Abstract
Recent trials in patients with neurodegenerative diseases documented the safety of gene therapy based on adeno-associated virus (AAV) vectors deposited into the brain. Inborn errors of the metabolism are the most frequent causes of neurodegeneration in pre-adulthood. In Sanfilippo syndrome, a lysosomal storage disease in which heparan sulfate oligosaccharides accumulate, the onset of clinical manifestation is before 5 years. Studies in the mouse model showed that gene therapy providing the missing enzyme α-N-acetyl-glucosaminidase to brain cells prevents neurodegeneration and improves behavior. We now document safety and efficacy in affected dogs. Animals received eight deposits of a serotype 5 AAV vector, including vector prepared in insect Sf9 cells. As shown previously in dogs with the closely related Hurler syndrome, immunosuppression was necessary to prevent neuroinflammation and elimination of transduced cells. In immunosuppressed dogs, vector was efficiently delivered throughout the brain, induced α-N-acetyl-glucosaminidase production, cleared stored compounds and storage lesions. The suitability of the procedure for clinical application was further assessed in Hurler dogs, providing information on reproducibility, tolerance, appropriate vector type and dosage, and optimal age for treatment in a total number of 25 treated dogs. Results strongly support projects of human trials aimed at assessing this treatment in Sanfilippo syndrome.
Introduction
Several recent human gene therapy trials for the treatment of neurodegenerative diseases relied on the deposit of adeno-associated virus (AAV) vectors directly into the brain. Results of phase I and phase II studies in Parkinson disease1,2,3 and of phase I studies in pediatric neurodegenerative diseases4,5 indicated that this procedure is safe.
Lysosomal storage diseases represent the most frequent cause of neurodegeneration in pre-adulthood. AAV-mediated direct gene transfer to the brain has been considered for the treatment of several neuropathic lysosomal storage diseases.6 This approach is a particularly appropriate treatment option for most forms of mucopolysaccharidosis type III (MPSIII, i.e., Sanfilippo syndrome). This disease is a heterogeneous condition caused by the deficiency of one of the lysosomal enzymes specifically required for the degradation of heparan sulfate glycosaminoglycans (GAGs).7,8 Four genetic forms of MPSIII have been described. Type A (MPSIIIA, OMIM #252900), due to sulfamidase (SGSH; EC 3.10.1.1) deficiency, and type B (MPSIIIB, OMIM #252920), due to α-N-acetylglucosaminidase (NAGLU; EC 3.2.1.50) deficiency, are the most frequent. The accumulation of incompletely degraded GAGs in affected cells and tissues is the unique cause of cascades of pathological events. They include the secondary accumulation of gangliosides (GM2 and GM3), which possibly participate in neuropathology,9 and the progressive storage of intracellular vacuoles with lysosomal characteristics throughout the central nervous system.10 Disease onset is before 5 years marked by hyperactivity and progressive mental impairment and loss of social interaction. The severe neurological consequences of the disease are in stark contrast to the relatively mild somatic disease. By 10–15 years of age, affected children are disabled, often nonambulatory, requiring nutritional support, and in a semivegetative state. They often succumb to their disease in their mid-teens. Correction of the enzyme deficiency in the brain and subsequent clearance of GAGs, GM2/GM3, and storage lesions will presumably prevent or reduce the most severe clinical manifestations of the disease. Indeed, AAV-mediated gene therapy in the brain of the mouse models of MPSIIIA11 or MPSIIIB12,13 improved neuropathology and animal behavior.
In order to translate proof-of-concept of efficacy from mouse models to human application, we previously explored AAV-mediated gene therapy in the brain in seven dogs with MPSI,14 a disease closely related to MPSIII, in which a defect of the lysosomal enzyme α-iduronidase (IDUA; EC 3.2.1.76) interrupts the degradation of both heparan and dermatan sulfate.15 MPSI (OMIM #607014) differs from MPSIII by severe skeletal involvement and by the relative efficacy of treatments based on hematopoietic stem cell transplantation.7,8 Therefore, MPSI is not as attractive a candidate for brain-directed AAV-mediated gene therapy as is MPSIII. The dog model of MPSI is nevertheless relevant for assessing tolerance and efficacy of gene therapy in a large animal developing MPS-related brain pathology. Herein, we describe completion of this treatment approach in 10 additional MPSI dogs, in which different treatment protocols were explored. Results reported here emphasize the efficacy and reproducibility of the procedure and provide useful information relative to the influence of vector type, vector dose, and age at treatment.
At the onset of AAV therapy evaluation in the MPSI dog, no large animal model of MPSIII was available. However, since that time a canine model has been identified and characterized for MPSIIIB,16 which we have used in the present study to assess the safety and efficacy of brain-directed AAV-mediated gene therapy for the treatment of children with MPSIIIB. Affected animals do not express clinical manifestations of the disease until ataxia occurs at late and varying age (18–30 months). Like MPSI dogs, MPSIIIB dogs tolerated AAV-mediated gene therapy well. Biochemical and pathological markers of the disease were improved in the entire brain of treated animals. We also confirmed that the combination of gene therapy with efficient immunosuppression was required for treatment efficacy.
Results
Treatment protocol and tolerance
The tolerance and efficacy of AAV-mediated gene therapy in the brain was assessed in 25 young adult dogs with MPSIIIB (n = 9), MPSI (n = 14), or not affected (n = 2). Analyses in MPSIIIB dogs were performed in 12.2–18.5-month-old animals. Studied animals belonged to three groups: untreated dogs (n = 3), dogs treated with the AAV2.5NAGLU vector without full immunosuppression (age at treatment: 9.5 and 10.8 months, n = 2), and dogs treated with AAV2.5NAGLU vector and immunosuppression (age at treatment: 7.6–14.1 months, n = 7). The latter group included two dogs that received vector prepared in insect Sf9 cells using baculovirus vectors (B12 and B15). Analyses in MPSI dogs were performed in 4.2–16.0-month-old animals. Studied animals belonged to four groups: untreated dogs (n = 5, including one dog that received immunosuppressant), dogs treated with the AAV5.5IDUA vector and immunosuppressant as young adults (age at treatment: 3.4–4.8 months, n = 4, partial data on these dogs have been previously reported but are shown again to facilitate comparison with new animals that received different treatment protocols15), dogs treated with AAV2.5IDUA vector and immunosuppressant as young adults (age at treatment: 3.9–5.2 months, n = 6), and dogs treated with the AAV2.5IDUA vector and immunosuppressant at older age (age at treatment: 7.3–10.0 months, n = 4). Analyses of nonaffected dogs were performed at 1.6–17.0 months of age (n = 7). Studied animals belonged to two groups: untreated dogs (n = 5), and dogs treated with the AAV2.5IDUA vector and immunosuppressant (age at treatment 3.6 and 3.8 months, n = 2).
