Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive loss of motoneurons. We have recently uncovered a new neurotrophic growth factor, granulocyte-colony stimulating factor (G-CSF), which protects α-motoneurons, improves functional outcome, and increases life expectancy of SOD-1 (G93A) mice when delivered subcutaneously. However, chronic systemic delivery of G-CSF is complicated by elevation of neutrophilic granulocytes. Here, we used adeno-associated virus (AAV) to directly target and confine G-CSF expression to the spinal cord. Whereas intramuscular injection of AAV failed to transduce motoneurons retrogradely, and caused a high systemic load of G-CSF, intraspinal delivery led to a highly specific enrichment of G-CSF in the spinal cord with moderate peripheral effects. Intraspinal delivery improved motor functions, delayed disease progression, and increased survival by 10%, longer than after systemic delivery. Mechanistically, we could show that G-CSF in addition to rescuing motoneurons improved neuromuscular junction (NMJ) integrity and enhanced motor axon regeneration after nerve crush injury. Collectively, our results show that intraspinal delivery improves efficacy of G-CSF treatment in an ALS mouse model while minimizing the systemic load of G-CSF, suggesting a new therapeutic option for ALS treatment.
Introduction
Amyotrophic lateral sclerosis (ALS) is an incurable fatal motoneuron disease, characterized by progressive weakness, muscle wasting and death ensuing 3–5 years after diagnosis.1 The etiopathogenesis and pathophysiology of ALS is complex with many players involved that lead to the functional decline of the motor pathway.2,3,4 Due to insufficient insights into the molecular pathway(s) crucial to ALS pathogenesis the most rational strategy at present remains to rescue and strengthen motor units with neurotrophic factors.5 A number of growth factors have been clinically tested in ALS without success so far, but major pharmacokinetic problems and unexpected peripheral effects preclude any conclusion as to the true therapeutic potential of this concept.5
Granulocyte-colony stimulating factor (G-CSF) is a 19.6 kd cytokine first described as a hematopoietic growth factor that stimulates proliferation and differentiation of myeloid precursors.6 We and others have uncovered neurotrophic properties of G-CSF. G-CSF and its receptor are expressed by neurons in many regions of the adult brain and spinal cord.7,8,9 G-CSF appears clinically attractive, because it is generally well-tolerated, crosses the intact blood–brain barrier,7 and its pharmacokinetic properties are well established. A large number of studies in various animal models of neurodegenerative diseases demonstrated that G-CSF is neuroprotective and proregenerative in models of stroke,7 Parkinson's disease,10 and of spinal cord injury.9
In 2008, we reported that G-CSF had beneficial effects in SOD-1 (G93A) transgenic mice, an animal model for ALS, after systemic delivery using subcutaneous pump delivery or transgenic expression of G-CSF.8 One key mechanism of G-CSF beneficial action is its direct antiapoptotic effect on motoneurons, as supported by G-CSF-mediated protection of motoneurons after neonatal sciatic nerve axotomy.11
One challenge for growth factor treatment in ALS is the likely need for a very chronic treatment with those proteins. In the case of G-CSF, any form of systemic delivery, such as subcutaneous delivery, has the inherent consequence of a chronic rise in white blood cells (WBC). The effects of such chronic elevation of WBCs have not been explored extensively in humans. In addition, the limited plasma half-life (~4 hours) would require repeated dosing or some sort of pump delivery of the protein.
To circumvent these problems, direct delivery of G-CSF to the central nervous system (CNS) using viral vectors might be advantageous. Recombinant adeno-associated virus12 is replication-deficient, derived from a nonpathogenic virus, and able to infect numerous cell types, including neurons, resulting in its presence in the nucleus as episomal concatamers.13 In nondividing neuronal cells, the virus may persist in that form for the lifetime of the cell. AAV serotype 2 was described to be able to transduce spinal motoneurons of SOD-1 (G93A) mice after intramuscular and intraspinal injections,14,15 and lead to the production of neurotrophic factors.
We used a chimeric AAV1/2, known to have enhanced transduction efficiency over AAV2 in the CNS to deliver G-CSF to spinal motor neurons by either intramuscular or direct intraspinal injection of AAV. We show here that intraspinal delivery improved efficacy of G-CSF treatment and decreased peripheral load and elevation of leukocyte count.
