Abstract
The elimination process of systemically administered small interfering RNA (siRNA) was investigated by using siRNA labeled with an infrared fluorescent dye. A novel siRNA elimination pathway was identified. In this pathway, liver-enriched siRNA is secreted into the gallbladder and then emptied into the intestine. Blocking this pathway resulted in the absence of siRNA fluorescence within the intestine, with greatly enhanced siRNA accumulation in liver and gallbladder at the same time. Furthermore, we demonstrated that delivery carriers play an essential role in siRNA distribution and elimination, highlighting their importance in siRNA therapeutics.
Introduction
RNA interference is a fundamental pathway in eukaryotic cells by which small interfering RNA (siRNA) is able to target and mediate cleavage of complementary mRNA transcripts, thereby repressing target gene expression and compromising the function of targeted genes.1 Since Tuschl and co-workers demonstrated that synthetic siRNA mediates efficient RNA interference in mammalian cells,2 considerable efforts have been made to develop siRNA-based therapeutic reagents for the treatment of various diseases.3,4,5,6 Theoretically, the RNA interference machinery can be exploited to silence any target gene, in particular targets not amenable to manipulation by conventional small molecule drugs, only if an appropriate siRNA can be designed and used. It is believed that systemic delivery of siRNA will be one of the main routes for in vivo application of siRNA. Thus, in order to translate their efficient gene silencing into a potent therapeutic strategy, it is crucial to understand the behavior and fate of systemically delivered siRNAs in vivo. This is often done by tracing the distribution of siRNA in model animals. After delivery into the bloodstream, the siRNA normally undergoes an initial distribution throughout the circulatory system in the first few minutes. Then the siRNA molecules are subjected to rapid clearance from the blood through liver accumulation and renal filtration, therefore displaying a patterned distribution in whole-animal imaging assays. Investigations on this topic indicate that, although the initial blood clearance of circulating siRNA occurs through liver accumulation and renal filtration, the final elimination appears to be only through the renal system in urine.2,7 The fate of siRNAs accumulated in the liver and their elimination from the body, another critical issue in siRNA drug development, is however rarely addressed.
In the latest in vivo siRNA studies, fluorescence dyes are preferred over isotopes due to their stability, safety, flexibility, and compatibility with high-throughput observation. Autofluorescence of dietary components, skin, and hemoglobin however, poses a significant challenge to the application of fluorescence dyes because the emission spectra of these biological milieus were found to overlap with those of most fluorescence dyes used in biological research. In the present study, we used Cy5-labeled siRNA and interrogated the process of siRNA elimination from the liver.
Results
Identification of novel siRNA elimination pathway
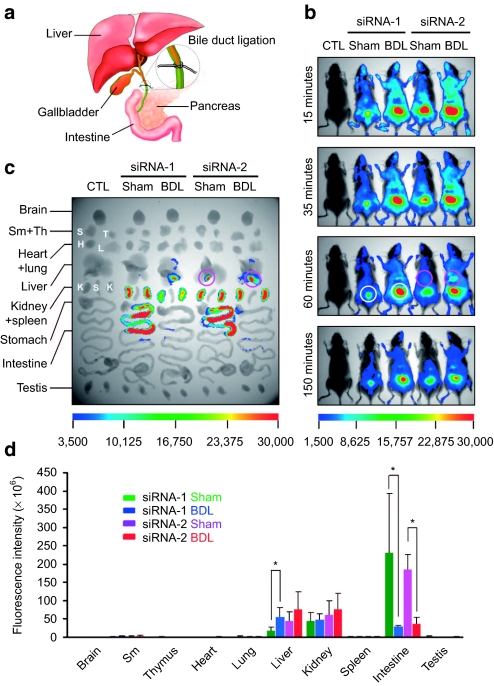
In the present study, Cy5 was used to monitor the in vivo behavior of systemically applied siRNA. The emission spectrum of this fluorophore is within the near-infrared range, rendering it distinguishable from background autofluorescence. Fluorophore-labeled siRNAs were injected into male C57 mice via tail vein at a dose of 2.5 mg/kg. Under a long-pass activation filter, the tissue distribution of the siRNAs was recorded over 4 hours, using a Kodak in vivo imaging system. The siRNAs exhibited a rapid circulatory distribution and tissue accumulation as reported in previous studies.2 To examine the distribution in detail, mice were sacrificed at the end of the experiments by cervical dislocation, and the major tissues were isolated and examined using the same equipment as for whole-animal assay. To our surprise, the experiment demonstrated intensive siRNA fluorescence in the gallbladder, indicating that the siRNAs accumulate