Abstract
The administration of antisense oligonucleotides (AOs) to skip one or more exons in mutated forms of the DMD gene and so restore the reading frame of the transcript is one of the most promising approaches to treat Duchenne muscular dystrophy (DMD). At present, preclinical studies demonstrating the efficacy and safety of long-term AO administration have not been conducted. Furthermore, it is essential to determine the minimal effective dose and frequency of administration. In this study, two different low doses (LDs) of phosphorodiamidate morpholino oligomer (PMO) designed to skip the mutated exon 23 in the mdx dystrophic mouse were administered for up to 12 months. Mice treated for 50 weeks showed a substantial dose-related amelioration of the pathology, particularly in the diaphragm. Moreover, the generalized physical activity was profoundly enhanced compared to untreated mdx mice showing that widespread, albeit partial, dystrophin expression restores the normal activity in mdx mice. Our results show for the first time that a chronic long-term administration of LDs of unmodified PMO, equivalent to doses in use in DMD boys, is safe, significantly ameliorates the muscular dystrophic phenotype and improves the activity of dystrophin-deficient mice, thus encouraging the further clinical translation of this approach in humans.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked inherited neuromuscular disease caused by mutations in the dystrophin gene, which cause a deficiency in production of the dystrophin protein leading to an increase in muscle fragility. Mutations generating shorter but in-frame, and therefore still partially functional proteins, lead to a milder myopathy, sometimes with a very late onset, known as Becker muscular dystrophy.1,2 The mdx mouse, which carries a nonsense point mutation in the exon 23, also does not produce dystrophin in its muscles, except for rare revertant dystrophin-positive fibers in skeletal and cardiac muscles.3,4 Exclusion of exon 23 from the mature mRNA (exon skipping) in the mdx mouse leads to the restoration of dystrophin expression and has been achieved by using different antisense oligonucleotides (AOs) chemistries, among which the most promising are the phosphorothioate-linked 2′-O-methyl RNA (2OMePS) and the phosphorodiamidate morpholino oligomer (PMO).5,6,7,8,9,10 The more prolonged resistance to endonucleases and the higher affinity to the sequence target make the PMO particularly suitable for in vivo applications.11,12 Furthermore, the PMO can be readily linked to cell-penetrating peptides or octaguanidine groups improving the efficiency of systemic delivery in skeletal13,14,15,16,17,18,19,20 and cardiac muscles.19,21,22 At present, safety for these latter modified compounds has not been demonstrated in the human.23 Currently only unmodified AOs are in use in clinical trials. Two independent clinical trials in the Netherlands and UK have already demonstrated safety and efficiency of 2OMePS and PMO AOs targeting the exon 51 after intramuscular injection in DMD patients24,25,26 and their systemic delivery has been investigated in further clinical trials (ref. 27 and NTC00844597). We have recently demonstrated that the choice of an effective dosing regimen for PMO administration is a key parameter in reducing the amount of naked PMO necessary for systemic delivery and that even a low dosage such as 4 weekly injections of 5 mg/kg PMO induces a significant increase in dystrophin expression.10 Due to the nature of the AO action, repeated chronic administration of AO is necessary to guarantee a continuous production of dystrophin. However, at present, no preclinical studies have been published about the effect of long-term systemic PMO administration in the mdx mouse and the efficacy and the safety of such a long treatment has not been proven. In the present study, we show that systemic delivery of low, clinically applicable, doses of PMO in mdx mice for up to 1 year is safe and ameliorates the pathology of skeletal muscles. Importantly, restored dystrophin expression partially recovered muscle functionality and limb strength. In mice treated for 1 year, the partial, body-wide dystrophin expression resulted in motor activity and movement behavior of mdx that was indistinguishable from normal wild-type C57BL10 mice. These encouraging results support the feasibility of a long-term PMO treatment in humans as a therapy for DMD.
Results
Chronic repeated treatment with intravenous PMO at both 50 and 5 mg/kg results in significant and widespread restoration of dystrophin in skeletal muscle of mdx mice
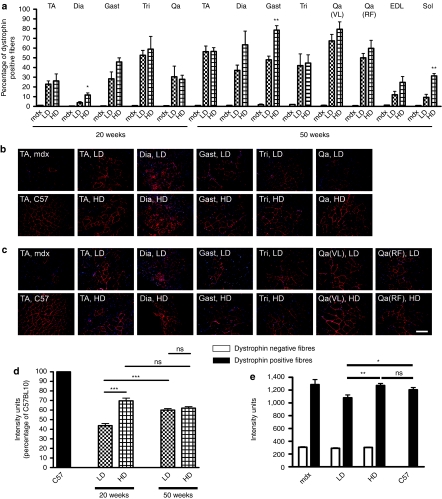
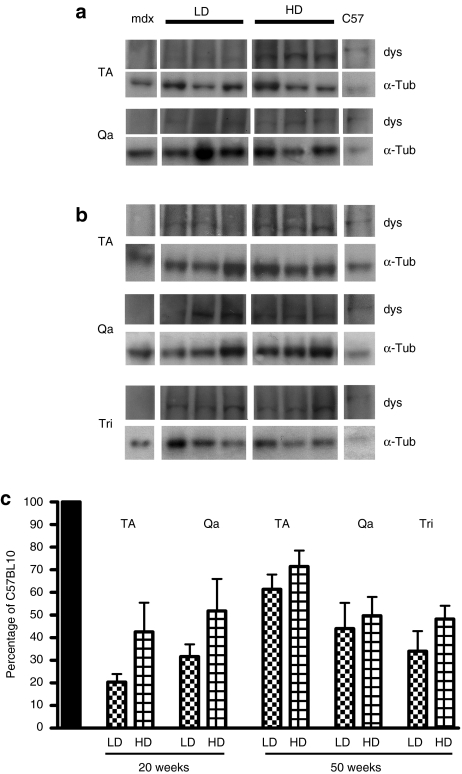
Six-week-old mdx mice were injected with PMO diluted in sterile saline via the tail vein. Two doses of PMO were tested in this study: 5 mg/kg/injection and 50 mg/kg/injection that we referred as low dose (LD) and high dose (HD) because respectively lower and higher compared to the highest dose currently under clinical trial in human DMD patients (20 mg/kg, NTC00844597). A regimen of 4 weekly injections (1 cycle) and 6 weeks of no treatment was adopted in each group of treated mice. The cycle of 4 weekly injections was repeated two or five times and animals were analyzed 6 weeks after the last injection (Supplementary Figure S1). After 20 weeks (two treatment cycles), dystrophin was widely expressed in all the muscles analyzed of treated mice achieving 23–27% of dystrophin-positive fibers in tibialis anterior (TA) and ~30% in quadriceps (Figure 1a). By prolonging the treatment to 50 weeks, the number of dystrophin-positive fibers remained unchanged in triceps (42 and 48% for LD and HD, respectively, n = 4–8) whereas they generally increased in the other muscles tested compared to the two cycle time point. In particular, vastus lateralis and gastrocnemius of HD-treated mice contained >80% dystrophin-positive fibers whereas thinner muscles