Results
Muscular injection fails to transduce motoneurons in SOD-1 (G93A) transgenic mice
An elegant and clinically feasible way to bring G-CSF to motoneurons would be to exploit the retrograde transport ability of AAV and inject the virus into skeletal muscles where it would be taken up by presynaptic neuromuscular junctions (NMJs). Indeed, successful targeting of motoneurons with AAV using this route has been described.15 Motoneurons are then expected to synthesize and secrete G-CSF that will bind to its neuronally expressed receptor and induce antiapoptotic pathways. Such a strategy would have the additional advantage of mimicking the endogenous autocrine behavior of the ligand in neurons.7
We injected a total of 0.9 × 1010 particles of G-CSF-expressing and control virus into the gastrocnemius and longissimus thoracis muscles of SOD-1 (G93A) transgenic mice. We next studied virus-mediated enhanced green fluorescent protein (eGFP) expression 4 weeks after injection, i.e., the reported time for the maximal expression of AAV-encoded proteins.16 Surprisingly, we were unable to detect any fluorescent signal in the spinal cord of intramuscularly (i.m.) injected mice (n = 10; Figure 1a,c), while there was strong eGFP expression in all injected muscles (Figure 1e), suggesting either that AAV particles were not retrogradely transported or that transported particles did not lead to detectable amounts of eGFP expression. For a more sensitive detection of virus presence in the spinal cord after i.m. delivery, we employed quantitative PCR analysis of DNA extracted from the thoracic and lumbar spinal cord. However, we were unable also with this method to detect viral DNA in the spinal cord of any of the animals studied (n = 4; Supplementary Figure S1).
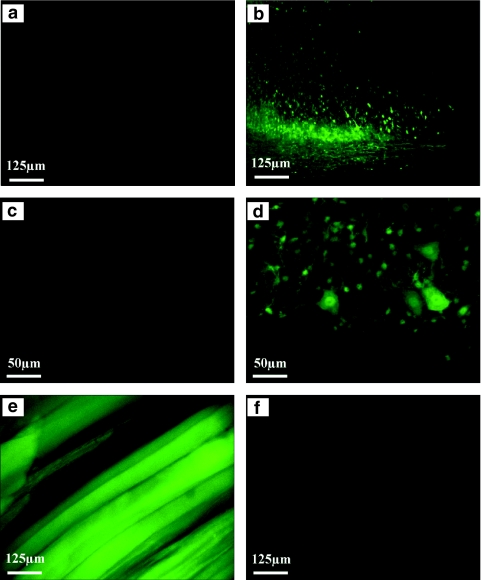
Figure 1.
G-CSF expression in motoneurons after intramuscular and intraspinal injection of AAV. (a,c) Following intramuscular injection no virus expression can be detected in the spinal cord. However, the virus expresses eGFP in the (e) skeletal muscle. (b,d) In contrast, intraspinal injection leads to a strong transduction of motoneurons. As expected, no virus expression is seen in the (f) musculature. AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; G-CSF, granulocyte-colony stimulating factor.
We therefore decided to directly inject viral particles into the spinal cord to transduce motoneurons.17 This mode of injection led to strong and mainly neuronal expression of the virus-derived eGFP in the ventral spinal cord (Figure 1d)18 over a length of at least 2 mm (Figure 1b,d), but not in the musculature (Figure 1f). Thus, intramuscular injection of AAV particles in our hands did not result in detectable retrograde transport of the virus, whereas intraspinal delivery appears as a very efficient way to deliver AAV particles to the spinal cord.
Intraspinal injection of AAV leads to a highly CNS-specific expression of G-CSF
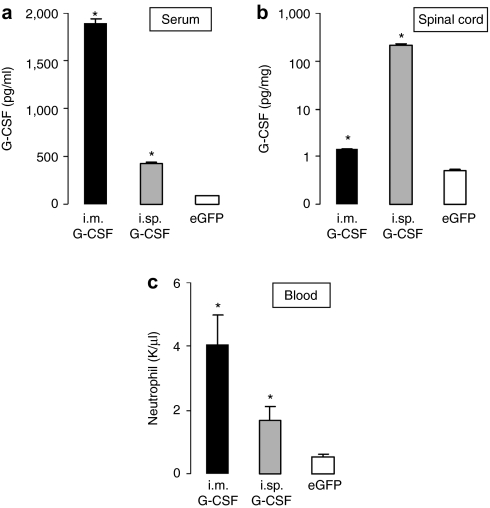
After intramuscular injection at week 15, we observed a high level of G-CSF in the serum accompanied by a moderate increase of G-CSF in spinal cord extracts. In contrast to this, intraspinal injection of AAV G-CSF strongly increased spinal G-CSF levels, but only moderately elevated serum G-CSF (serum: i.m. G-CSF: 1.890 pg/ml; intraspinal G-CSF: 420.1 pg/ml; eGFP: 92.5 pg/ml; P < 0.05 for both injections; spinal cord: i.m. G-CSF: 1.25 pg/mg; intraspinal G-CSF: 229.2 pg/mg; eGFP: 0.3 pg/mg; P < 0.05 for both injections Figure 2a,b).
Figure 2.