in this organ (Figure 1c, siRNA-2, sham). In addition, a diffuse siRNA signal was seen in the intestine (Figure 1c, siRNA-1 and siRNA-2, sham). Accumulation of siRNAs throughout the hepatobiliary system as well as the intestine led us to speculate that circulating siRNA is also cleared from the body through liver accumulation followed by liver secretion. This therefore might constitute an additional elimination pathway. For proof of concept, bile duct ligation was performed in mice before systemic siRNA delivery (Figure 1a). In comparison to the sham group, blockade of the bile duct in the ligation group was found to result in the virtual absence of siRNA signals within the intestine, whereas at the same time, greatly enhanced fluorescence in the liver and gallbladder, suggesting exceptional siRNA retention in these two organs (Figure 1b,c, bile duct ligation).
Figure 1.
Characterization of a novel siRNA elimination pathway. (a) Illustration of bile duct ligation (BDL). Ligation was performed at the distal end of the bile duct in male C57 mice. (b) Whole-animal imaging of siRNA distribution after in vivo administration. The siRNA was administered to saline-treated, sham, and ligation mice via tail-vein at 2.5 mg/kg. Fluorescence images were taken at 15, 35, 60, and 150 minutes after delivery. White circles highlight siRNA accumulation in the bladder, pink circles indicate siRNA distribution in the liver. (c) Fluorescence images of siRNA accumulation in isolated mouse tissues. Pink circles indicate accumulation of siRNA in the gallbladder. (d) Quantification of siRNA accumulated in mouse tissues using a molecular imaging software package (Kodak, Woodbridge, CT). Data were normalized to corresponding tissues from saline-treated animals. (n = 3, *P < 0.05, ligation versus sham). siRNA, small interfering RNA; Sm, submandibular gland; Th, thymus.
To further characterize this finding, the siRNA accumulated in each tissue was quantified using a molecular imaging software package (Figure 1d). Consistent with the whole-animal assay, a twofold increase in liver fluorescence, as well as a correlated decrease in intestine fluorescence, was revealed in the ligation group. To exclude the influence of siRNA sequence and animals used, two siRNAs and independent experiments were carried out, resulting in similar distribution and accumulation profiles. It should be noted that isolated kidneys displayed the highest fluorescence but it was undetectable in whole-animal imaging because they are located deep inside the body. In contrast, siRNA accumulation was readily detected in the bladder, which has a superficial location. Although ligation significantly enhanced siRNA accumulation in the bladder, siRNA levels in the kidneys did not significantly change when isolated tissues were studied at the end point of experiments.
Intact siRNA duplexes identified in urine
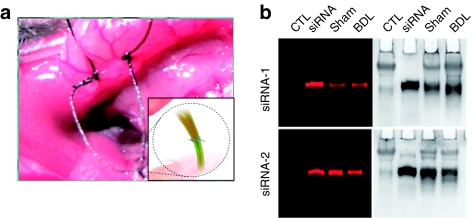
As siRNA is readily degraded by serum ribonucleases, the observed fluorescence might have come from degraded siRNA fragments bearing the label, rather than from full-length siRNA molecules. Two serum-stable siRNAs were used to minimize possible bias caused by potential siRNA degradation (Figure 2). These siRNAs remain intact in 90% serum for up to 6 hours without chemical modification.8 We further confirmed the exceptional in vivo stability of these natural stable siRNAs by collecting and analyzing the urine of mice injected with the siRNAs through a tail vein. The urine samples were subjected to gel electrophoresis (Figure 3), revealing a great proportion of intact siRNA at the end point of siRNA in vivo processing. This provided the first evidence showing that the distribution and the fate of the Cy5 dye molecules do reflect the in vivo behavior of intact siRNA at least through the urine elimination pathway. We further carried out real-time PCR to quantify the full-length siRNA from the intestine and were able to demonstrate that significant amounts of full-length siRNA does coexist with the Cy5 dyes in the intestine, and ligation of the bile duct reduced the amount of such siRNA to basal levels.