like soleus and EDL generally expressed smaller percentages of dystrophin-positive fibers (Figure 1a). After either 20 or 50 weeks of treatment both dose regimens gave rise to approximately the same number of dystrophin-positive fibers in most muscles even though a stronger signal was observed after immunofluorescence for dystrophin in muscles treated for 20 weeks with the HD (Figure 1b,c). A semiquantitative intensity analysis corroborated these observations confirming that the averaged dystrophin intensity in TA fibers after 20 weeks of HD treatment (70 ± 3% of the intensity of control C57BL10) was higher than in LD-treated muscles (44 ± 2% of control) (n = 4–5, P < 0.0001) (Figure 1d). In TA of 50 weeks treated mice, patches of dystrophin negative fibers were substantially reduced but not entirely absent even though an extended general fluorescent signal, clearly detectable above the threshold set up on the untreated muscles might be the result of a low dystrophin expression in the majority of fibers (Figure 1c). Accordingly, after 50 weeks of treatment a similar averaged level of intensity in TA sections stained for dystrophin was measured for the two doses (60 ± 1.6 and 62 ± 1.5% for LD and HD compared to C57BL10 fiber intensity, n = 3–5, P = 0.35, Figure 1d). However, when fluorescent intensity was measured specifically in the dystrophin-positive fibers of these muscles, the myofibers of HD-treated mice gave rise to similar amount of dystrophin compared to C57BL10 fibers (1,230 ± 30 compared to 1,205 ± 35 intensity units, n = 5, P = 0.16), whereas the fibers of LD-treated mice expressed significantly less dystrophin compared to the HD treatment (1,095 ± 40 intensity units) [n = 3–5, P < 0.0001 (Figure 1e)]. Western blot analysis confirmed the results obtained by dystrophin intensity study showing that after 20 weeks of PMO administration, TA muscles of HD-treated mice expressed generally more dystrophin compared to the muscles of LD-treated mice. However, the difference was not significant (20 ± 3 and 43 ± 13% of dystrophin expressed by C57 for LD and HD, respectively, n = 4–5, P = 0.17). A similar result was found for total quadriceps muscles (32 ± 5 and 52 ± 14% for LD and HD, respectively, n = 4–5, P = 0.27) (Figure 2a,c). After 50 weeks of PMO treatment, no obvious difference in the levels of dystrophin expression was found between the LD and HD doses in TA (61 ± 6 and 71 ± 7%, respectively, n = 5, P = 0.32), total quadriceps (44 ± 11 and 50 ± 8%, n = 5, P = 0.7), and Tri (34 ± 9 and 48 ± 6%, n = 5, P = 0.21) (Figure 2b,c). No dystrophin expression was observed in cardiac muscles of PMO-treated mice (data not shown). These data suggest that the 20 weeks LD and HD PMO administration produced a different amount of dystrophin in mdx skeletal muscles, but this difference tends to decrease with a longer administration.
Figure 1.
Immunohistological evaluation of significant and widespread restoration of dystrophin in skeletal muscles of mdx mice following chronic repeated treatment with intravenous PMO. Mdx mice were treated for a period of 20 or 50 weeks over which respectively two or five cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. (a) Morphometric quantitation of numbers of dystrophin-positive fibers in immunolabeled cryosections of treated mdx tibialis anterior (TA), total quadriceps (Qa), vastus lateralis Qa(VL), rectus femoris Qa(RF), diaphragm (Dia), gastrocnemius (Gast), triceps brachii (Tri), Soleus (Sol), and EDL muscles (mean ± SEM, n = 4–8, t-test between treated and untreated mdx mice, **P < 0.01, *P < 0.05). (b,c) Representative histological fields of immunolabeled muscle cryosections from (b) 20 weeks or (c) 50 weeks PMO-treated mice and from untreated mice. Bar = 200 µm. (d) Morphometric quantitation of staining intensity in dystrophin-positive fibers from immunolabeled cryosections of treated mdx TA muscles using Metamorph software. Values for LD- and HD-treated samples were normalized as a percentage of that found for control C57BL10 myofibers, and were significantly different after 20 weeks of treatment (mean ± SEM, n = 4–8, t-test, ***P < 0.001, ns: not significant). After 50 weeks of PMO administration, dystrophin was similarly expressed in TA of LD- and HD-treated mice. (e) Intensity of dystrophin positive or negative fibers in 50 weeks treated and untreated muscles. A significant difference was present between the two administered doses and between the LD treatment and the C57BL10. (Mean ± SEM, n = 4–8, t-test, **P < 0.01, *P < 0.05, ns: not significant). PMO, phosphorodiamidate morpholino oligomer; TA, tibialis anterior.
Figure 2.
Western immunoblotting evaluation of dystrophin expression in skeletal muscles of mdx mice following chronic repeated treatment with intravenous PMO. Mdx mice were treated for a period of 20 or 50 weeks over which respectively two or five cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week). PMO were administered intravenously. (a) Dystrophin (dys) and α-tubulin (α-tub) in TA and Qa muscles from mdx mice treated for 20 weeks with PMO: three representative samples are shown for each treatment. (b) Dystrophin (dys) and α-tubulin (α-tub) in TA, Qa, and Tri muscles from mdx mice treated for 50 weeks with PMO: three representative samples are shown for each treatment. (c) Densitometric analysis of dystrophin expression from western immunoblot of PMO-treated TA, Qa, and Tri muscles. Forty microgram for mdx, or twenty-five microgram for C57 were loaded. Values for dystrophin densitometry were normalized internally against α-tubulin and presented as a percentage of the level found in control C57BL10 mouse muscle. No statistically significant difference in dystrophin expression between LD- and HD-treated samples was observed at the end of either the 20- or 50-week treatment period. PMO, phosphorodiamidate morpholino oligomer; Qa, total quadriceps; TA, tibialis anterior; Tri, triceps brachii.