Distribution of G-CSF after intramuscular and intraspinal injection of AAV vector. (a) The level of G-CSF in the serum is increased in mice injected with AAV G-CSF compared to mice injected with the control vector (n = 10, *P < 0.05). Muscular injection of AAV G-CSF leads to a higher level of circulating G-CSF compared to the spinal injection (P < 0.05). (b) Level of G-CSF in the total spinal cord is increased in mice injected with AAV G-CSF compared to mice injected with the control vector (n = 6, P < 0.05). The increase following intraspinal injection is 150-fold higher than after intramuscular injection (P < 0.05). (c) Neutrophil numbers following intramuscular and intraspinal injections of AAV G-CSF. The number of neutrophils increases after both muscular and spinal injections. Neutrophil counting was performed using an automatic counting system (n = 7, *P < 0.05). AAV, adeno-associated virus; G-CSF, granulocyte-colony stimulating factor; i.m., intramuscular; i.sp., intraspinal.
Serum G-CSF is able to stimulate the proliferation of neutrophil precursors and their differentiation into mature neutrophilic granulocytes.19 Indeed, we noted a significant elevation of neutrophils after both intramuscular and intraspinal injections when compared to the control SOD-1 (G93A) mice. Whereas neutrophil count was eightfold elevated with i.m. injection (4.04 neutrophils/nl), intraspinal delivery resulted only in a threefold elevation (1.68 neutrophils/nl), still within the normal value range for mice20 (Figure 2c). Thus, muscular injection of AAV predominantly led to a systemic delivery of G-CSF produced in the injected muscles, along with its expected consequences in terms of neutrophil elevation and did not pose any advantage over systemic subcutaneous delivery. In contrast, intraspinal injection led to a highly CNS-specific delivery with low systemic levels, and a moderate increase of neutrophils, suggesting that this mode of delivery could maximize the neuroprotective effects, and minimize the peripheral effects. We thus focused our efforts on this mode of delivery.
Spinal delivery of G-CSF is beneficial for SOD-1 (G93A) mice
Since intraspinal injection in contrast to i.m. injection requires a relatively lengthy surgery, we studied postoperative behavior, weight, and motor performance of the mice and found no obvious difference between mice before and 1 week after surgery (Supplementary Figure S2), suggesting that the surgery did not have a major impact on general mouse health and motor functions.
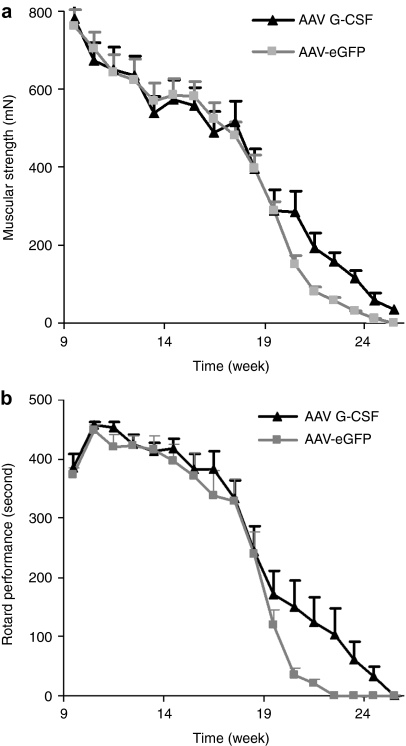
We monitored Rotarod and grip strength performance as indicators of muscular endurance and strength weekly in AAV G-CSF or control injected animals (n = 12 female mice per group, litter-matched). Our experimental settings comply with the guidelines for preclinical studies in ALS established by the European ALS/MND group.21 From week 20 on we noted a relative improvement in muscular strength in the G-CSF versus the control group (AAV- eGFP: 151 mN; AAV G-CSF: 285 mN; P < 0.05 by repeated measures analysis of variance and Fisher's LSD) and a better performance on the Rotarod (AAV-eGFP: 34 seconds; AAV G-CSF: 135 seconds, P < 0.05 by repeated measures analysis of variance and Fisher's LSD) (Figure 3).
Figure 3.
AAV G-CSF improves motor functions of SOD-1 (G93A) mice. (a) Muscular strength measured by the grip strength test. AAV G-CSF treatment leads to a relative preservation of the muscular strength of SOD-1 (G93A) mice at the mid- to end-point of the disease (n = 12). (b) Performance on Rotarod. AAV G-CSF treatment leads to an improvement of endurance/coordination performance at the mid- to end-point of the disease (n = 12). AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; G-CSF, granulocyte-colony stimulating factor.