Treatment consisted of intracerebral deposits of AAV vector. The surgical procedure was similar in all treated dogs. It consisted of four stereotactic tracks with two 50-µl vector deposits at different depth per track (total volume 320 µl, flow 2 µl/mn). The eight successive vector deposits necessitated animal anesthesia for about 4 hours. Surgery was well tolerated. Minor side effects were observed (hypotension, bradycardia, tachycardia, and hyperthermia), which were transitory and easily accommodated by anesthesia protocol adjustment. Animals experiencing this episode had not significant postoperative issues. Transient diminution of palpebral reflexes, fever and unstable blood pressure, and respiration frequency, which spontaneously reversed after 2–3 days, were observed in three animals. One dog (B99) with a suspected hemostatic disorder not related to MPSIIIB after a completely unremarkable surgery experienced semiparesis and proprioceptive deficit that were fully resolved at 1 month.
Based on our previous observations, the administration of immunosuppressant (a combination of cyclosporine and mycophenolate mofetil) was mandatory to prevent immune response against the therapeutic enzyme in MPSI dogs.15 Immunosuppression was associated with frequent side effects, including the development of papillomas (13 dogs, 52%), gingivitis (4 dogs, 16%), diarrhea (19 dogs, 76%). Disability resulting from papillomas necessitated euthanasia in five MPSI dogs with severe skeletal manifestations of the disease. Reactions to immunosuppression had less severe consequences in MPSIIIB dogs (papillomas in three dogs), which appeared healthy at the time of treatment and during follow-up.
Treated MPSIIIB dogs were killed after 3.7–4.4 months. The duration of follow-up of treated MPSI dogs was determined by the health status and varied from 0.7 to 8.3 months. Brain tissue was investigated to assess gene transfer efficiency and treatment efficacy. Analyses consisted of vector genome (vg) quantification by quantitative PCR (qPCR), assay of therapeutic enzyme activity, dosage of primary and secondary storage products (GAG, GM2 and GM3), and histopathology examination. Storage lesion density was determined on histological sections stained by hematein–eosin or luxol fast blue in order to provide an exhaustive view of pathology throughout the brain. A semiquantitative scoring system (0 indicating the absence of lesion, with 3 indicating severe pathology) was used for cerebrum and cerebellum. The number of luxol-positive storage lesions was determined in a defined surface area of the caudate nucleus. Results are summarized in Table 1, shown in Figures 1–4, and commented upon below.
Table 1. Overview of investigated dog groups, treatment conditions, and disease markers.
Figure 1.
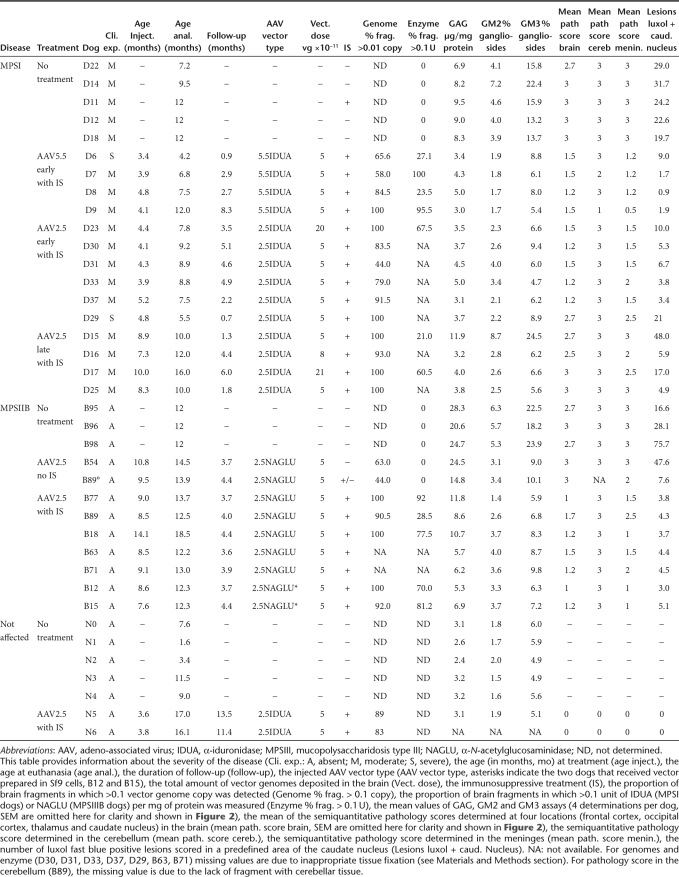
AAV vector genome spreading in dog brains. MPSI or MPSIIIB dogs received AAV-mediated gene therapy and immunosuppressive treatment. Brains were cut in 4–8 coronal slabs, which were further divided in four fragments, producing 16–32 fragments per dog brain. Copy numbers of the endogenous β-glucuronidase gene or AAV vector genomes were determined by quantitative PCR in DNA extracted from each fragment. The ratio of these two values indicates the number of vector genome copies/cell. Supplementary Figure S1 shows the distribution in the brain of fragments containing >0.1 vector genome copies/cell. (a) Histograms indicate the proportion of brain fragments in which >0.1 vector genome copy/cell was measured in each group of treated dog: MPSI dogs treated with AAV5.5IDUA vector (n = 4), MPSI dogs treated with AAV2.5IDUA vector (n = 10), MPSIIIB dogs treated with AAV2.5NaGlu vector (n = 7). Data are means ± SEM and not significantly different between groups. Equivalent proportions indicate equivalent spreading of the different vectors used in this study in dog brain. (b) Mean ± SEM of the proportion of fragments in which >0.1 vector genome copy/cell was measured in MPSI dogs treated with AAV2.5IDUA vector before 5.2 months (D23, D29, D30, D31, D33, D37) or after 7.3 months (D15, D16, D17, D25) of age. Data are not significantly different between groups. AAV, adeno-associated virus; IDUA, α-iduronidase; MPSIII, mucopolysaccharidosis type III; NAGLU, α-N-acetylglucosaminidase.
Figure 4.
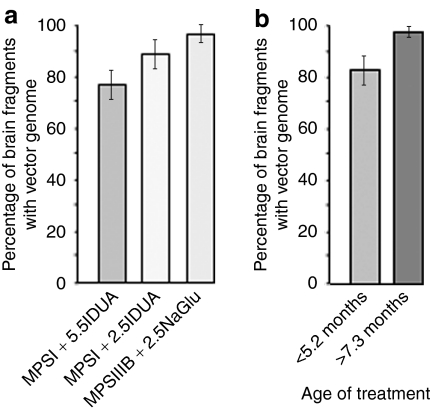
Residual lesions in dog brains. Four-micrometer paraffin sections of the caudate nucleus were stained with silver impregnation and luxol fast blue, which reveals intracytoplasmic accumulation of glycosaminoglycans and gangliosides. Luxol fast blue positive accumulation is visible in untreated MPSIIIB (D98) and MPSI (D14) dogs (left column), as well as in B54, a MPSIIIB dogs that did not receive immunosuppressant (middle, upper row) and in D17, a MPSI dog that was treated at the age of 10.0 months (right, bottom row). In contrast, luxol fast blue positive staining is not visible in B18, a MPSIIIB treated dog that received immunosuppressant (right, upper row) and D30, a MPSI dog that was treated at the age of 4.1 months (bottom, middle row). Bar = 50 µm. AAV, adeno-associated virus; IDUA, α-iduronidase; IS, immunosuppression; MPSIII, mucopolysaccharidosis type III; NAGLU, α-N-acetylglucosaminidase.