Widespread dystrophin expression after chronic PMO treatment correlates with a substantial histological improvement in mdx skeletal muscles
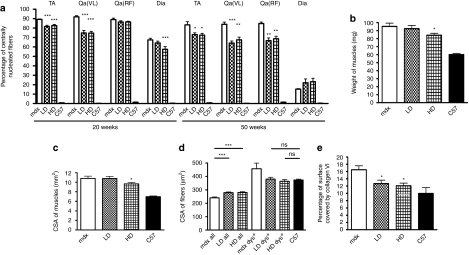
The considerable dystrophin restoration observed after 20 weeks of treatment in diaphragm of HD-treated mice, TA, and vastus lateralis was in keeping with a reduced number of centrally nucleated fibers compared with untreated mdx mice (in TA 82.5 ± 1.4% compared to 89 ± 0.5%, in vastus lateralis 74.6 ± 1.9% compared to 91.9 ± 1.3, in diaphragm after HD treatment 57.4 ± 2.9% compared to 67.4 ± 1.8%, n = 4–6, P < 0.0001) (Figure 3a). In muscles presenting a lower number of dystrophin-positive fibers after LD administration, such as the rectus femoris (about 10–15%, not shown in the graph) and in diaphragm (3.8 ± 1.1%), no change in centrally nucleated fiber number was observed (Figure 3a). The extension of the treatment to 50 weeks further reduced the percentage of centrally nucleated fibers compared to age-matched mdx controls (in TA 72.4 ± 1.9 and 74 ± 1.7% for HD and LD, respectively compared to 83.3 ± 3.4% in mdx, n = 8, P = 0.02 for LD, P = 0.012 for HD; in vastus lateralis 68 ± 1.8 and 65.3 ± 1.7% for HD and LD, respectively compared to 84.4 ± 1.2% in mdx, P = 0.0032 for LD and P < 0.0001 for HD; in rectus femoris 67.5 ± 3 and 66.7 ± 2.8% for HD and LD, respectively compared to 84 ± 2.2% in mdx, P < 0.0001 for LD and HD) indicating lower fiber turnover. In TA of HD-treated animals for 50 weeks, weight (84.2 ± 2.4 mg compared to 95.3 ± 4 mg in mdx, n = 8, P = 0.03) (Figure 3b) and cross-sectional area (9.6 ± 0.3 mm2 compared to 10.8 ± 0.5 mm2 in mdx, n = 8, P = 0.048) (Figure 3c) significantly decreased, indicating a reduction of the typical mdx hypertrophy. This was accompanied by a significant increase in average fiber cross-sectional area (327 ± 10 µm2 and 349 ± 11 µm2 for LD and HD compared to 270 ± 7 µm2 in mdx, n = 8, P < 0.0001) (Figure 3d) in all the fibers suggesting a decrease in fiber splitting. Importantly, the cross-sectional area of the dystrophin-positive fibers reached values comparable to the normal fibers (Figure 3d), confirming a protective role for the expressed dystrophin. Finally, the fibrotic tissue in TA muscles, measured as the collagen VI positive area in representative transverse sections of treated muscles, was comparable to that of normal mice (12.7 ± 0.9 and 12 ± 0.7% for LD and HD compared to 9.9 ± 1.6% in wild-type mice, n = 8, P= 0.18 and P = 0.2 for LD and HD, respectively) and significantly lower than the collagen VI found in mdx muscle (16.5 ± 1.1%, Figure 3e). These data demonstrate that the chronic administration of low, clinically applicable doses of PMO ameliorates the histology of dystrophic muscles.
Figure 3.
Widespread dystrophin expression after chronic PMO treatment correlates with substantial histological improvement in mdx skeletal muscles. Mdx mice were treated for a period of 20 or 50 weeks over which respectively two or five cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. (a) Percentage of centrally nucleated fibers in tibialis anterior (TA), vastus lateralis Qa(VL), rectus femoris Qa(RF), and diaphragm (Dia) muscles after treatment for 20 or 50 weeks with PMO. Both the doses generally induced a decrease in percentage of centrally nucleated fibers. Values given are mean ± SEM (n = 4–8: t-test between treated and untreated mdx mice, ***P < 0.001, **P < 0.01, *P < 0.05). (b) Tibialis anterior (TA) of HD 50 weeks treated mice showed a reduction in weight compared to the muscles of untreated mice whereas no difference was observed after the LD treatment (mean ± SEM n = 8, t-test, *P < 0.05). (c) The CSA of TA muscles from HD 50 weeks treated mice was smaller than in TA muscles of untreated mdx mice. Overall CSA was estimated using the following formula: muscle weight (g)/[Lo (cm) × 1.06 (g/cm3)] where the muscle length was measured using digital callipers (mean ± SEM n = 8, t-test, *P < 0.05). (d) Cross-sectional area (CSA) of myofibers in 50 weeks PMO-treated and -untreated controls. The average CSA of dystrophin-positive fibers in treated muscles was larger than the average of all the muscle fibers indicating a protective role for the ex-novo produced dystrophin (mean ± SEM n = 8, t-test, ***P < 0.001). (e) Percentage of muscle section covered by collagen type VI in TA muscles of 50 weeks PMO-treated mice. The values were compared to that seen in untreated mdx mice (mean ± SEM n = 8, t-test, *P < 0.05, ns: not significant). CSA, cross-sectional area; PMO, phosphorodiamidate morpholino oligomer; TA, tibialis anterior.
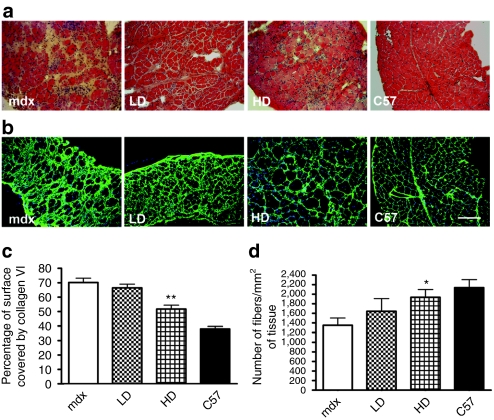
Chronic PMO administration reduces fibrosis and prevents the loss of myofibers in the diaphragm of mdx mice
In mice treated for 50 weeks, the diaphragm showed the most obvious morphological improvement particularly after HD treatment. In contrast to limb muscles in mdx mouse, the diaphragm undergoes continuous degeneration with a loss of myofibers starting from 6 months of age. The diaphragms of treated mice showed less infiltrate with more closely packed myofibers compared to the diaphragms of untreated mdx mice (Figure 4a). In agreement with these findings, the fibrotic tissue in diaphragms of HD PMO-treated mice occupied significantly less area compared to the tissue of untreated mice as shown by immunostaining for collagen VI (51.7% of the total area in HD compared to 70.2 ± 2.9% in mdx mice, n = 8, P = 0.0018) (Figure 4b,c). Although no significant difference was observed in the number of centrally nucleated fiber between treated and untreated mdx mice (Figure 3a) the number of fibers per unit area of diaphragm in HD-treated mice was comparable to that of C57BL10 mice (1,935 ± 163 fibers/mm2 in HD-treated mice compared to 2,137 ± 168 fibers/mm2 in wild-type mice, n = 8, P = 0.23) (Figure 4d). These data demonstrate that the considerable muscle fiber loss occurring in diaphragms of 13-month-old mdx mice can be delayed by administering clinically applicable doses of PMOs.
Figure 4.
Chronic PMO administration reduces fibrosis and prevents the loss of myofibers in the diaphragm of mdx mice mice. Mdx mice were treated for a period of 50 weeks over which five cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. Diaphragm muscles were evaluated histologically and immunocytochemically. (a) H&E staining suggests an improved histology in diaphragms of treated mdx mice with more fibers and less connective tissue. (b) Immunocytochemistry for collagen VI in diaphragms of treated and untreated mice. Bar = 200 µm. (c) Morphometric evaluation of collagen VI immunostaining in diaphragms of treated and untreated mice shows a significant reduction in fibrosis in HD treated compared to untreated mdx mice (mean ± SEM n = 8, t-test, **P < 0.01). (d) Evaluation of fiber density in the diaphragm of HD-treated mdx and -untreated mdx mice shows delayed loss of fibers (mean ± SEM n = 8, Mann–Whitney test, *P < 0.05). H&E, hematoxylin and eosin; PMO, phosphorodiamidate morpholino oligomer.