Figure 2.
siRNA sequences and serum stability. (a) Sequences of siRNAs and peptide, and schematic representation of Cy5-cholesterol-conjugated siRNA-1. (b) Serum stability of the siRNAs. siRNA, small interfering RNA.
Figure 3.
Integrity of siRNA processed in vivo. (a) Photograph of ligation procedure. (b) Integrity of siRNA in urine. siRNA, small interfering RNA.
No enterohepatic circulation revealed in siRNA processing
For some drugs such as digitoxin and chloromycetin, enterohepatic circulation occurs after systemic administration. In this process, drug molecules eliminated by liver excretion are absorbed in the lower small intestine and thereby re-enter the circulation. To determine whether this scenario also occurs with siRNA eliminated by the liver, siRNA was delivered intragastrically to mice. At 4 and 24 hours after administration, the distribution of siRNA was examined, and showed no siRNA accumulation other than in the stomach and intestine (Figure 4). This result indicated that, similar to renal filtration, liver elimination of circulating siRNA is a terminal process. Taken together, this evidence has characterized a novel siRNA elimination pathway, in which liver-enriched siRNA is secreted into the gallbladder and then emptied into the intestine.
Figure 4.
Distribution of intragastrically delivered siRNA. (a) Whole-animal imaging of siRNA distribution. (b) Fluorescence images of siRNA accumulation in isolated mouse tissues. (c) Quantification of siRNA accumulated in mouse tissues using imaging software. Data were normalized to corresponding tissues from saline-treated animals. (n = 3, * P < 0.05). siRNA, small interfering RNA.
Delivery carriers play essential roles in siRNA distribution and elimination
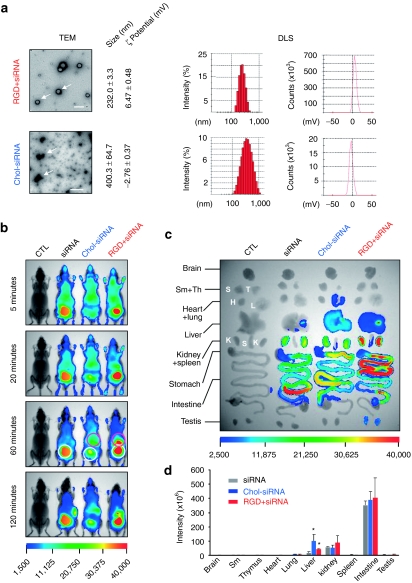
Owing to their large size and high negative charge, siRNAs do not readily enter cells.2 Therefore, siRNA administered in vivo is often formulated with various carriers to facilitate this process, as well as improve its pharmaceutical properties, such as extended circulation time or targeted delivery.9,10 Besides these desirable effects, siRNA formulation very likely modulates its in vivo distribution and elimination. To this end, differently formulated siRNAs (naked siRNA, cholesterol-conjugated siRNA, and RGD peptide/siRNA complex) were administered to mice via tail vein. In a recent study,7 cholesterol-conjugated siRNA targeting apolipoprotein B was injected into mice, resulting in suppression of apolipoprotein B mRNA as well as decreased levels of plasma apolipoprotein B and serum cholesterol. In consideration of the fact that cholesterol is transported in the circulation by binding to lipoprotein particles, cholesterol-siRNA conjugates are therefore likely to be delivered in a chaperone-mediated manner. When siRNA was mixed with RGD peptide at a molar ratio of 1:20, it assembled into uniform spherical siRNA/peptide nanoparticles with a size of 232 ± 3.3 nm, as measured by transmission electron microscopy and dynamic light scattering assay (Figure 5a). The dynamic light scattering assay further showed that these nanoparticles were positively charged with a zeta-potential of 6.47 mV, reflecting the predominance of peptide in the particle. Interestingly, when chol-siRNA was dispersed in saline, it aggregated into irregularly shaped nanoparticles with a large particle dispersion index (Figure 5a).