AAV genome delivery
Efficient treatment of neuropathology in lysosomal storage diseases supposes broad vector delivery to brain tissues. We previously showed in MPSI dogs (D6, D7, D8, and D9) that AAV5 capsids allow delivery of vg to large portions of the brain.15 We confirmed these findings in the additional MPSI dogs enrolled in the present study and showed a similarly efficient spreading of the AAV2.5NAGLU vector in the brain of MPSIIIB dogs (Table 1 and Supplementary Figure S1).
The proportion of brain tissue samples in which vg were detected by qPCR did not significantly differ between treated MPSI, MPSIIIB, and normal dogs (Figure 1a, P = 0.057). We nevertheless noticed that vg were more frequently detected in the most caudal brain sections in MPSIIIB than in MPSI dogs. Vector distribution did not differ between MPSI dogs treated with AAV5.5IDUA or AAV2.5IDUA vectors (Figure 1a, P = 0.32), and when the AAV2.5IDUA vector was injected in young or older MPSI dogs (Figure 1b, P = 0.136).
All dogs received a total vector dose of 5 × 1011 vg, with three exceptions (D16, D17, and D23). The quantification of vg copy numbers in brain tissue showed large variations depending on the animal (Supplementary Figure S1). Maximal values ranged from 81 to 3,289 genome copies/cell in MPSI dogs and from 52 to 2,480 genome copies/cell in MPSIIIB dogs, without significant difference between these groups. Very low genome copy numbers were detected in B54 (<1 genome copy/cell), a MPSIIIB dog that did not receive immunosuppressant. Genome copy numbers were also low in B89 (maximal value 39 genome copies/cell), a MPSIIIB dog in which immunosuppression was halted 2 months before sacrifice due to digital papillomatosis. The highest vg copy numbers among treated MPSIIIB dogs were measured in the two animals that received vector prepared in Sf9 cells, indicating that this preparation method generates particles capable of efficient penetration of target cells. Whereas vg copy number in D16 (dose: 8 × 1011 vg, maximal value 109 genome copies/cell) and D23 (dose: 20 × 1011 vg, maximal value 602 genome copies/cell) did not differ from the mean value in dogs treated with the standard dose (5 × 1011 vg, maximal value 477 ± 261 genome copies/cell), significantly higher copy numbers were measured in D17 (dose 21 × 1011 vg, maximal value 1,603 genome copies/cell, P = 0.001, one-sample t-test).
Disease correction in MPSIIIB dogs
When compared to nonaffected animals, pathology in the brain of 1-year-old untreated MPSIIIB dogs was characterized by high levels of GAG, GM2/GM3 gangliosides, high scores in semiquantitative scoring of storage lesions, and a high density of quantitatively measured luxol-positive storage lesions in the caudate nucleus (Table 1 and Figures 2–4).
Figure 2.
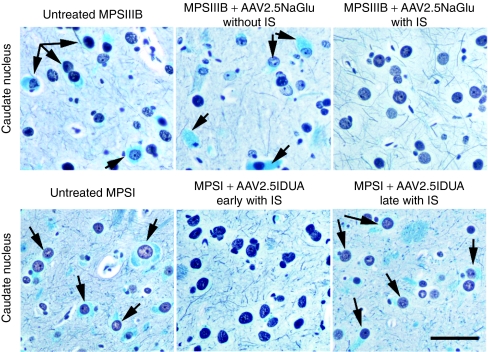
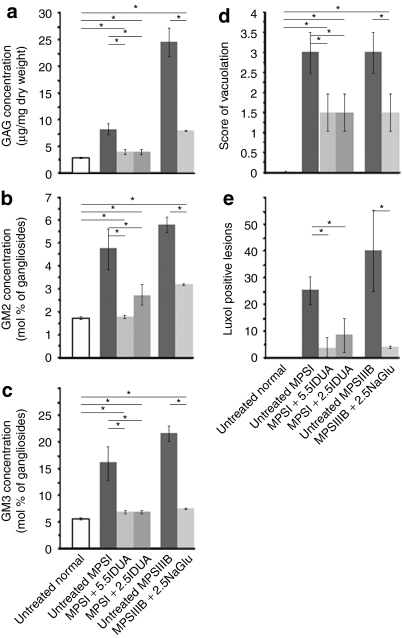
Improvement of disease markers in treated dog brains. Biochemical and histological markers of disease severity in the brain were measured in untreated normal dogs (n = 5), untreated MPSI dogs (n = 5), MPSI dogs treated with AAV5.5IDUA vector (n = 4), MPSI dogs treated with AAV2.5IDUA vector (n = 10), untreated MPSIIIB dogs (n = 3), and MPSIIIB dogs treated with AAV2.5NaGlu vector (n = 7). All treated dogs received immunosuppressants (data from B54 and B89, which were not fully immunosuppressed, are not included). (a–c) Biochemical markers. (a) GAG, (b) GM2, and (c) GM3 amounts were measured in brain extracts. Four values corresponding to four brain regions were determined in each dog. Data are means ± SEM of all determined values in each dog group. (d,e) Histological markers. Brain coronal slabs were processed for 4-µm paraffin sections. (d) Sections from the frontal cortex, the occipital cortex, the thalamus, or the caudate nucleus were stained with hematoxylin–eosin. Cell vacuolation was scored semiquantitatively from zero to three for each of these four regions in each dog, zero indicating absence of observed storage lesions, 1 up to 20% of affected cells, 2 up to 50%, 3 >50% (scores of individual animals at each location are shown in Figure 3). A global score represented by the mean of the four values was attributed to each dog. Histograms indicate means ± SEM of global scores in each dog group. (e) Sections of the caudate nucleus were stained with luxol fast blue, which stains storage lesions in blue. The total number of luxol fast blue positive lesions was scored in a surface of 10.45 ± 0.47 mm2 covering a region located between the internal capsule to the brain surface. Data are means ± SEM of values determined in each dog group. Asterisks indicate significant differences between groups (P < 0.05, Mann and Whitney test). AAV, adeno-associated virus; GAG, glycosaminoglycan; IDUA, α-iduronidase; MPSIII, mucopolysaccharidosis type III; NAGLU, α-N-acetylglucosaminidase.
As previously observed for IDUA delivery in the brain of MPSI dogs,15 NAGLU was detected in large parts of the brain in treated MPSIIIB dogs that received immunosuppression (>70% in four out of the five dogs in which analysis was performed (Table 1 and Supplementary Figure S2). However, NAGLU activity remained low or undetectable in the most rostral and most caudal regions of the brain (especially in the cerebellum). Surprisingly, certain distal areas in which NAGLU was not detected contained vg, as shown by qPCR, suggesting that catalytic activity was too low for detection by the enzyme assay, the sensitivity of which is actually poor (≥0.1 U/mg of protein).