Chronic PMO administration substantially improves the physiological properties and function of mdx skeletal muscles
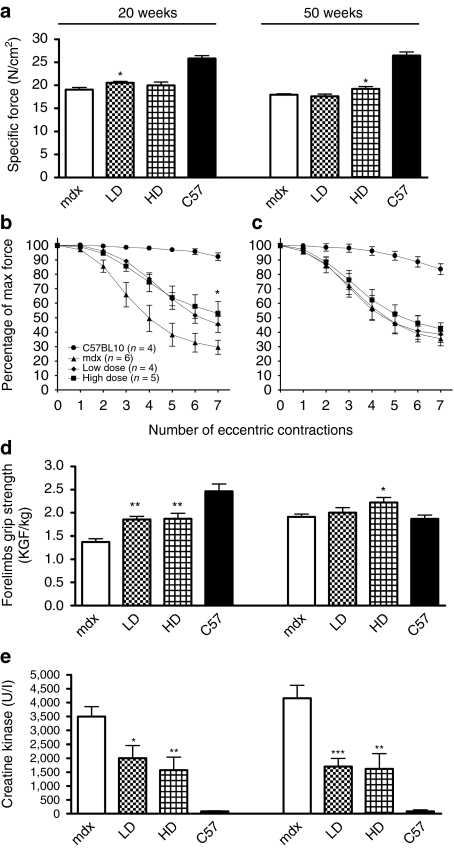
To examine whether dystrophin expression in muscle of LD- and HD-treated mice was sufficient to improve muscle function, we measured in vivo both the contractile properties of TA muscles and their susceptibility to contraction-induced injury. We found that the 20 weeks administration of PMO followed by dystrophin expression in ~25% of TA muscle fibers, only resulted in a tendency to improve specific force after LD treatment [20.6 ± 0.3 N/cm2 compared to 19 ± 0.4 N/cm2 in mdx, n = 4–6 (P = 0.046)] (Figure 5a). The 50 weeks PMO administration induced a significant increase in specific force in TA of HD-treated mice (19.2 ± 0.5 N/cm2 in HD compared to 17.9 ± 0.2 N/cm2 in mdx mice, n = 8, P = 0.046) (Figure 5a). Interestingly, treating mdx mice for 20 weeks at both doses of PMO induced sufficient levels of dystrophin to statistically improve the muscle resistance to seven eccentric contractions compared to mdx controls (41.6 ± 4.9 and 50.4 ± 5.7% of initial force for LD and HD, respectively compared to 27.2 ± 3.8% in mdx mice, n = 4–6, P < 0.05) (Figure 5b). However, the resistance to eccentric contractions was not improved in TA muscles after the 50 weeks treatments (Figure 5c). The high amount of dystrophin-positive fibers observed in the triceps of mice treated for 20 weeks correlated with a statistically significant 30% increase in forelimb force of treated mice compared to mdx control mice as measured by grip strength test (1.86 ± 0.06 and 1.87 ± 0.11 kilogram-force/kg for LD and HD, respectively compared to 1.37 ± 0.07 kilogram-force/kg in mdx, n = 4–6, P = 0.0015 for LD and P = 0.0046 for HD compared to mdx mice) (Figure 5d). The same type of analysis in 50 weeks-treated mice showed a significant increase in limb muscle strength in HD-treated mice (2.22 ± 0.11 kilogram-force/kg for HD compared to 1.91 ± 0.06 kilogram-force/kg in mdx n = 8, P = 0.03) (Figure 5d). The analysis of creatine kinase concentration in the blood-derived serum of both dose-treated mice for 20 weeks (Figure 5e) confirmed the improvement in sarcolemmal function (2,004 ± 457 and 1,570 ± 478 U/l for LD and HD, respectively compared to 3,494 ± 358 U/l in mdx, n = 4–6, P = 0.0322 and P = 0.0089, respectively for LD and HD). After five cycles of treatment, the levels of creatine kinase concentration in serum were kept low after both the treatments indicating a functional effect of the restored dystrophin (1,700 ± 192 and 1,619 ± 544 U/l for LD and HD compared to 4,155 ± 468 U/l in mdx mice, n = 8, P = 0.0005 and P = 0.005 for LD and HD, respectively) (Figure 5e). This was confirmed by coimmunostaining for dystrophin and mouse immunoglobulin G (IgG) showing that no necrotic fibers containing IgG fibers were present in treated muscles after two or five cycles compared to the untreated mdx controls (Supplementary Figure S2a). The dystrophin-associated protein complex showed a dose–response restoration with a uniform and wide expression in the sarcolemma of dystrophin-positive fibers after HD treatment (Supplementary Figure S2b). In particular, the neuronal nitric oxide synthase protein was correctly associated with the sarcolemma of 80–90% of dystrophin-positive fibers (45–50% of the total fibers in TA, Supplementary Figure S2c). Importantly, no difference was observed in neuronal nitric oxide synthase expression between two or five cycles of PMO treatment showing that the force transfer mechanism mediated by dystrophin-neuronal nitric oxide synthase was similarly restored. Taken together, these data suggest that there is a substantial functional improvement at the end of the treatment. Dosages that in young muscles were sufficient to provide resistance to eccentric exercise do not appear sufficient in adult/aged muscles. However, when the force generated by all the muscles of the same limb is considered, long-term PMO-treated mice show a substantial improvement compared to untreated mdx mice.
Figure 5.
Chronic PMO administration substantially improves the physiological properties and functionality of mdx skeletal muscles. Mdx mice were treated for a period of 20 or 50 weeks over which respectively two or five cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. (a) Specific force measured in TA of treated and untreated mdx mice. Twenty weeks administration of low doses of PMO resulted in a tendency to improve specific force (20.6 ± 0.3 and 20 ± 0.7 N/cm2 for LD and HD, respectively compared to 19 ± 0.4 N/cm2 in mdx). After 50 weeks, the HD administration induced a significant increase in specific force (19.2 ± 0.5 N/cm2 in HD compared to 17.9 ± 0.2 N/cm2 in mdx mice) (mean ± SEM n = 4–6 for 20 weeks treatment and n = 8 for 50 weeks treatment, t-test between treated and untreated mdx mice, *P < 0.05). (b,c) Tibialis anterior muscles assessed for force deficit following a series of seven eccentric contractions showed a significant improvement in their resistance to contraction-induced injury after both 20 weeks LD and HD treatments. No change was observed after 50 weeks of treatment (mean ± SEM n = 4–6, one-way ANOVA test, *P < 0.05). (d) Forelimbs grip strength analysis showed a significant increase of strength after LD and HD treatment compared to the untreated mdx after 20 weeks of treatment. After 50 weeks of treatment, only the HD improved the forelimbs strength. The values are expressed as force normalized for the weight of the mice (mean ± SEM n = 8, t-test between treated and untreated mdx mice, **P < 0.01, *P < 0.05). (e) Creatine kinase concentration measured in serum significantly decreased after 20 and 50 weeks of treatments with both the PMO dosage regimens. (Mean ± SEM n = 8, t-test between treated and untreated mdx mice, ***P < 0.001, **P < 0.01, *P < 0.05.) PMO, phosphorodiamidate morpholino oligomer; TA, tibialis anterior.