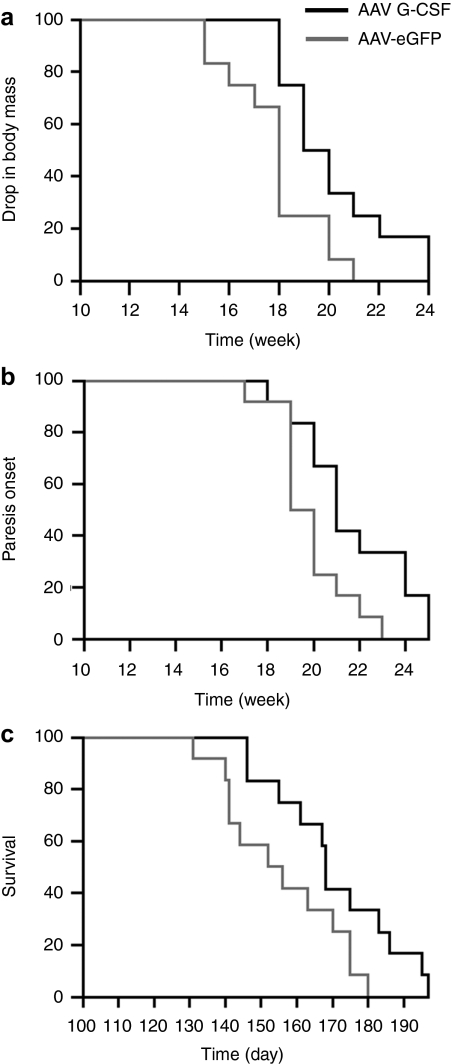
The disease progression in SOD-1 (G93A) mice is well described. The disease at its onset is characterized by a slight hind limb tremor, the midpoint of disease is defined by gait impairment and weight loss, and the end stage of disease is marked by paresis. Onset of body mass decrease, defined as a drop of 5% of the mouse maximal weight (around 1 g), was significantly delayed by G-CSF treatment by >2 weeks (P < 0.05; Figure 4a). The onset of gait impairment, defined as abnormal limb movement in at least one hind limb, was not significantly different between the two groups despite a trend (P < 0.17), but the onset of paresis, defined as the inability to use one limb in the coordinated stride, was significantly delayed after G-CSF treatment (P < 0.05; Figure 4b). Most importantly, the clinical end point of the disease was delayed by 15 days, increasing the survival by 10% (Figure 4c). This gain in survival was higher than the increases observed after subcutaneous delivery (7% increased survival8). An increase of 7% in survival was also seen in the mice treated by i.m. injection, which was essentially a systemic delivery (Supplementary Figure S3).
Figure 4.
AAV G-CSF delays symptoms progression and enhances survival of SOD-1 (G93A) mice. Kaplan–Meyer graphs showing time to (a) body mass decrease, (b) onset of paresis, and (c) clinical endstage of the disease. (a) Body mass decrease, associated with muscular atrophy, is delayed after AAV G-CSF treatment (P < 0.05). (b) First manifestation of paresis of the hind limbs is delayed after AAV G-CSF treatment (P < 0.05). (c) Survival of SOD-1 (G93A) mice is increased by 10% after AAV G-CSF treatment (P < 0.05). AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; G-CSF, granulocyte-colony stimulating factor.
Thus, G-CSF delivered by AAV to the spinal cord is able to delay disease progression and improve survival in SOD-1 (G93A) mice.
Spinal delivery of G-CSF maintains motor-unit integrity in SOD-1 (G93A) tg mice
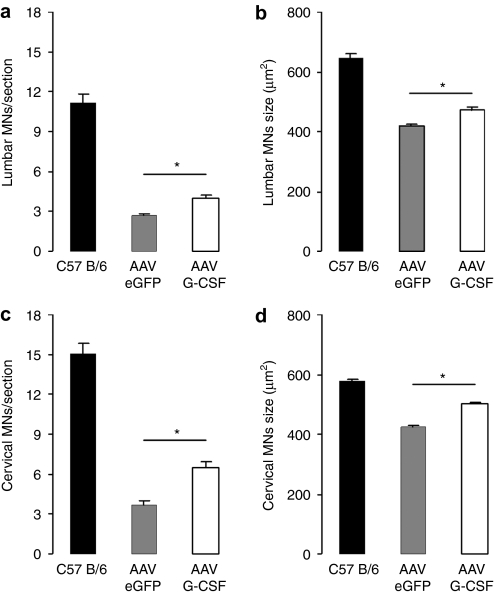
Motoneuron survival. G-CSF is known to protect α-motoneurons under proapoptotic conditions.8,11 We have previously shown an increase by 30% in the total number of α-motoneurons at midpoint of the disease (week 15) after systemic delivery of G-CSF.8 Here, we sought to determine the effect of a direct delivery of G-CSF to motor neurons at two different spinal segments: the cervical (C3–C4) and lumbar level (L3–L4) of 15-week-old SOD-1 (G93A) mice. We used the previously described criteria based on localization, size, and ChAT positivity.8 We noted a loss of α-motoneurons at both lumbar and cervical spinal segments for the SOD-1 (G93A) mice when compared to the littermate wild types (P < 0.05; Figure 5). After G-CSF treatment, we noted a rescue of α-motoneurons at both the cervical (+50% motoneurons; P < 0.05; Figure 5a) and lumbar level (+35% motoneurons; P < 0.05; Figure 5c). The analysis of the size distribution of the remaining motoneurons indicates that large α-motoneurons are particularly protected by G-CSF treatment at both levels (Figure 5b,d; P < 0.05). Supplementary Figure S4 shows a histogram size distribution of cervical and lumbar motoneurons. In addition, we assayed microglial numbers in the spinal cord as a possible cellular element contributing to disease pathophysiology.22 While microglial numbers were increased in the SOD-1 (G93A) model at week 15 in contrast to wild-type littermates, we could not detect any influence of G-CSF on this elevation, a result in concordance with our previous study8 (Supplementary Figure S5).