All pathology markers were improved in MPSIIIB dogs that received immunosuppression, in comparison to untreated MPSIIIB dogs (Table 1 and Figures 2 and 3). However, values remained higher than in nonaffected dogs (GAG, P = 0.003; GM2, P = 0.048; GM3, P = 0.005; storage lesions in the caudate nucleus, P = 0.018, Figure 2). Moreover, storage lesions persisted unchanged in the cerebellum and were partially and inconsistently improved in the meninges (Table 1).
Figure 3.
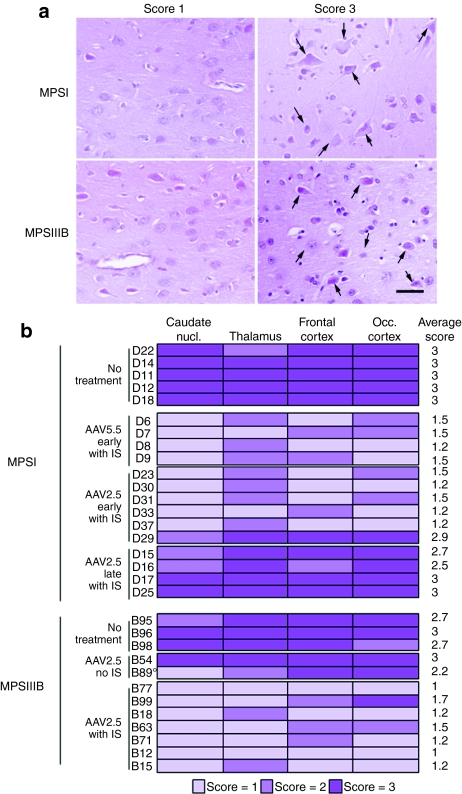
Reduction of cell vacuolation in treated dog brains. Brain coronal slabs were processed for 4-µm paraffin sections. Sections were stained with hematoxylin–eosin and cell vacuolation was scored semiquantitatively from zero to three in the frontal cortex, the occipital cortex, the thalamus and the caudate nucleus (1: up to 20% of affected cells; 2: up to 50%; 3: >50%). (a) Examples of frontal cortex sections scored 1 or 3 in treated (D30) or untreated (D14) MPSI dog, respectively, and in treated (B18) or untreated (B98) MPSIIIB dogs, respectively. (b) A color code indicates scores at each location for each treated or untreated dog examined in this study. A global score represented by the mean of the four determined scores is shown on the right (mean score). This score is used in Table 1 and Figure 2d. All treated dogs received immunosuppressant except B54, which did not receive immunosuppressant, and B89, in which immunosuppressant was halted two months before analysis. AAV, adeno-associated virus; MPSIII, mucopolysaccharidosis type III.
Consistently with our previous observations in treated MPSI dogs that received low immunosuppressant regimen,15 NAGLU activity was not detected in the brain of two dogs (B54 and B89) that did not receive full immunosuppressant regimen (Table 1 and Supplementary Figure S2). In B54, which never received immunosuppressant, GAG levels and storage lesions were comparable to untreated MPSIIIB dogs. GM2 and GM3 accumulations were nevertheless significantly reduced (Table 1, P = 0.012 and P = 0.018, respectively, one-sample t-test). However, pathology was not corrected and intense inflammation was observed in the thalamus (Figure 3). In B89, which received immunosuppressant for 2 months followed by 2 months without immunosuppressant, GAG (P = 0.048), GM2 (P = 0.015), GM3 (P = 0.022), and storage lesions in the caudate nucleus (P = 0.032, one-sample t-test) were significantly reduced, as compared to untreated MPSIIIB dogs. However, inflammation was prominent in the thalamus, perivascular spaces were infiltrated with macrophages, and storage lesions were unchanged as compared to untreated MPSIIIB dogs in the frontal and occipital cortex. These results confirmed that immunosuppression was mandatory to preventing inflammatory response and allowing disease correction in the brain of treated MPSIIIB dogs.
Disease correction in MPSI dogs
When compared to nonaffected animals, pathology in the brain of untreated MPSI dogs was marked by high levels of GAG, GM2/GM3 gangliosides, high scores in the semiquantitative scoring of storage lesions, and high density of luxol fast blue positive storage lesions in the caudate nucleus (Table 1 and Figures 2 and 3). When comparing approximately age-matched animals (7–12 months), GAG storage and GM3 accumulation were more marked in MPSIIIB than in MPSI dog (Figure 2, P = 0.036 and P = 0.053, respectively).
With the exception of one dog (D29), all pathology markers were improved in MPSI dogs treated at early stage of the disease. Values were not significantly different between MPSI dogs treated with AAV5.5IDUA or AAV2.5IDUA (Table 1 and Figure 2). However, considering these two groups of treated MPSI dogs together, as observed for treated MPSIIIB dogs, values remained higher than in nonaffected dogs, indicating persistent low level of GAG storage (P = 0.005), GM2/GM3 ganglioside accumulation (P = 0.001 and P = 0.040) and storage lesions in the caudate nucleus (P = 0.003). Storage lesions persisted in the cerebellum and were only partially improved in the meninges (Table 1).
One animal (D29) showed a contrasting result. Whereas histology was not improved compared to untreated MPSI dogs (Table 1 and Figure 3), GAG, GM2, and GM3 values were lower than in untreated MPSI dogs (Table 1, P < 0.001, P = 0.015, P = 0.011, and P = 0.011, respectively, one-sample t-test). We assume that this dissociated phenotype was related to the short delay between treatment and analysis (0.7 months).
Noticeably, whereas disease correction was fully effective in MPSIIIB dogs treated between 7 and 14.1 months of age, markers of pathology were either not improved or only partially improved in MPSI dogs that received treatment after the age of 7 months, especially with regards to histological lesions (Table 1 and Figure 3). In D15, which was treated at 8.9 months and analyzed shortly after (1.3 months), biomarkers and histology did not show improvement as compared with untreated MPSI dogs. In D17, which was treated at 10 months and followed up for 6 months, biomarkers were improved but histology indicated major residual lesions. In D16 and D25, which were treated at 7.3 and 8.3 months and followed up for 4.4 and 1.8 months, respectively, biomarkers were normalized, residual lesions were minimal in the caudate nucleus, but storage lesions persisted at other locations.
Discussion
We report herein on a comprehensive study of 25 adult dogs that underwent AAV-mediated direct gene therapy in the brain and 13 untreated control dogs. This study extends and confirms our previous conclusions about the safety and efficacy of this treatment in dog models of MPS.15 We provide evidence for tolerance and phenotypic improvement in MPSIIIB, a disease that represents a good candidate for clinical evaluation of gene therapy. Results obtained in the two investigated dog models are fully consistent. The high number of animals that were safely and successfully treated emphasizes the reproducibility of the procedure and its suitability for human application.