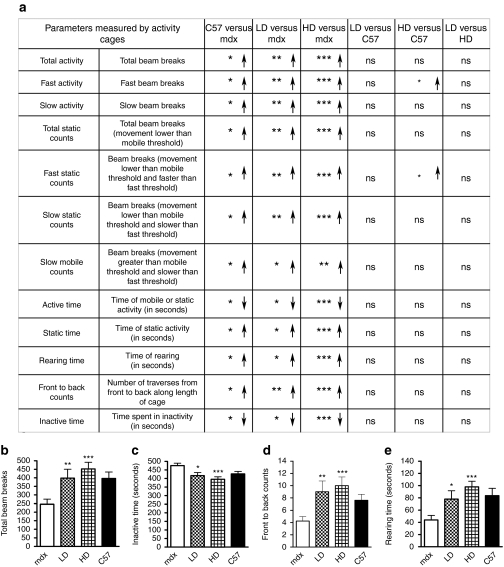
Physical activity and locomotor behavior is completely normalized in mdx mice following chronic PMO administration despite only a partial restoration of dystrophin levels
Mice were monitored using open-field behavioral activity cages and 20 different parameters were recorded at the end of the experiment (the complete set of data is available upon request). In 12 out of 20 parameters, 13-month-old-untreated mdx mice showed significant differences in locomotor behavior compared to age- and sex-matched normal mice (Figure 6a). After five cycles, treated mice exhibited a dose-dependent increase in horizontal (active time, total activity, front to back counts) and vertical (rearing time) activities and a substantial decrease in inactive time with a consequent normalization compared to the age-matched C57BL10 mice (Figure 6b–d). In most of the parameters, the HD-treated mdx mice had higher performances compared to C57BL10 with statistically significant changes in “fast activity ” and “fast static counts ” (Figure 6a). However, 13-month-old C57BL10 mice weighed more due to a greater body fat content than age-matched mdx28 and this probably reduced the fast activity of wild-type mice. These data suggest that the prolonged administration of PMO produces a beneficial effect in skeletal muscle of treated mice restoring the wild-type activity.
Figure 6.
Physical activity and behavior is completely normalized in mdx mice following chronic PMO administration and despite only a partial restoration of dystrophin levels. Mdx mice were treated for a period of 50 weeks over which five cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. Mice were analyzed after 50 weeks of treatment with open-field behavioral activity cages. (a) 12 out of 20 parameters that show a difference between mdx and C57BL10 mice are reported. *Significance differences in the measured parameters (mean ± SEM, Mann–Whitney test, ***P < 0.001, **P < 0.01, *P < 0.05). Arrows indicate an increase (↑) or a decrease (↓) in the value of the parameter measured for the first group compared to the second in any single category. All the parameters were significantly changed between treated and untreated mdx mice with a dose-dependent effect and almost all the parameters were normalized to that of the C57BL10 mice. In the bottom panel graphs show the detailed measurement related to the parameters (b) “total activity, ” (c) “inactive time, ” (d) “front to back counts, ” and (e) “rearing time. ” (Mean ± SEM, Mann–Whitney test between treated and untreated mdx mice, ***P < 0.001, **P < 0.01, *P < 0.05.) PMO, phosphorodiamidate morpholino oligomer.
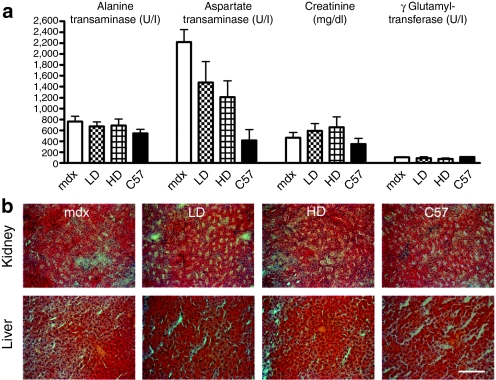
Indicators of renal and hepatic toxicity are unaffected by chronic PMO administration in mdx mice
No deaths or loss of weight due to PMO administration were detected during the course of the experiment (Supplementary Figure S3). To investigate possible toxic effect due to long-term treatment, the concentrations of kidney and liver enzymes were evaluated in serum. The long-term PMO delivery did not modify the concentration of aspartate aminotransferase, alanine aminotransferase, and gamma glutamyltransferase in serum, which suggests that there are no detrimental effects of PMO in the liver. Creatinine concentration was recorded in the same range as untreated mice indicating no adverse effect of PMO on the kidney (Figure 7a). The histological structure of both organs was also evaluated by hematoxylin and eosin staining and no infiltrate or vacuolization was observed confirming the safety of PMO treatment (Figure 7b). All these data showed that 50 weeks chronic administration of PMO is safe in mdx mice.
Figure 7.
Indicators of renal and hepatic toxicity are unaffected by chronic PMO administration in mdx mice. Mdx mice were treated for a period of 50 weeks over which five cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. (a) The concentrations of serum alanine transaminase, aspartate transaminase, creatinine, and γ glutamyltransferase were evaluated. The concentrations in treated mice were comparable to those in untreated mice (mean ± SEM). (b) H&E staining of sections of liver and kidney showed a similar histology in control and PMO-treated mice. Bar = 200 µm. H&E, hematoxylin and eosin; PMO, phosphorodiamidate morpholino oligomer.
Discussion
The choice of an optimal dosing regimen plays a pivotal role in clinical applications of any medicinal compound, including AOs for the treatment of DMD. PMO is one of the most promising AO chemistries due to the high efficiency and safety profile in in vivo applications.5,26 However, at present, only short-term studies have been performed5,26,29 and both safety and final outcome of a long-term treatment are still unknown. We postulated that repeated cycles of LD PMO injections may represent an efficient dosing regimen for a potential clinical approach for DMD, allowing significant dystrophin production during the treatment. As we previously reported, a cycle of 4 weekly injections of 5 or 50 mg/kg of PMO induced substantial levels of dystrophin in skeletal muscles lasting for at least 8–10 weeks.10 These PMO doses are very low compared to the extremely HDs of PMO that can be tolerated without serious adverse effects (up to 3,000 mg/kg/injection in mice and to 320 mg/kg/injection in nonhuman primates).26,30,31 Furthermore, similar doses have been recently used in a UK-based clinical trial (NTC00844597). Here, we demonstrate that two cycles of the proposed treatment induced a body wide and robust expression of dystrophin. In particular, the histological and functional outcome previously obtained in a study where a high amount of PMO (100 mg/kg/injection) was delivered in 3–7 weekly injections,5 was similarly obtained here with lower doses of PMO. Importantly, we found that a dosing regimen based on a very low, clinically applicable dose of PMO (cumulative dose of only 40 mg/kg) significantly strengthens the muscles of treated mdx mice and considerably decreases the CK concentration in serum. The extension of the treatment to five cycles further increased dystrophin expression and ameliorated the dystrophic phenotype. In adult/aged mdx mice, the most affected muscle is the diaphragm, which most closely recapitulates the severe feature of dystrophic human muscle.32,33 Following repeated injections of PMO, the diaphragm showed significant histological improvement. By starting the treatment in young mice, the PMO delayed the progression of the pathology by reducing the collagen deposition and avoiding loss of myofibers. In TA muscles, even though we observed a similar dystrophin expression after both treatments, the level of dystrophin in fibers of HD-treated mice was higher and correlated with the amelioration of pathology. These observations suggest that higher doses improve the histological and functional outcomes inducing more protection to the fibers than lower doses. However, the dystrophin produced in TA muscles following 50 weeks of treatment was not sufficient to protect the muscle against eccentric contraction-induced damage even though fibrosis was reduced after PMO administration. The presence of many centrally nucleated fibers suggests that muscle degeneration and regeneration had occurred during the treatment. It is possible that the intermittent PMO dosing regimen may have resulted in significant fluctuations in dystrophin expression over time leading to periods of muscle damage. The consequent chronic remodeling of muscle may have led to degradation in resistance to contraction-induced injury. An alternative, but not mutually exclusive explanation is that the doses we used were sufficient to prevent the development of pathology in relatively sedentary caged mdx mice but might not be sufficient to counteract the effects of a more demanding regular exercise and this could explain the lack of protection against eccentric exercise. The latter finding suggests that functional