Figure 5.
AAV G-CSF improves α-motoneuron survival in SOD-1 (G93A) mice. (a,c) Quantification of surviving α-motoneurons after AAV G-CSF treatment, in SOD-1 (G93A) mice at 15 weeks of age. Motoneuron survival is increased after spinal injection of AAV G-CSF at both the (a) cervical (+50%; P < 0.05; n = 9) and (c) lumbar (+35%; P < 0.05; n = 9) level of the spinal cord when compared to control. (b,d) Size evaluation of all ChAT+ cells in SOD-1 (G93A) mice at 15 weeks of age. There is an upward shift in mean size distribution by AAV G-CSF treatment at both the (b) cervical and (d) lumbar level (P < 0.05). AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; G-CSF, granulocyte-colony stimulating factor.
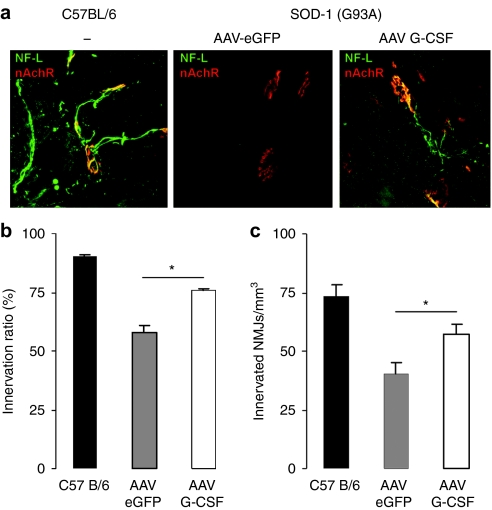
Preservation of NMJs. In ALS, the disruption of the NMJs occurs long before the degeneration of the cell body of motoneurons.23 Therefore, the rescue of α-motoneuron cell bodies is not sufficient to explain a therapeutic effect that inherently implies preserved muscle innervation.24 To determine whether G-CSF treatment can preserve muscular innervation, we investigated the state of the NMJs in the gastrocnemius muscle of 15-week-old mice. At this age, mice present clear gait impairment and decreased performance in both Rotarod and grip strength analyses. At first, we determined the innervation fraction of the gastrocnemius muscle, defined as the number of innervated NMJs per total NMJs (innervated and denervated). We found that the gastrocnemius muscle in SOD1 (G93A) mice showed clear denervation when compared to the littermate control wild-type mice where virtually all muscular endplates were found innervated (Figure 6a). The severity of this denervation is reduced by G-CSF treatment (AAV-eGFP: 57.7% innervation ratio; AAV G-CSF: 75.7%; wild type: 90.2%; P < 0.05; Figure 6b). This effect is also seen when comparing the total number of innervated NMJs per muscle volume (40% increase in the total number of NMJs after AAV G-CSF transduction; AAV-eGFP: 40.6 innervated NMJs/mm3; AAV G-CSF: 57.4; P < 0.01; Figure 6c).
Figure 6.
AAV G-CSF enhances muscular innervation in SOD-1 (G93A) mice. (a) Double fluorescence immunostaining of nicotinic acetylcholine receptors (AChr, red) and axons (neurofilament-L, green) in the gastrocnemius muscle of 15-week-old mice (AAV-eGFP, AAV G-CSF, and wild-type mice). (b) Innervated NMJs in percentage of total NMJs, in the gastrocnemius muscle of 15-week-old mice. AAV G-CSF treatment increases the fraction of innervated sites (P < 0.0005; n = 5). (c) Number of innervated NMJs normalized to muscle volume. AAV G-CSF treatment increases the number of innervated NMJs per volume (P < 0.0005; n = 5). AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; G-CSF, granulocyte-colony stimulating factor; NMJ, neuromuscular junction.
