAP, weight and swimming latency were analyzed using a two-way (group × time) repeated measures analysis of variance followed by a Duncan multiple range post hoc test. Disease survival was plotted on Kaplan–Meier curves and analyzed using the Mantel–Haenszel log-rank test. All statistics were performed using Statistica (Statsoft, Maisons-Alfort, France). Data is expressed in mean ± SE of the mean (SEM).
SUPPLEMENTARY MATERIAL Figure S1. MN-specific transduction after rAAV intramuscular delivery. Colocalization of eGFP+ cells in lumbar spinal cord with the motor neuron marker, vesicular acetylcholine transporter, VAChT, after intramuscular injection of AAV:shSOD1. Arrows indicate double labeled cells. Scale bar = 50 μm. Figure S2. Laser-capture of transduced cells. Spinal cord sections before and after laser-capture microdissection of eGFP+ cells. Scale bar = 60 μm. Figure S3. Neuromuscular junction quantification on the triceps surae muscle. Fluorescent labeling using Alexa Fluor 647-conjugated bungarotoxin (647-Btx) (green channel) and immunodetection against vesicular acetylcholine transporter (VAChT) (red channel). Left panel: Tile reconstruction of whole triceps surae longitudinal section demonstrating “zigzag” pattern of end plate (647-Btx) across the tissue. Scale bar = 1 mm. Right panels: Higher magnification of end plate staining (647-Btx) with corresponding nerve terminal (VAChT) in a fALS mouse with partial denervation. Scale bar = 50 μm. Figure S4. MN transduction profile after bilateral AAV:shSOD1 delivery into the triceps surae. Longitudinal transduction rate across lumbar spinal cord expressed as eGFP+ MNs against the total number of MNs per section as determined by VAChT staining. eGFP+ MNs were counted from every sixth section. Figure S5. MN protection as demonstrated by remaining eGFP+ cells. Infected MNs remaining at 110 days in wild-type and fALS mice after bilateral injections of AAV:shSOD1 or AAV:shSOD1mis into the triceps surae muscles. eGFP+ MNs were counted from every sixth section. *P < 0.05 between AAV:shSOD1 and AAV:shSOD1mis-injected fALS mice. Table S1. Vector dose per muscle used for multiple injection study.
Acknowledgments
The authors thank Smita Saxena, Pico Caroni, Artem Kaplan, Philippe Colin, Elisabeth Dirren, Dairin Kieran, Vivianne Padrun, Fabienne Pidoux, Christel Sadeghi, and Juan-Carlos Sarria for their expert technical assistance. This work was supported by the ALS Association (ALSA), the Sixth Research Framework Programs of the European Union, Project RIGHT (LSHB-CT-2004 005276), and Project NeuroNE (LSHM-CT-2004-512039). The authors declared no conflict of interest.
Supplementary Material
MN-specific transduction after rAAV intramuscular delivery. Colocalization of eGFP+ cells in lumbar spinal cord with the motor neuron marker, vesicular acetylcholine transporter, VAChT, after intramuscular injection of AAV:shSOD1. Arrows indicate double labeled cells. Scale bar = 50 μm.
Laser-capture of transduced cells. Spinal cord sections before and after laser-capture microdissection of eGFP+ cells. Scale bar = 60 μm.
Neuromuscular junction quantification on the triceps surae muscle. Fluorescent labeling using Alexa Fluor 647-conjugated bungarotoxin (647-Btx) (green channel) and immunodetection against vesicular acetylcholine transporter (VAChT) (red channel). Left panel: Tile reconstruction of whole triceps surae longitudinal section demonstrating “zigzag” pattern of end plate (647-Btx) across the tissue. Scale bar = 1 mm. Right panels: Higher magnification of end plate staining (647-Btx) with corresponding nerve terminal (VAChT) in a fALS mouse with partial denervation. Scale bar = 50 μm.
MN transduction profile after bilateral AAV:shSOD1 delivery into the triceps surae. Longitudinal transduction rate across lumbar spinal cord expressed as eGFP+ MNs against the total number of MNs per section as determined by VAChT staining. eGFP+ MNs were counted from every sixth section.
MN protection as demonstrated by remaining eGFP+ cells. Infected MNs remaining at 110 days in wild-type and fALS mice after bilateral injections of AAV:shSOD1 or AAV:shSOD1mis into the triceps surae muscles. eGFP+ MNs were counted from every sixth section. *P < 0.05 between AAV:shSOD1 and AAV:shSOD1mis-injected fALS mice.