tests like the measure of protection to eccentric contractions more than a mere quantification of protein expression should be used to validate a treatment based on dystrophin restoration in preclinical models. This is particularly relevant in the case of exon-skipping approach where different mutations are associated to the production of different quasidystrophins that could be clearly detectable while potentially associated with a very low functionality. Thus, the dose of PMO might need to be adjusted to reflect the severity of the pathology, the level of activity of the DMD patient and the functionality of the quasidystrophin produced. The de novo dystrophin expressed following PMO administration was associated with a profound improvement of the activity of treated mdx mice. Mice were monitored with a noninvasive open field apparatus which has been recently used to verify the difference in activity between C57BL10 mice and mdx mice34 but it was never been used before to measure the improvement in activity after a treatment aimed to induce dystrophin restoration. At the end of the study, we observed a normalization of the parameters associated with horizontal and vertical activity in treated mdx mice, with values which were indistinguishable from wild-type C57BL10 mice. Treated mice exhibited normal locomotion inside the cage and normal rearing activity. Particularly intriguing was the improvement in vertical activity, which has been recently demonstrated to be strictly linked with the resistance to fatigue due to restoration of sarcolemma localization of neuronal nitric oxide synthase in mdx mice.35 Moreover, the significant improvement in forelimb grip strength indicated a generalized major improvement in the whole body muscular performance. Because the PMO cannot cross the blood–brain barrier,31 it is unlikely that the PMO administration directly interfered with behavior in treated mice via effects at the central nervous system level. To our knowledge, this is the first report showing a clear normalization in motor activity and locomotor behavior of mdx mice after a treatment to restore the dystrophin expression. Although the efficiency of some PMOs to induce exon skipping in human DMD RNAs or the functionality of derived human quasidystrophin forms may be relatively low, our current findings suggest that reduced dystrophin expression may nevertheless be sufficient to allow improvement of the day to day activities in treated DMD patients. In agreement with previous short-term studies based on systemic AOs delivery,5,9,10 we did not observe dystrophin expression in cardiac muscles. However, early diagnosis, careful follow-up and timely coadministration in patients treated with PMOs, of angiotensin-converting enzyme inhibitor, antioxidant β-blocker or corticosteroid should be beneficial in the heart.36,37 In conclusion, our data demonstrate extensive amelioration of the dystrophic pathology in mdx mice after 1 year of chronic administration of low, clinically applicable dosages of antisense exon-skipping PMOs, suggesting that this approach may provide a safe and beneficial long-term treatment in DMD patients.
Materials and Methods
Administration of PMO to mdx mice. PMO with the following sequence, 5′-GGCCAAACCTCGGCTTACCTGAAAT-3′, which has been previously shown to be biologically active in the induction of dystrophin exon 23 skipping6 was purchased from GeneTools (Philomath, OR). PMO was formulated in pyrogen-free saline before injection via the tail vein in C57BL/10ScSn-Dmdmdx (mdx) male mice starting at 6 weeks of age, and following the regimen indicated schematically in Supplementary Figure S1. Animals were bred in-house. Food and water was provided ad libitum. Mouse husbandry and experimentation adhered to statutory UK Home Office regulatory, ethical, and licensing procedures, and under the Animals (Scientific Procedures) Act 1986 (project licences PPL 70/7008 and PPL 70/6797).
Histology and immunocytochemistry. As indicated in Supplementary Figure S1, muscles and other organs were harvested and processed for cryosectioning. Immunofluorescence for dystrophin, dystrophin protein complex members, nitric oxide synthase, and laminin were performed as previously described.10 Fibrosis was evaluated by immunostaining for collagen VI (Abcam ab6588 rabbit polyclonal, diluted 1:200) in combination with goat anti-rabbit IgG (Alexafluor 568; Invitrogen, Paisley, UK). The percentage of the area stained for collagen VI in cryosections was measured using SigmaScan Pro image analysis software (Systat Software, Chicago, IL) in the largest section of individual muscles analyzed. The same program was used to manually count dystrophin-positive fibers in six randomly captured fields (×20 magnification) from the same section.10 All immunofluorescence images were captured and digitized using identical parameters of exposure, saturation, and γ-levels between treated and untreated specimens. The intensity of dystrophin immune-stained fibers were analyzed by Metamorph software as reported in ref. 38 using a rabbit polyclonal antibody to detect dystrophin (P6) and antilaminin-2 (4HB2) for normalization. As an indicator of sarcolemmal permeability, endogenous intracellular IgG was evaluated in muscle fibers by direct staining using rabbit anti-mouse IgG-488 (Invitrogen).
Western blotting. Western blot analyses were performed on a range of skeletal muscles from the treated mice. Tissues were homogenized in loading buffer (125 mmol/l Tris–HCl, pH 6.8, 10% sodium dodecyl sulfate, 2 mol/l urea, 5% 2-mercaptoethanol, 20% glycerol) and samples (40 µg for mdx or 25 µg for C57) resolved on Tris-acetate sodium dodecyl sulfate-polyacrylamide gel electrophoresis (3–8% gradient gels: Invitrogen, Paisley, UK) and then transferred to nitrocellulose (Hybond; GE Healthcare, Little Chalfont, UK) for 3 hours at 30 V. Membranes were blocked with 5% dried milk powder in phosphate-buffered saline with Tween 20 for 2 hours at room temperature. Expression of α-tubulin was detected as a loading control using a mouse monoclonal anti-α-tubulin antibody (diluted 1:2,500; Sigma-Aldrich, Dorset, UK), and dystrophin expression was detected using the mouse monoclonal antibody DYS1 (1:100; Novocastra, Newcastle upon tynes, UK). Both primary antibodies were diluted in phosphate-buffered saline with Tween 20 and membranes incubated overnight at 4 °C, followed by anti-mouse-horseradish peroxidase (Dako, Ely, UK, 1:1,000 in phosphate-buffered saline with Tween 20, 1 hour at room temperature). Immunoreactive bands on western blot membranes were detected by incubation with an ECL reagent (GE Healthcare) and exposure to X-ray film (Hyperfilm-ECL; GE Healthcare). Densitometric analysis of dystrophin and α-tubulin bands was performed using the ImageJ 1.4 software (National Institutes of Health, Bethesda, MD). The averages were obtained using the values of dystrophin normalized for the corresponding bands for α-tubulin, and then comparing these values with the ratio obtained in the same way for a muscle of C57BL/10 (which was considered as 100%).
Measurement of serum creatine kinase, alanine transaminase, aspartate transaminase, creatinine, and γ-glutamyl transpeptidase. Mouse blood was taken from the tail vein immediately before cervical dislocation and after clotting, serum collected, centrifuged at 350g for 10 minutes at 4 °C. The concentration of the various serum enzymes and components was determined using Randox laboratories kits according to the manufacturer's instructions.
Mouse forelimb grip strength evaluation. Mouse forelimb grip strength evaluation was performed as previously described.20 Mice were allowed to grip a horizontal bar connected to a commercial grip strength monitor (Linton Instruments, Palgrave Diss, Norfolk, UK). Five forelimb strength measurements were recorded within 2 minutes for each mouse each day over a 3-day period and all the values were averaged.21 Data were expressed as kilogram force and normalized to the body weight.