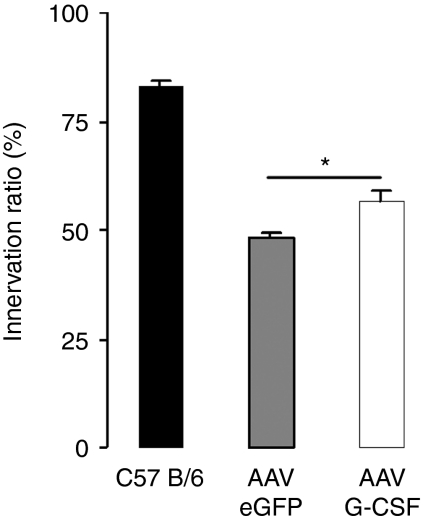
Motor axon regeneration. An important intrinsic compensatory mechanism in ALS is that surviving motoneurons partially reinnervate postsynaptic NMJ sites that belonged to the motor unit of a damaged neighboring motoneuron.25 A G-CSF-induced higher propensity for motor axon outgrowth may therefore be an additional mechanism that leads to a higher number of innervated NMJs, especially since G-CSF enhances neurite outgrowth in vitro.9,26 To approach this question, we performed sciatic nerve crush injury on SOD-1 (G93A) mice at 15 weeks of age. Sciatic nerve crush in adult mice results in axonal degeneration and in muscular denervation. Due to the small length of the sciatic nerve and a high regenerative potential in mice, regeneration usually occurs fast and is complete within 2 weeks in wild-type mice.27 Six days after nerve crush, we counted the percentage of innervated NMJs in the gastrocnemius muscle, ipsilateral to the nerve injury. Reinnervation was almost complete in wild-type mice after 6 days, whereas for the SOD-1 (G93A) mice it was strongly impaired (P < 0.005) possibly due to axonal transport disturbances.28 AAV-mediated G-CSF treatment led to a higher reinnervation rate in the SOD-1 (G93A) mice (AAV-eGFP: 48.0%; AAV G-CSF: 56.4%; wild type: 82.3%; P < 0.005; Figure 7).
Figure 7.
AAV G-CSF enhances reinnervation after sciatic nerve crush in SOD-1 (G93A) mice. Innervated NMJs in percentage of total NMJs, in the gastrocnemius muscle of 15-week-old mice, 6 days after sciatic nerve crush injury. AAV G-CSF treatment increases reinnervation (P < 0.005; n = 4). AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; G-CSF, granulocyte-colony stimulating factor; NMJ, neuromuscular junction.
In conclusion, intraspinal delivery of G-CSF was able to potently preserve NMJs and stimulate axonal regeneration.
Discussion
The results of this study demonstrate that intraspinal delivery of G-CSF through viral gene therapy improves treatment effects of this neurotrophic protein, while minimizing unwanted systemic effects. These data also further solidify our chain of arguments for a direct motoneuronal mode-of-action of G-CSF versus indirect effects mediated by its hematopoietic effects. Our results also suggest that the chimeric AAV1/2 serotype employed here is not a highly efficient means for retrograde transport to motor neurons even if this serotype is better at transducing neurons than AAV2 alone.
Retrograde AAV delivery by intramuscular injections
We could not detect any retrograde transport of AAV after intramuscular injection, both measured by eGFP expression and PCR, and confirmed by the distribution profile of G-CSF. This is unexpected, and at odds with several published studies reporting successful retrograde transduction of motoneurons.15,29 The amount of virus used has been comparable between our work and published studies.15,29
One possible reason for the discrepancy to published reports may lie in the AAV serotype used. Originally, retrograde transduction after muscle injection has been demonstrated for serotype 2.15,30 In a systematic comparison of retrograde transduction efficiency of different (self-complimentary) serotypes AAV1 performed much better than AAV2 which did not result in detectable spinal cord transduction after i.m. injection.29 We have used the chimeric recombinant AAV 1/2 because of its superior transduction efficiency and neuronal preference in contrast to AAV218,31 matching the transduction efficiency and tropism of AAV1 reported by others.32,33 AAV1/2 was also reported to be transsynaptically transported in the nigrostriatal pathway.34 Our failure to transduce neurons is therefore not easily explained by the chosen serotype.
We believe that the most likely explanation for our failure to detect any retrograde transport is the very low retrograde transduction efficiency resulting in a borderline success rate of the process. In addition to the above-mentioned data from Hollis with AAV2, at least one other group has been unable to detect retrograde transduction after i.m. injection.35 Novel self-complimentary viruses and partial nerve demyelination appear to produce higher transduction efficiencies.29,36 Overall, the low efficiency of this application mode makes it unsuited for any clinical considerations.