AAV vector delivery to the brain
Efficient spreading of vector particles in the brain is crucial for the treatment of diseases like many of the MPSs in which pathology is widespread in the central nervous system. We previously showed in mice,13 dogs,15 and monkeys17 that surgical deposits of AAV5 vectors particles in the brain ensures efficient spreading of vg throughout the cerebrum. These observations were fully confirmed in the present study performed in a large number of dogs. Results showed that after eight deposits of AAV5 particles in the brain through four stereotactic tracks, the average proportion of the dog brain in which vg were detected was in the range of 85%. Eight dogs showed vg at all investigated territories. Similar spreading was observed when AAV2 or AAV5 genomes were packaged in AAV5 particles. Two dogs that received a fourfold higher vector dose were positive throughout the brain, but only one showed higher mean vg copy numbers than the mean value in the 23 animals treated with the regular dose of 5 × 1011 vg, suggesting that the efficiency of vector delivery to brain tissues was not strictly related to the amount of deposited vector particles. For the same vector batch, vg copy numbers in tissues were actually highly variable depending on the animal and the examined brain territory. The analysis of two normal dogs, which received the AAV2.5IDUA vector and were followed up for ~1 year, indicated that high-vector copy number and widespread distribution in the brain persisted over the long term.
Vector particle deposition was well tolerated. Examination of injection sites at various time points after vector deposition (from 3 weeks to 13.5 months) did not reveal, in the presence of concurrent immunosuppression, either persistent local inflammation, or sequel of inflammatory reaction, even in the animals that received the highest vector dose (2 × 1011 vg at each deposit site). Thus, a deposit of 2 × 1011 vg was well tolerated. This finding is important when discussing the adequate vector dosing for treating human patients. Vector tolerance was equally good and spreading equally efficient in the two MPSIIIB dogs that received vector particles produced in insect cells using baculovirus vectors, as compared to dogs that received AAV vector prepared by co-transfection of human embryonic kidney 293 human cells with plasmid DNA. The AAV vector production method based on the use of baculovirus vectors to deliver packaging components to Sf9 cells is well adapted to large scale manufacturing processes required for clinical batches preparation.18 Our results indicate that this method of production should be considered for human trials.
Disease correction
MPSI or MPSIIIB dogs do not express clinical manifestations of central nervous system disease at the ages animals were treated and followed-up. Whereas ataxia occurs at advanced stage of the disease in MPSIIIB dogs, animal age at onset is not a reliable clinical marker because of major variations among affected animals and of the absence of an appropriate description of the natural history of the canine disease. Life span, which depends on the affection of peripheral organs in MPSI dogs and of humane decision in MPSIIIB dogs developing ataxia, is also not an adequate clinical marker. We therefore relied on biochemical markers (GAGs and GM2/GM3 levels) and histological markers to assessing disease severity in dog brain. As pathology associated to MPSI and MPSIIIB is spread throughout the central nervous system, these markers were investigated in the entire brain using adequate methodologies.
Biochemical and histological markers were consistently improved in the almost entire brain in all treated dogs that received immunosuppressant (16 dogs). Improvement of biochemical markers with persistent histological lesions was observed in few dogs, D29 which was examined early after treatment (0.7 months), three MPSI dogs that were treated after the age of 7.3 months of age (D16, D17, D25), and two MPSIIIB dogs that did not receive immunosuppressant (B54, B89). These results suggest that clearance was more rapid and more easily obtained for stored products than for storage lesions. We do not know which of these markers is the most relevant to clinical manifestations in children. With respect to future therapeutic trials, GAG and GM2/GM3 ganglioside concentrations in the cerebrospinal fluid (CSF) represent potential surrogate markers of pathology severity in the brain, and therefore potential end points for the assessment of treatment efficacy in human trials. The observation of a possible dissociation between biochemical and histological markers emphasizes the importance of considering clinical end points in complement to biomarker assays on CSF to assess treatment efficacy in children.
Although biochemical and histological markers were consistently improved in 16 treated dogs that received immunosuppressant, treatment did not result in the complete resolution of disease-associated storage in the brain. Whereas GAG and GM2/GM3 gangliosides levels were close to normal values, they remained elevated relative to those of normal dogs, and residual histological lesions persisted. We do not know whether this residual disease will result in clinical symptoms in treated children. Treatment efficacy was also limited by the lack of improvement in the cerebellum, which was consistent with the almost total absence of vg in this tissue. We presume that an additional local vector deposit will be necessary to treat cerebellar disease and associated signs in children.
The age of MPSI dogs at the time of treatment affected efficacy. The four MPSI dogs treated after the age of 7.3 months showed either no improvement (D15), improvement of biological markers only (D17), or improvement of biological markers and histological lesions near vector deposition sites but not at distant locations (D16 and D25). These results may represent an age corollary in the canine model to young severely affected MPSI patients, where it is recognized that hematopoietic stem cell transplantation is not efficacious for patients older than 24 months.8 In contrast, MPSIIIB dogs responded fully to treatment when treatment was initiated at 9.2 ± 2.2 months. This result suggests different kinetics of disease progression may apply to canine MPSI and MPSIIIB.
Immunosuppression
A major determinant of treatment safety and efficacy was immunosuppression. Confirming our previous observations in MPSI dogs that received low immunosuppressant regimen,15 we show that absence (B54), or interruption (B89) of immunosuppressant in MPSIIIB dogs resulted in low vector copy numbers, absence of detectable NAGLU activity in brain extracts, almost unchanged pathology severity and marked inflammatory reaction. These results unambiguously indicate that effective immunosuppression will be mandatory for treatment safety and efficacy in children. Immunization against the therapeutic recombinant enzyme is frequent in children receiving enzyme replacement therapy, including in patients with residual enzyme activity.19 Thus, the synthesis of a mutant protein does not necessarily induce tolerance to the wild-type molecule. We previously showed that the immune response was directed against both the vector components and the therapeutic enzyme IDUA in treated MPSI dogs.15 We therefore presume that a similar reaction occurred against NAGLU in treated MPSIIIB dogs. However, the presence of anti-NAGLU antibodies in treated dog brain extracts could not be documented because antibodies are not available to designing appropriate assays. Low vg copy numbers in dogs that did not receive immunosuppressant is consistent with the immune elimination of transgenic cells. Interestingly, the improvement of biological markers in B54 and B89 suggests that transient delivery of the therapeutic enzyme preceding immune reaction might have been sufficient to clear primary storage products (GAGs), at least partially and transiently.
This extensive study in dogs provides large animal validation for a treatment of central nervous system manifestations of MPSI and MPSIIIB in affected children using AAV-mediated gene therapy directed to the brain. Patients with MPSIII represent the group of neuropathic MPS patients with the most urgent need for an effective treatment that targets the brain. Indeed, in contrast to MPSI, MPSIII does not appear to be improved by hematopoietic stem cell transplantation and is not associated with severe peripheral manifestations. The devastating natural course of this disease may mitigate consideration of such an approach to therapy, in spite of the known or potential and unforeseeable risks of gene therapy, in addition to the well-documented risks of immunosuppression.