Mouse open-field behavioral activity evaluation. Mouse open-field behavioral activity was evaluated as previously described34 using activity cages from Linton Instruments. Data were collected by using the Amonlite software (version 1.4). Briefly, mice were acclimatized to the test chamber during an undisturbed period of 60 minutes every day for four consecutive days. On the day of data collection, mice were acclimatized for 30 minutes before data acquisition. Data were then acquired every 10 minutes for six times in a session lasting 1 hour. Mice were analyzed like this 1 hour/day for four following days. Data obtained per each mouse were averaged. During the analysis, particular care was taken to minimize noise and movement into the room.
Muscle electrophysiology and resistance to eccentric contraction. TA muscles were examined in vivo for both force generation and resistance to eccentric contractions as previously described.39 Briefly, under deep anesthesia the distal tendon of the TA muscle was attached to the lever arm of a 305B dual-mode servomotor transducer and TA muscle contractions were elicited by stimulating the distal part of common peroneal nerve. Maximum isometric tetanic force (Po) was determined from the plateau of the force–frequency relationship following a series of stimulations from 10–150 Hz. The specific force (N/cm2) was calculated by dividing Po by TA muscle cross-sectional area. Following the assessment of muscle force, TA muscles were tested for their susceptibility to contraction-induced injury. This consisted of stimulating the muscle at 150 Hz for 700 ms. After 500 ms of stimulation the muscle was lengthened by 15% of Lo at a velocity of 0.75 Lo s/1. At the end of stimulation the muscle was returned to Lo at a rate of -0.75 Lo s/1. The stimulation-stretch cycle was repeated every 3 minutes for a total of seven cycles. Maximum force was measured after each eccentric contraction and expressed as a percentage of the initial maximum force.
Statistical analyses employed. Data are expressed as mean ± SE of the mean. One-way ANOVA test, two-tail parametric t-test or nonparametric Mann–Whitney test were used for the analyses as indicated. For all statistical analyses, GraphPad Prism software package (version 4; GraphPad Software, La Jolla, CA) was used.
SUPPLEMENTARY MATERIAL Figure S1. Schematic of the experimental regimen used for the chronic administration of PMOs by intravenous delivery in mdx mice. Mdx mice were systemically treated with 2 or 5 cycles of 4 weekly intravenous injections of 50 mg/kg or 5 mg/kg of PMO, with a 6 week period of rest. Treatment with PMO commenced when the mdx mice were 6 weeks old. Mice were analysed 6 weeks after the last injection. Untreated mdx and C57 mice were used as age-matched control. The weeks of injections are showed in red. In blue the numbers of mice sacrificed at each time point are shown. Figure S2. Evaluation of sarcolemmal permeability and dystrophin protein complex (DPC) restoration in DAPC following chronic administration of PMOs by intravenous delivery in mdx mice. Mdx mice were treated for a period of 20 or 50 weeks over which 2 or 5 cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. TA muscles were processed and cryosections stained for dystrophin, intracellular endogenous mouse IgG, β-dystroglycan and α-sarcoglycan. (a) IgG/dystrophin co-immunostaining demonstrates the sarcolemmal integrity in treated mice compared to mdx mice. Representative fields of untreated, 20 and 50 weeks treated mdx and C57BL10 controls stained for dystrophin (red) and IgG (green) are shown. (b) The dystrophin associated protein complex was correctly restored in dystrophin positive fibres after both treatments. White asterisks identify the same fibres in serial sections that are stained positive for the proteins indicated. (c) A similar number of fibres co-expressed dystrophin and nNOS following after both 2 and 5 cycles of administration. Scale bar: 200 μm. Figure S3. Evaluation of body mass following chronic administration of PMOs by intravenous delivery in mdx mice. During the first 20 weeks, treated and untreated mdx and C57BL10 mice had a similar body weight. In the following 30 weeks the difference in weight between C57BL10 and all the other mice considerably increases.
Acknowledgments
We gratefully acknowledge Jennifer E. Morgan for the helpful comments and the critical reading of the manuscript. We acknowledge the other members of the MDEX Consortium for helpful input and advice. This work was supported by the Muscular Dystrophy Campaign, Muscular Dystrophy Ireland, the EC Clinigene Network of Excellence, the Medical Research Council, and the UK Department of Health. The authors declared no conflict of interest.
Supplementary Material
Schematic of the experimental regimen used for the chronic administration of PMOs by intravenous delivery in mdx mice. Mdx mice were systemically treated with 2 or 5 cycles of 4 weekly intravenous injections of 50 mg/kg or 5 mg/kg of PMO, with a 6 week period of rest. Treatment with PMO commenced when the mdx mice were 6 weeks old. Mice were analysed 6 weeks after the last injection. Untreated mdx and C57 mice were used as age-matched control. The weeks of injections are showed in red. In blue the numbers of mice sacrificed at each time point are shown.
Evaluation of sarcolemmal permeability and dystrophin protein complex (DPC) restoration in DAPC following chronic administration of PMOs by intravenous delivery in mdx mice. Mdx mice were treated for a period of 20 or 50 weeks over which 2 or 5 cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. TA muscles were processed and cryosections stained for dystrophin, intracellular endogenous mouse IgG, β-dystroglycan and α-sarcoglycan. (a) IgG/dystrophin co-immunostaining demonstrates the sarcolemmal integrity in treated mice compared to mdx mice. Representative fields of untreated, 20 and 50 weeks treated mdx and C57BL10 controls stained for dystrophin (red) and IgG (green) are shown. (b) The dystrophin associated protein complex was correctly restored in dystrophin positive fibres after both treatments. White asterisks identify the same fibres in serial sections that are stained positive for the proteins indicated. (c) A similar number of fibres co-expressed dystrophin and nNOS following after both 2 and 5 cycles of administration. Scale bar: 200 μm.
Evaluation of body mass following chronic administration of PMOs by intravenous delivery in mdx mice. During the first 20 weeks, treated and untreated mdx and C57BL10 mice had a similar body weight. In the following 30 weeks the difference in weight between C57BL10 and all the other mice considerably increases.