Despite the failure of direct CNS transduction, we found G-CSF elevated in the spinal cord since G-CSF is able to cross the blood–brain barrier. Predictably, this also leads to a therapeutic effect on survival similar to systemic subcutaneous pump delivery (7% increase in survival).8 At the same time, constant and massive production of the virally expressed G-CSF from muscle led to high serum concentrations and clear elevations of WBC count. This would generate a considerable safety risk to patients, as the virus cannot be shut off. Most of the time, quantification and distribution of the synthesized proteins are not indicated in studies with AAV,14,15,17 making the comparison between our results with previous works difficult with regard to relative CNS specificity of delivery. Our results conclusively show that intramuscular injection of AAV is not a feasible route for CNS-targeted therapy with G-CSF.
Intraspinal injections have a favorable efficacy and specificity profile
Injection into the spinal cord led to a rather specific CNS delivery. Elevation of G-CSF concentration in the serum was moderate (about threefold) and the elevation of WBCs still in the normal range for mice.20 The peripheral load of G-CSF was still more than twofold lower after intraspinal injection when normalizing for the 2.4-fold lower total virus load injected intraspinally versus i.m. At the same time, CNS levels of G-CSF were 200-fold higher, generating a very favorable specificity profile for this delivery mode. It is unclear at present whether the systemic elevation of G-CSF after intraspinal delivery originates from spurious transduction of muscles (e.g., in the injection canal), or from leakage or secretion of intrathecally produced G-CSF into the blood stream.
Although only injected at the lumbar level, motoneuron protection was also seen in the cervical spinal cord, presumably because of sufficient distribution of the secreted protein within the spinal extracellular space. Injections were made at week 10, likely resulting in relevant protein expression at week 12–13 (no expression seen 1 week after delivery; data not shown), and resulted in clear benefits on motor function and survival. Although expression of G-CSF and therefore therapy started in the symptomatic phase, ~2 weeks later than done previously,8 survival was increased from 7 to 10% by specific CNS delivery. The higher survival after a highly specific CNS delivery suggests that the benefit after G-CSF treatment is caused by its direct neuroprotective activity rather than a stimulation of the hematopoietic system or influences on microglia.
Thus, direct intraspinal injection of AAV appears as a preferred approach for G-CSF delivery with a minimum of systemic side effects.
Effect size and translatability of findings in the mouse model
Do the effects seen in this mouse model justify clinical testing of the G-CSF concept in patients suffering from ALS? We have seen strong beneficial effects on motor function, and increases in survival in the range of 7–10% using various application modes of G-CSF. It is however, fully unclear at present how an increase in survival seen in the mouse may translate to the human. Translated linearly, a prolongation of life expectancy of 10% in human patients would certainly be a clinically highly meaningful benefit (~6 years).
The real issue with therapeutic experiments in the SOD-1 mouse is however the low reliability of animal efficacy data due to insufficient rigorousness (blinding, randomization, SOPs, sample size, control for confounding factors) in the conduction of the animal studies.37 Indeed, most of the positive results reported in the literature cannot be reproduced under conditions of standardized testing. Our studies have been conducted under highly standardized conditions including rigorous blinding and randomization procedures, and have been reproduced in multiple studies with different application modes. Since G-CSF is also the first growth factor which appears clinically feasible in terms of safety and pharmacokinetics, we believe that this approach is worthwhile to be tested in human patients.
Clinical relevance of AAV therapy for ALS
AAV vectors, particularly AAV2, have been evaluated in clinical trials in a considerable number of diseases, among those Parkinson's disease,38 cystic fibrosis,39 muscular dystrophy,40 Alzheimer's disease,41 Leber's congenital amaurosis,42 and hemophilia B.43 Although the total number of patients treated is still small, AAV therapy has not presented major safety issues yet. Findings of liver carcinogenesis in neonatal mice are likely an isolated finding.44
AAV therapy for ALS patients appears attractive, even if it has to be done by intraspinal injections. The main problem of chronic G-CSF therapy, increased neutrophil counts, would be avoided by this approach if the findings in mouse can be translated to human patients. A caveat in this approach is that intrathecal G-CSF production cannot be easily turned off in case of any CNS-specific problems. The virus can synthesize therapeutic proteins for a long time period after only one injection, e.g., up to 2 years in nonhuman primates.45 After monkey studies, an initial safety trial using regulatable promoters, for instance the tet-off system, could answer the question if there are safety issues of long-term G-CSF delivery to the CNS.46
Recently, novel reports have suggested the transduction of the CNS via intravenous delivery of the AAV serotype 9.47 This appears as a novel interesting delivery route, and needs to be tested in ALS models.