Figure 5.
Effects of delivery carrier on siRNA distribution and elimination. (a) Biochemical properties of siRNA nanoparticles formed by chol-siRNA-1 or siRNA (si-NC67-M) and RGD10-10R peptide. Formation of particles was examined by transmission electron microscopy (Tecnai G2 20 S-TWIN, FEI). Bar = 0.5 µm. Size distribution and zeta-potential were measured by dynamic light scattering assay (Nano-ZS; Malvern). (b) Whole-animal imaging of siRNA distribution. White circles highlight siRNA accumulation in the bladder, pink circles indicate siRNA distribution in the liver. (c) Fluorescence images of siRNA accumulation in isolated mouse tissues. (d) Quantified siRNA accumulation in mouse tissues. Data were normalized to corresponding tissues from saline-treated animals (n = 3, *P < 0.05 versus siRNA). chol, cholesterol; siRNA, small interfering RNA.
In vivo delivery of the siRNA as well as the conjugates was performed via tail vein, resulting in distinct tissue distribution profiles (Figure 5b,c). While naked siRNA was rapidly eliminated from the body through renal filtration and liver excretion, significant circulatory retention and liver accumulation occurred for the cholesterol-conjugated siRNA (Figure 5b,c). This finding is in agreement with a previous study,7 which demonstrated liver targeting of cholesterol-modified siRNA. In contrast, siRNA complexed with RGD peptide greatly enhanced siRNA accumulation in the liver, as well as accelerating siRNA clearance by renal filtration compared with naked siRNA (Figure 5b), which was further confirmed by the strongest fluorescence identified in isolated kidneys (Figure 5c). Quantitative tissue distribution further demonstrated that the highest liver accumulation was achieved by chol-siRNA delivery (Figure 5d). In addition to tissue distribution, checking the fluorescence patterns along the length of intestine indicated that siRNA elimination was also altered by siRNA formulation. While the fastest clearance occurred with naked siRNA delivery, the clearance process was significantly hindered by using peptide/siRNA delivery (Figure 5c).
Discussion
In summary, the present study characterized a novel siRNA elimination pathway, and further showed that delivery carriers play an essential role in siRNA distribution and elimination. Although only siRNAs were evaluated in the present study, the liver clearance is likely of general significance for the in vivo metabolism of nucleic acid reagents such as antisense DNA, considering their common properties and similar tissue distribution after intravenous injection.11
Materials and Methods
siRNA oligonucleotides and peptide. Two siRNAs targeting firefly luciferase and superoxide dismutase were used. Chemically synthetic siRNAs were from RiboBio (Guangzhou, China) or Suzhou Ribo Life Science (Jiangsu, China). Fluorophore-labeling and cholesterol modification was made at the 5′ and 3′ end of the antisense strand respectively. siRNA-2: sense strand, 5′-GGAA CCUCACAUCAACGCGCAtt-3′ antisense strand, 5′-UGCGCGUUGAU GUGAGGUUCCtt-3′ siRNA-1: sense strand, 5′-GCUGUUUCUGAGGA GCCUUtt-3′ antisense strand, 5′-AAGGCUCCUCAGAAACAGCtt-3′. RGD10-10R cyclic peptide with a sequence of DGARYCRGDCFDGRRRR RRRRRR was from Invitrogen (Shanghai, China), and a disulfide bond formed between the two cysteines.