Materials and Methods
AAV vector structure, construction, and production. The structure of the AAV5.5-hIDUA vector was previously reported.20 Briefly, this vector contains the murine phosphoglycerate kinase (pgk) gene promoter, the human complementary DNA (cDNA) coding for IDUA, the woodchuck post-translational response element and the bovine hormone polyadenylation site. The AAV2.5-hIDUA has a similar structure in an AAV2 backbone. The AAV2.5-hNAGLU contains the murine phosphoglycerate kinase gene promoter, the human cDNA coding for NAGLU and the bovine hormone polyadenylation site, but does not contain woodchuck post-translational response element. Vector production in human embryonic kidney 293 cells was performed as previously described.20
For production in Sf9 cells, the human NAGLU cDNA was previously cloned into a pUC-derived plasmid, pKAAV-PGK-hNAGLU-pA, which contains the hNAGLU cDNA under the control of the mouse PGK gene promoter and the bovine hormone polyadenylation site. The fragment containing the ITR and the hNAGLU expression cassette was cloned into the pPSC10 transfer plasmid (Protein Science, Meriden, CT). The sequence of the final clone was verified by DNA sequencing. A recombinant hNAGLU baculovirus (BAC-hNAGLU) was generated by co-transfection of Sf9 cells with the pPSC10-hNAGLU transfer plasmid and the linearized polyhedrin gene-deficient AcNPV virus genome. Titration and integrity testing of BAC-hNAGLU was performed by qPCR. The rAAV5-hNAGLU vector was produced by triple-infection of Sf9 cells with BAC-Rep, BAC-Cap5 and the BAC-hNAGLU, as described.18 Seventy-two hours before infection, baculovirus stock was amplified by infection of Sf9 cells. Seventy-two hours postinfection, Sf9 cells were counted to determine the percentage of viability, and were harvested by centrifugation (1,900g for 15 minutes at 4 °C). Cells were lysed at 28 °C for 1 hour under agitation and then treated with Benzonase (Merck, Lyon, France) for 1 hour at 37 °C. Supernatant was clarified by centrifugation at 1,900g for 15 minutes at 4 °C and further purified by affinity chromatography using AVB Sepharose High-Performance affinity medium (GE Healthcare, Velizy, France). A second purification step was added using a discontinuous iodixanol gradient protocol as previously described.21 The fraction containing the higher quantity of vg (determined by qPCR) was ultrafiltrated trough Vivaspin 15 centrifugal tubes (30000 MWCO; Sartorius Stedim, Aubagne, France) and diafiltrated. The final product was formulated in a solution of phosphate-buffered saline with 5% sucrose.
MPSIIIB dogs. The canine model of MPSIIIB has been previously described.16 All animal studies involving MPSIIIB were conducted at Iowa State University and followed both National Institutes of Health and United States Department of Agriculture guidelines for the care and use of dogs in research. All protocols were conducted according to protocols reviewed and approved by the Iowa State University IACUC committee. Heterozygous and affected MPSIIIB breeders were used to produce affected MPSIIIB dogs. Dogs were diagnosed at birth by a PCR assay for the mutant NAGLU allele. The natural history of the disease in these dogs is such that animals are apparently healthy until 18–30 months, after which they progressively develop severe ataxia. This dog colony carries an additional genetic defect (von Willebrand deficiency), that is unrelated to MPSIIIB and which was identified after the first three dogs were treated (B54, B77, and B99). Subsequent animals were tested to confirm their nonaffected status with regard to von Willebrand factor.
MPSI dogs. Heterozygous MPSI breeders were obtained from Dr E. Kakkis (BioMarin Pharm, Novato, CA) and Dr H.P. Kiem (Fred Hutchinson Cancer Research Center, Seattle, WA). Dogs carrying the recessive mutation underlying canine MPSI22 were bred at the Centre de Boisbonne of the National Veterinary School of Nantes. Homozygous puppies (MPSI dogs) were diagnosed at birth by IDUA assay showing the absence of detectable activity in peripheral blood lymphocyte extracts. Clinical symptoms of MPSI were noticed soon after birth in most severely affected dogs and before 3 months of age in all MPSI dogs. Symptoms included corneal clouding, facial dysmorphism, dysostosis multiplex, umbilical hernia, liver failure, and holosystolic cardiac murmur. The Institutional Animal Care and Use Committee of the National Veterinary School of Nantes and the University of Nantes approved experiments, which were performed by authorized investigators.
Surgery. MPSI dogs were anesthetized with a combination of medetomidine (Domitor; Pfizer, Paris, France), morphine (Morphine; Aguettant, Lyon, France), and ketamine (Imalgène; Merial, Villeurbanne, France) given intravenously as induction drugs. After endotracheal intubation, anesthesia was maintained with 1.5% isoflurane. For MPSIIIB dogs, anesthesia consisted of isoflurane inhalation (3% vol/vol) and morphine injection (0.1 mg/kg). Dogs were monitored by clinical observations, respiratory rate measurement, temperature measurement, electrocardiography, pulse oximetry, and capnography. Vector was deposited at two locations (four deposits) in each brain hemisphere during a single session. Tracks targeted the putamen and the centrum semiovalae (0.2 mm rostral and 10 mm caudal, respectively, 15 mm lateral to the bregma, depth 10 and 20 mm). All treated animals received the same vector suspension volume (8 × 40 µl) at the same injection flow (2 µl/minute). Amounts of deposited vector depended on vector concentrations in the deposited suspension (5 × 1011 vg, 1.5 × 1012 vg/ml; 8 × 1011 vg, 2.5 × 1012 vg/ml; 20 × 1011 vg, 6.5 × 1012 vg/ml). No immediate or early severe side effect presumed to be directly related to surgery or vector deposits was noticed.
Immunosuppression. Immunosuppressants consisted in the combination of mycophenolate mofetil (800 mg/m2/day) and a weekly-adjusted dose of cyclosporine to maintain cyclosporinemia >300 ng/ml.
Tissue processing. MPSI dogs were killed by over anesthesia when they become unable to stand on their legs or stop taking food. MPSIIIB dogs were killed ~4 months after surgery. Anesthetized animals were perfused with 150 ml of phosphate-buffered saline and 600 ml of 4% paraformaldehyde in phosphate-buffered saline. Brain hemispheres were immediately cut into 4-mm thick coronal slabs (n = 15–17). Every third slab was used for vg and NAGLU or IDUA activity detection, GAG and ganglioside assays, and histology, respectively. Slabs used for genome detection and enzyme assays were divided in dorsal lateral, dorsal median, ventral lateral, and ventral median quarters. Each quarter was cut into ~100 mg fragments, which were submitted to three freeze-thaw cycles after the addition of water (200 µl). DNA was extracted from nuclear pellets, and pools corresponding to each entire quarter were used for qPCR. Pools of supernatants were similarly used for enzyme assay. We usually recovered in the range of 40 µg of DNA and 15–20 mg of protein/gram of tissue. Much lower values suggested that tissue fixation had been too intense to allow efficient extraction and did not allow reliable vector detection (B63, B71), or enzyme assay (D29, D30, D31, D33, D37, B63, B71). For GAG and gangliosides assays, four samples were analyzed for each dog brain. Each sample consisted in the mixture of two slabs (two rostral left, two rostral right, two dorsal left, and two dorsal right). Values indicated in Table 1 and Figure 2 are means ± SEM of the four measured values.