Vector dose per muscle used for multiple injection study.
REFERENCES
- Bruijn LI, Miller TM., and, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Franz CK, Federici T, Yang J, Backus C, Oh SS, Teng Q, et al. Intraspinal cord delivery of IGF-I mediated by adeno-associated virus 2 is neuroprotective in a rat model of familial ALS. Neurobiol Dis. 2009;33:473–481. doi: 10.1016/j.nbd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P., and, Büeler H. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Genet. 2000;9:803–811. doi: 10.1093/hmg/9.5.803. [DOI] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, et al. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13:1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar BK, Lladó J, Sherkat N, Rothstein JD., and, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Bordet T, Lesbordes JC, Rouhani S, Castelnau-Ptakhine L, Schmalbruch H, Haase G, et al. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum Mol Genet. 2001;10:1925–1933. doi: 10.1093/hmg/10.18.1925. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- Daya S., and, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel RJ, Manfredsson FP, Foust KD, Rising A, Reimsnider S, Nash K, et al. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther. 2006;13:463–483. doi: 10.1016/j.ymthe.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Towne C, Schneider BL, Kieran D, Redmond DE., Jr, and, Aebischer P. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17:141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- Towne C, Raoul C, Schneider BL., and, Aebischer P. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol Ther. 2008;16:1018–1025. doi: 10.1038/mt.2008.73. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L., and, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Mah C, Fraites TJ, Jr, Cresawn KO, Zolotukhin I, Lewis MA., and, Byrne BJ. A new method for recombinant adeno-associated virus vector delivery to murine diaphragm. Mol Ther. 2004;9:458–463. doi: 10.1016/j.ymthe.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Miller TM, Kim SH, Yamanaka K, Hester M, Umapathi P, Arnson H, et al. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:19546–19551. doi: 10.1073/pnas.0609411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, et al. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS ONE. 2009;4:e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez de Aguilar JL, Niederhauser-Wiederkehr C, Halter B, De Tapia M, Di Scala F, Demougin P, et al. Gene profiling of skeletal muscle in an amyotrophic lateral sclerosis mouse model. Physiol Genomics. 2008;32:207–218. doi: 10.1152/physiolgenomics.00017.2007. [DOI] [PubMed] [Google Scholar]
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, et al. Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res. 2004;130:7–15. doi: 10.1016/j.molbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Kay MA., and, Kleinschmidt JA. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol Ther. 2003;7:839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Towne C., and, Aebischer P. Lentiviral and adeno-associated vector-based therapy for motor neuron disease through RNAi. Methods Mol Biol. 2009;555:87–108. doi: 10.1007/978-1-60327-295-7_7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MN-specific transduction after rAAV intramuscular delivery. Colocalization of eGFP+ cells in lumbar spinal cord with the motor neuron marker, vesicular acetylcholine transporter, VAChT, after intramuscular injection of AAV:shSOD1. Arrows indicate double labeled cells. Scale bar = 50 μm.
Laser-capture of transduced cells. Spinal cord sections before and after laser-capture microdissection of eGFP+ cells. Scale bar = 60 μm.
Neuromuscular junction quantification on the triceps surae muscle. Fluorescent labeling using Alexa Fluor 647-conjugated bungarotoxin (647-Btx) (green channel) and immunodetection against vesicular acetylcholine transporter (VAChT) (red channel). Left panel: Tile reconstruction of whole triceps surae longitudinal section demonstrating “zigzag” pattern of end plate (647-Btx) across the tissue. Scale bar = 1 mm. Right panels: Higher magnification of end plate staining (647-Btx) with corresponding nerve terminal (VAChT) in a fALS mouse with partial denervation. Scale bar = 50 μm.
MN transduction profile after bilateral AAV:shSOD1 delivery into the triceps surae. Longitudinal transduction rate across lumbar spinal cord expressed as eGFP+ MNs against the total number of MNs per section as determined by VAChT staining. eGFP+ MNs were counted from every sixth section.
MN protection as demonstrated by remaining eGFP+ cells. Infected MNs remaining at 110 days in wild-type and fALS mice after bilateral injections of AAV:shSOD1 or AAV:shSOD1mis into the triceps surae muscles. eGFP+ MNs were counted from every sixth section. *P < 0.05 between AAV:shSOD1 and AAV:shSOD1mis-injected fALS mice.
Vector dose per muscle used for multiple injection study.