Serum stability assay. Serum degradation assays were performed in phosphate-buffered saline solution containing 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO). One microliter of 20 µmol/l siRNA was incubated in 10 µl 10% fetal bovine serum at 37 °C for the indicated time. After treatment, the solution was rapidly frozen in liquid nitrogen and stored at −80 °C until gel electrophoresis. In this, 1 µl of sample was separated in a 20% nondenaturing PAGE-TBE gel together with untreated control, and then visualized by SYBR Gold staining (Invitrogen, Eugene, OR).
Whole-animal imaging. Animals were maintained in the Center for Experimental Animals (an AAALAC accredited experimental animal facility) at Peking University (Beijing, China). All procedures involving experimental animals were performed in accordance with protocols approved by the Committee for Animal Research of Peking University, China, and conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1985). Male mice of 8–10 weeks weighed 18–22 g at the time of the study. For monitoring the in vivo siRNA distribution, conjugated or unconjugated siRNAs were prepared as described and injected into mice via a tail vein. Whole-animal imaging was recorded by the Kodak in vivo imaging system FX Pro. At the end of the experiment, mice were sacrificed for tissue examination.
Physiochemical properties of nanoparticles. Electron micrographs were obtained with a transmission electron microscope (Tecnai G2 20 S-TWIN, FEI). Samples were prepared by depositing 7.5 µl siRNA/peptide polyplex or chol-siRNA conjugate onto a carbon-coated copper grid and staining with 2% wt uranyl acetate.
The size and zeta-potential of formed nanoparticles were determined on a Nano-ZS (Malvern, Worcestershire, UK), at a scattering angle of 173° and analyzed using the DTS (Nano) program. Three independent measurements (each with 20 subruns) were performed.
Statistical analysis. Data are expressed as the mean ± SD. Statistical comparisons used t-test analysis of variance. P < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30873187, 30771085, and 30871385), the Beijing Natural Science Foundation (5092011), the National High-tech R&D Program of China (2007AA02Z165), the National Basic Research Program of China (2007CB512100), the National 985 Program, and grants from the Department of Education of China (20070001011 and 20090001110052) as well as 2009ZX09503 from the National Drug Program.
REFERENCES
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K., and, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Bartlett DW, Su H, Hildebrandt IJ, Weber WA., and, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacque JM, Triques K., and, Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature. 2002;418:435–438. doi: 10.1038/nature00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- Ptasznik A, Nakata Y, Kalota A, Emerson SG., and, Gewirtz AM. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drug-resistant, BCR-ABL1(+) leukemia cells. Nat Med. 2004;10:1187–1189. doi: 10.1038/nm1127. [DOI] [PubMed] [Google Scholar]
- Kim SH, Jeong JH, Lee SH, Kim SW., and, Park TG. PEG conjugated VEGF siRNA for anti-angiogenic gene therapy. J Control Release. 2006;116:123–129. doi: 10.1016/j.jconrel.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Hong, J, Huang, Y, Li, J, Yi, F, Zheng, J, Huang, H, et al. FASEB J. epub ahead of print; 2010. Comprehensive analysis of sequence-specific stability of siRNA. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- Liu N, Ding H, Vanderheyden JL, Zhu Z., and, Zhang Y. Radiolabeling small RNA with technetium-99m for visualizing cellular delivery and mouse biodistribution. Nucl Med Biol. 2007;34:399–404. doi: 10.1016/j.nucmedbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Lendvai G, Velikyan I, Bergström M, Estrada S, Laryea D, Välilä M, et al. Biodistribution of 68Ga-labelled phosphodiester, phosphorothioate, and 2'-O-methyl phosphodiester oligonucleotides in normal rats. Eur J Pharm Sci. 2005;26:26–38. doi: 10.1016/j.ejps.2005.04.017. [DOI] [PubMed] [Google Scholar]