Determination of vg copy numbers by qPCR analysis. Sequences from the human NAGLU cDNA, the human IDUA cDNA and the canine β-glucuronidase gene were amplified. Vg copy numbers were determined by qPCR. Sequences from the human NAGLU cDNA or human IDUA cDNA were amplified using either SyberGreen (D6, D7, D8, D9), or Taqman (other dogs) qPCR methods. Equivalent efficiency of the Taqman and SyberGreen methods was previously documented in this context.15 Reference curves were established by determining cycle threshold (Ct) values for the amplification of serial dilutions of plasmid DNA containing the human NAGLU23 or IDUA24 cDNA (38–6 × 105 copies). The same sequences were simultaneously amplified from serial dilutions of dog brain genomic DNA (0.156–50 ng of DNA/reaction). Vg copy numbers were calculated for every Ct according to reference curves and expressed for 2N genomes according to measured canine β-glucuronidase gene copy numbers. Primers and probes: human NAGLU cDNA, 5′-CAGTGCTGCCTGCATTCG-3′ (forward), 5′-GTGACATTGACCTGCATTCG-3′ (reverse), 5′-FAM- TTCCCGAGGCTGTCACCAGG-TAMRA-3′ (probe); human IDUA cDNA, 5′-ACTTGGACCTTCTCAGGGAGAAC-3′ (forward), 5′-CACCTGCTTGTCCTCAAAGTCA-3′ (reverse), 5′-FAM-CAGCGCCTCGGGCCACTTCA-TAMRA-3′ (probe); canine β-glucuronidase gene exon 9, 5′-ACGCTGATTGCTCACACCAA-3′ (forward), 5′-CCCCAGGTCTGCTTCATAGTTG-3′ (reverse), 5′-FAM-CCCGGCCCGTGACCTTTGTGA-TAM RA-3′ (probe). Amplification parameters were as previously described.15 The assay sensitivity threshold was 0.1 copies per diploid genome.
α-N-acetyl glucosaminidase and α-l-iduronidase asays. NAGLU and IDUA assays were performed as described.25,26 Tissue was homogenized in water, submitted to three freeze/thaw cycles and centrifuged at 13,000 r.p.m. for 3 minutes. Detection threshold enzyme activity in tissue extract was 0.1 U/mg of protein.
Histology. Neuropathology was assessed on full-hemisphere coronal slabs that had been fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4-µm sections. In treated dogs, sections were prepared from slabs adjacent to those in which vg and IDUA or NAGLU were assayed, providing information about gene and enzyme delivery at the examined locations. Sections were stained either with hematoxylin–eosin for semiquantitative analyses or with periodic acid-Schiff APS and luxol fast blue associated to Bodian's silver impregnation for quantitative analysis of residual lesions.
Storage lesions quantification. Semiquantitative analyses were performed in four neuroanatomic areas (frontal cortex, caudate nucleus, thalamus, and occipital cortex). Lysosomal distension was scored according to the percentage of vacuolated cells (absence = 0; presence in up to 20% of the cells = 1; up to 50% = 2; >50% = 3). For quantitative analysis of storage lesions, neuronal profiles were scored as positive when their cytoplasm contained luxol-stained granules. A 97.7% scoring reliability was measured using materials from five untreated MPSI dogs. Lesions were not detected in nonaffected dogs.
Analysis of gangliosides. Samples were homogenized with a minimum volume of water (10% vol./weight). Total lipids were extracted from water homogenates27 isolated and desalted using reverse-phase Bond Elut C18 columns (Varian, Buc, France).28 Gangliosides were separated on G60 high-performance thin-layer chromatography plates (Merck), visualized by resorcinol/HCl and quantified by densitometry. Data (mean of duplicates) were expressed as molar percentage of total gangliosides.
GAG assay. Frozen samples were homogenized with a minimum volume of water (10% vol./weight). Defatted pellets were dried and weighed. Dried residues were digested overnight at 65 °C with papain (0.3% wt.:vol.) in 3 ml of 100 mmol/l sodium acetate buffer pH 5.5 containing 5 mmol/l cysteine and 5 mmol/l EDTA. After centrifugation, GAGs were measured in the supernatant with a dimethylmethylene blue dye-binding assay. Briefly, 200 µl of the supernatant was added to 2.5 ml of dimethylmethylene blue reagent29 and the absorbance at 535 nm was measured. Heparan sulfate (H7640; Sigma-Aldrich, St Quentin-Fallavier, France) was used as standard. Data (mean of duplicates) were expressed as µg of GAGs per mg of dried pellet.
Statistical analysis. Statistics were performed using the SPSS software (SPSS). The assumption that the values follow normal distribution was verified by the Shapiro–Wilk's test. Indicated P values were calculated using Student's t-test, except when specified otherwise.
SUPPLEMENTARY MATERIAL Figure S1. Spreading of AAV vectors in treated dog brains. Pictures are schematic representations of dog brain left and right hemispheres. Coronal slab numbers are indicated on the left. Small boxes correspond to latero-dorsal (LD), latero-ventral (LV), median-dorsal (MD) and median-ventral (MV) fragments generated form each slab. Fragments with more than 0.1 vector genome copy per cell are shadowed. White boxes state for values below 0.1 copy per cell. Figure S2. Spreading of IDUA or NaGlu activity in treated dog brains. Fragments with more than 0.1 U/mg of protein of IDUA or NAGLU activity are shadowed. White boxes state for values below 0.1 U/mg of protein.
Acknowledgments
We are grateful to Drs E. Kakkis and H.P. Kiem for the generous gift of MPSI breeder dogs. We thank Dr L. Vérot, Dr M. Perrocheau, F. Thouron, Jérêrome Amiaud, and Johan Deniaud for technical help, and a cadre of Iowa State University undergraduate students for assistance with animal production and care. This work was supported by a join program of the Association Française contre les Myopathies, the Institut Pasteur and the Institut National de la Santé et de la Recherche Médicale (Programme AAVMPS), by a grant from the Conny Maeva Charitable Foundation, the Sanfilippo Children's Research Foundation, and by Amsterdam Molecular Therapeutics.
Supplementary Material
Spreading of AAV vectors in treated dog brains. Pictures are schematic representations of dog brain left and right hemispheres. Coronal slab numbers are indicated on the left. Small boxes correspond to latero-dorsal (LD), latero-ventral (LV), median-dorsal (MD) and median-ventral (MV) fragments generated form each slab. Fragments with more than 0.1 vector genome copy per cell are shadowed. White boxes state for values below 0.1 copy per cell.
Spreading of IDUA or NaGlu activity in treated dog brains. Fragments with more than 0.1 U/mg of protein of IDUA or NAGLU activity are shadowed. White boxes state for values below 0.1 U/mg of protein.