REFERENCES
- England SB, Nicholson LV, Johnson MA, Forrest SM, Love DR, Zubrzycka-Gaarn EE, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H., and, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA., and, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Morgan JE, Watkins SC., and, Partridge TA. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990;99:9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Gebski BL, Mann CJ, Fletcher S., and, Wilton SD. Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum Mol Genet. 2003;12:1801–1811. doi: 10.1093/hmg/ddg196. [DOI] [PubMed] [Google Scholar]
- Heemskerk H, de Winter C, van Kuik P, Heuvelmans N, Sabatelli P, Rimessi P, et al. Preclinical PK and PD studies on 2'-O-methyl-phosphorothioate RNA antisense oligonucleotides in the mdx mouse model. Mol Ther. 2010;18:1210–1217. doi: 10.1038/mt.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba A, Thorogood FC, Dickson G., and, Graham IR. Dosing regimen has a significant impact on the efficiency of morpholino oligomer-induced exon skipping in mdx mice. Hum Gene Ther. 2009;20:955–965. doi: 10.1089/hum.2008.157. [DOI] [PubMed] [Google Scholar]
- Popplewell LJ, Trollet C, Dickson G., and, Graham IR. Design of phosphorodiamidate morpholino oligomers (PMOs) for the induction of exon skipping of the human DMD gene. Mol Ther. 2009;17:554–561. doi: 10.1038/mt.2008.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantana A., and, Iversen PL. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr Opin Pharmacol. 2005;5:550–555. doi: 10.1016/j.coph.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Steinhaus JP, et al. Morpholino oligomer-mediated exon skipping averts the onset of dystrophic pathology in the mdx mouse. Mol Ther. 2007;15:1587–1592. doi: 10.1038/sj.mt.6300245. [DOI] [PubMed] [Google Scholar]
- Moulton HM, Fletcher S, Neuman BW, McClorey G, Stein DA, Abes S, et al. Cell-penetrating peptide-morpholino conjugates alter pre-mRNA splicing of DMD (Duchenne muscular dystrophy) and inhibit murine coronavirus replication in vivo. Biochem Soc Trans. 2007;35 Pt 4:826–828. doi: 10.1042/BST0350826. [DOI] [PubMed] [Google Scholar]
- Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S, et al. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos PA, Li Y., and, Jiang S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques. 2008;45:613–4, 616, 618 passim. doi: 10.2144/000113005. [DOI] [PubMed] [Google Scholar]
- Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P, et al. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet. 2008;17:3909–3918. doi: 10.1093/hmg/ddn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton HM, Wu B, Jearawiriyapaisarn N, Sazani P, Lu QL., and, Kole R. Peptide-morpholino conjugate: a promising therapeutic for Duchenne muscular dystrophy. Ann N Y Acad Sci. 2009;1175:55–60. doi: 10.1111/j.1749-6632.2009.04976.x. [DOI] [PubMed] [Google Scholar]
- Wu B, Li Y, Morcos PA, Doran TJ, Lu P., and, Lu QL. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther. 2009;17:864–871. doi: 10.1038/mt.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Moulton HM, Betts C, Seow Y, Boutilier J, Iverson PL, et al. A fusion peptide directs enhanced systemic dystrophin exon skipping and functional restoration in dystrophin-deficient mdx mice. Hum Mol Genet. 2009;18:4405–4414. doi: 10.1093/hmg/ddp395. [DOI] [PubMed] [Google Scholar]
- Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Li J, et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jearawiriyapaisarn N, Moulton HM, Sazani P, Kole R., and, Willis MS. Long-term improvement in mdx cardiomyopathy after therapy with peptide-conjugated morpholino oligomers. Cardiovasc Res. 2010;85:444–453. doi: 10.1093/cvr/cvp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton HM., and, Moulton JD. Morpholinos and their peptide conjugates: Therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim Biophys Acta. 2010;1798:2296–2303. doi: 10.1016/j.bbamem.2010.02.012. [DOI] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazani P, Weller DL., and, Shrewsbury SB. Safety pharmacology and genotoxicity evaluation of AVI-4658. Int J Toxicol. 2010;29:143–156. doi: 10.1177/1091581809359206. [DOI] [PubMed] [Google Scholar]
- Goemans NM, Buyse G, Tulinius M, Verschuuren JJG, de Kimpe SJ., and, van Deutekom JCT. T.O.4 A phase I/IIa study on antisense compound PRO051 in patients with Duchenne muscular dystrophy. Neuromuscular Disorders. 2009;19:659–660. [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM., and, Chamberlain JS. Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol Ther. 2008;16:657–664. doi: 10.1038/mt.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD., and, Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 2006;8:207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- Wu B, Lu P, Benrashid E, Malik S, Ashar J, Doran TJ, et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 2010;17:132–140. doi: 10.1038/gt.2009.120. [DOI] [PubMed] [Google Scholar]
- Sazani P, Gemignani F, Kang SH, Maier MA, Manoharan M, Persmark M, et al. Systemically delivered antisense oligomers upregulate gene expression in mouse tissues. Nat Biotechnol. 2002;20:1228–1233. doi: 10.1038/nbt759. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Morrison J, Lu QL, Pastoret C, Partridge T., and, Bou-Gharios G. T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Invest. 2000;80:881–891. doi: 10.1038/labinvest.3780092. [DOI] [PubMed] [Google Scholar]
- Spurney CF, Gordish-Dressman H, Guerron AD, Sali A, Pandey GS, Rawat R, et al. Preclinical drug trials in the mdx mouse: assessment of reliable and sensitive outcome measures. Muscle Nerve. 2009;39:591–602. doi: 10.1002/mus.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Bach JR., and, Minami R. Cardioprotection for Duchenne's muscular dystrophy. Am Heart J. 1999;137:895–902. doi: 10.1016/s0002-8703(99)70414-x. [DOI] [PubMed] [Google Scholar]
- Markham LW, Kinnett K, Wong BL, Woodrow Benson D., and, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365–370. doi: 10.1016/j.nmd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Arechavala-Gomeza V, Kinali M, Feng L, Brown SC, Sewry C, Morgan JE, et al. Immunohistological intensity measurements as a tool to assess sarcolemma-associated protein expression. Neuropathol Appl Neurobiol. 2010;36:265–274. doi: 10.1111/j.1365-2990.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- Foster H, Sharp PS, Athanasopoulos T, Trollet C, Graham IR, Foster K, et al. Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol Ther. 2008;16:1825–1832. doi: 10.1038/mt.2008.186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic of the experimental regimen used for the chronic administration of PMOs by intravenous delivery in mdx mice. Mdx mice were systemically treated with 2 or 5 cycles of 4 weekly intravenous injections of 50 mg/kg or 5 mg/kg of PMO, with a 6 week period of rest. Treatment with PMO commenced when the mdx mice were 6 weeks old. Mice were analysed 6 weeks after the last injection. Untreated mdx and C57 mice were used as age-matched control. The weeks of injections are showed in red. In blue the numbers of mice sacrificed at each time point are shown.
Evaluation of sarcolemmal permeability and dystrophin protein complex (DPC) restoration in DAPC following chronic administration of PMOs by intravenous delivery in mdx mice. Mdx mice were treated for a period of 20 or 50 weeks over which 2 or 5 cycles of repeated low dose (LD: 5 mg/kg/week) and high dose (HD: 50 mg/kg/week) PMO were administered intravenously. TA muscles were processed and cryosections stained for dystrophin, intracellular endogenous mouse IgG, β-dystroglycan and α-sarcoglycan. (a) IgG/dystrophin co-immunostaining demonstrates the sarcolemmal integrity in treated mice compared to mdx mice. Representative fields of untreated, 20 and 50 weeks treated mdx and C57BL10 controls stained for dystrophin (red) and IgG (green) are shown. (b) The dystrophin associated protein complex was correctly restored in dystrophin positive fibres after both treatments. White asterisks identify the same fibres in serial sections that are stained positive for the proteins indicated. (c) A similar number of fibres co-expressed dystrophin and nNOS following after both 2 and 5 cycles of administration. Scale bar: 200 μm.
Evaluation of body mass following chronic administration of PMOs by intravenous delivery in mdx mice. During the first 20 weeks, treated and untreated mdx and C57BL10 mice had a similar body weight. In the following 30 weeks the difference in weight between C57BL10 and all the other mice considerably increases.