REFERENCES
- Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- Ellinwood NM, Vite CH., and, Haskins ME. Gene therapy for lysosomal storage diseases: the lessons and promise of animal models. J Gene Med. 2004;6:481–506. doi: 10.1002/jgm.581. [DOI] [PubMed] [Google Scholar]
- Neufeld EF., and, Muenzer J. Scriver CR, Beaudet AL, Sly WS, Valle D. The Metabolic and Molecular Basis of Inherited Disease, 8th edn. McGraw-Hill: New York. Pp. 3421–3452; 2001. The mucopolysaccharidoses. [Google Scholar]
- Pastores GM, Arn P, Beck M, Clarke JT, Guffon N, Kaplan P, et al. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol Genet Metab. 2007;91:37–47. doi: 10.1016/j.ymgme.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Walkley SU. Secondary accumulation of gangliosides in lysosomal storage disorders. Semin Cell Dev Biol. 2004;15:433–444. doi: 10.1016/j.semcdb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Li HH, Yu WH, Rozengurt N, Zhao HZ, Lyons KM, Anagnostaras S, et al. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding α-N-acetylglucosaminidase. Proc Natl Acad Sci USA. 1999;96:14505–14510. doi: 10.1073/pnas.96.25.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraldi A, Hemsley K, Crawley A, Lombardi A, Lau A, Sutherland L, et al. Functional correction of CNS lesions in an MPS-IIIA mouse model by intracerebral AAV-mediated delivery of sulfamidase and SUMF1 genes. Hum Mol Genet. 2007;16:2693–2702. doi: 10.1093/hmg/ddm223. [DOI] [PubMed] [Google Scholar]
- Fu H, Samulski RJ, McCown TJ, Picornell YJ, Fletcher D., and, Muenzer J. Neurological correction of lysosomal storage in a mucopolysaccharidosis IIIB mouse model by adeno-associated virus-mediated gene delivery. Mol Ther. 2002;5:42–49. doi: 10.1006/mthe.2001.0514. [DOI] [PubMed] [Google Scholar]
- Cressant A, Desmaris N, Verot L, Bréjot T, Froissart R, Vanier MT, et al. Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum. J Neurosci. 2004;24:10229–10239. doi: 10.1523/JNEUROSCI.3558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull RM, Munger RJ, Spellacy E, Hall CW, Constantopoulos G., and, Neufeld EF. Canine α-L-iduronidase deficiency. A model of mucopolysaccharidosis I. Am J Pathol. 1982;109:244–248. [PMC free article] [PubMed] [Google Scholar]
- Ciron C, Desmaris N, Colle MA, Raoul S, Joussemet B, Vérot L, et al. Gene therapy of the brain in the dog model of Hurler's syndrome. Ann Neurol. 2006;60:204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- Ellinwood NM, Wang P, Skeen T, Sharp NJ, Cesta M, Decker S, et al. A model of mucopolysaccharidosis IIIB (Sanfilippo syndrome type IIIB): N-acetyl-α-D-glucosaminidase deficiency in Schipperke dogs. J Inherit Metab Dis. 2003;26:489–504. doi: 10.1023/a:1025177411938. [DOI] [PubMed] [Google Scholar]
- Ciron C, Cressant A, Roux F, Raoul S, Cherel Y, Hantraye P, et al. Human α-iduronidase gene transfer mediated by adeno-associated virus types 1, 2, and 5 in the brain of nonhuman primates: vector diffusion and biodistribution. Hum Gene Ther. 2009;20:350–360. doi: 10.1089/hum.2008.155. [DOI] [PubMed] [Google Scholar]
- Urabe M, Ding C., and, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- Kakavanos R, Turner CT, Hopwood JJ, Kakkis ED., and, Brooks DA. Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I. Lancet. 2003;361:1608–1613. doi: 10.1016/S0140-6736(03)13311-9. [DOI] [PubMed] [Google Scholar]
- Desmaris N, Verot L, Puech JP, Caillaud C, Vanier MT., and, Heard JM. Prevention of neuropathology in the mouse model of Hurler syndrome. Ann Neurol. 2004;56:68–76. doi: 10.1002/ana.20150. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- Menon KP, Tieu PT., and, Neufeld EF. Architecture of the canine IDUA gene and mutation underlying canine mucopolysaccharidosis I. Genomics. 1992;14:763–768. doi: 10.1016/s0888-7543(05)80182-x. [DOI] [PubMed] [Google Scholar]
- Weber B, Blanch L, Clements PR, Scott HS., and, Hopwood JJ. Cloning and expression of the gene involved in Sanfilippo B syndrome (mucopolysaccharidosis III B) Hum Mol Genet. 1996;5:771–777. doi: 10.1093/hmg/5.6.771. [DOI] [PubMed] [Google Scholar]
- Scott HS, Anson DS, Orsborn AM, Nelson PV, Clements PR, Morris CP, et al. Human α-L-iduronidase: cDNA isolation and expression. Proc Natl Acad Sci USA. 1991;88:9695–9699. doi: 10.1073/pnas.88.21.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkis ED, Matynia A, Jonas AJ., and, Neufeld EF. Overexpression of the human lysosomal enzyme α-L-iduronidase in Chinese hamster ovary cells. Protein Expr Purif. 1994;5:225–232. doi: 10.1006/prep.1994.1035. [DOI] [PubMed] [Google Scholar]
- Marsh J., and, Fensom AH. 4-Methylumbelliferyl α-N-acetylglucosaminidase activity for diagnosis of Sanfilippo B disease. Clin Genet. 1985;27:258–262. doi: 10.1111/j.1399-0004.1985.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Fujita N, Suzuki K, Vanier MT, Popko B, Maeda N, Klein A, et al. Targeted disruption of the mouse sphingolipid activator protein gene: a complex phenotype, including severe leukodystrophy and wide-spread storage of multiple sphingolipids. Hum Mol Genet. 1996;5:711–725. doi: 10.1093/hmg/5.6.711. [DOI] [PubMed] [Google Scholar]
- Kyrklund T. Two procedures to remove polar contaminants from a crude brain lipid extract by using prepacked reversed-phase columns. Lipids. 1987;22:274–277. doi: 10.1007/BF02533991. [DOI] [PubMed] [Google Scholar]
- Whitley CB, Ridnour MD, Draper KA, Dutton CM., and, Neglia JP. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989;35:374–379. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spreading of AAV vectors in treated dog brains. Pictures are schematic representations of dog brain left and right hemispheres. Coronal slab numbers are indicated on the left. Small boxes correspond to latero-dorsal (LD), latero-ventral (LV), median-dorsal (MD) and median-ventral (MV) fragments generated form each slab. Fragments with more than 0.1 vector genome copy per cell are shadowed. White boxes state for values below 0.1 copy per cell.
Spreading of IDUA or NaGlu activity in treated dog brains. Fragments with more than 0.1 U/mg of protein of IDUA or NAGLU activity are shadowed. White boxes state for values below 0.1 U/mg of protein.