Mechanism of action of G-CSF: motor-unit preservation
Besides improving the survival of motoneurons, G-CSF treatment preserves NMJs in SOD-1 (G93A) Tg mice. Axonopathy and loss of NMJs is known to occur in ALS long before motor neuron degeneration and initiation of symptoms.23,48 Many NMJs are lost in the SOD-1 (G93A) mice from P50 on, before detectable loss of motor axons in the ventral roots exiting the spinal cord, and long before the first symptoms of paralysis. Preservation of NMJs by G-CSF treatment may therefore constitute a complementary protective mechanism, independent of antiapoptotic protection of the α-motoneurons.
The higher innervation rate of the NMJs under G-CSF treatment may be caused by stabilization of the NMJs, by a higher reinnervation of depleted postsynaptic sites, or by both. The present experiment does not allow us to distinguish between these possibilities. Likely, the effect seen is a combination of these mechanisms.
A very recent paper claims that subcutaneously applied G-CSF elevates microglial numbers in the spinal cord of SOD1 (G93A) transgenic animals, and suggests this as a mechanism of action for G-CSF.49 We have not observed any alterations of microglial numbers after intraspinal delivery of G-CSF, consistent with earlier work on systemic delivery of G-CSF in ALS models.8 Although a number of differences exist between Yamasaki et al. and our studies (glycosylated versus nonglycosylated G-CSF, continuous versus once daily delivery, different doses of G-CSF used), none of those appears fundamental enough to offer a clear explanation of this discrepancy at present. From a general perspective, it appears however rather unlikely that elevation of SOD1-transgenic microglia would make a major contribution to the beneficial G-CSF effects seen in ALS models.22,50
In conclusion, the data shown here further support our concept that the direct action of G-CSF on motoneurons is the major mode-of-action responsible for its beneficial effects in the SOD-1 (G93A) model.7,8,11
Materials and Methods
ALS model. The animals used for the experiments were transgenic for the SOD1 (G93A) mutation on a C57BL/6 background [B6.Cg-Tg(SOD1-G93A)1Gur/J strain; Jackson Laboratory, Bar Harbor, ME]. They harbor a high copy number of the mutant human SOD1 transgene. For animals with a delay in their onset of disease (no symptom at week 12 of age), the transgene copy number was determined by quantitative PCR to control against drops in copy number that might modify the disease phenotype. The heterozygous line was maintained by mating transgenic males with C57BL/6 wild-type females. Transgenic females were used in all experiments. The animals were age-matched with equally distributed siblings to treatment and control groups.
Recombinant AAV G-CSF vector. Generation of AAV G-CSF was performed by subcloning the murine G-CSF complementary DNA sequence into the AAV2 backbone plasmid containing the chicken β-actin promoter and an IRES-eGFP sequence, flanked by AAV2 ITR sequences. AAV-eGFP as control vector was generated by inserting the coding sequence of eGFP into the same AAV expression cassette. HEK293 cells were used for the production of pseudotyped chimeric AAV1/2 vectors (containing a 1:1 ratio of capsid proteins serotype 1 and 2) as described previously.31 Cultured cells (80% confluent) propagated in complete Dulbecco's modified Eagle's medium were transfected with the AAV construct and helper plasmids (pH21, pRV1, and pFΔ6) using calcium phosphate. Forty-eight hours later, cells were harvested in phosphate-buffered saline, centrifuged, and pellets from five plates were pooled in 25 ml of a buffer consisting of 150 mmol/l NaCl, 20 mmol/l Tris pH 8, 1.25 ml of 10% natrium deoxycholate and 50 U/ml of benzonase. After an incubation of 1 hour at 37 °C, 25 ml of 150 ml NaCl, and 1.25 ml of 10% natrium deoxycholate were added and the solution was centrifuged. The supernatant was collected and filtered with 450 mmol/l NaCl, 20 mmol/l Tris pH 8 through a high-affinity heparin column (1 ml HiTrap Heparin; Sigma, Munich, Germany) previously equilibrated with buffer (150 mmol/l NaCl, 20 mmol/l Tris pH 8), at a speed of 1 ml/minute as described. The genomic titer of the viral solutions was determined by real-time PCR (light cycler; Roche Diagnostics, Mannheim, Germany).
Injection of AAV G-CSF. All injections were done in symptomatic 70-day-old female mice.
Intramuscular injections: Mice were anesthetized by inhalation of 70% N2O, 30% O2, and 1% halothane. The gastrocnemius and the longissimus thoracis were chosen as target muscles. Injections were done using a nanofil syringe (WPI, Berlin, Germany) and a 33-gauge needle. After incision of the skin at the level of the thoracic spinal column, the longissimus thoracis was exposed. A total of 6 µl of viral solution was injected bilaterally (total of 9 × 109 AAV particles) in one site per gastrocnemius and two sites per longissimus thoracis (1 µl per site).
Intraspinal injections: Mice were anesthetized by injection of a mixture of ketamine (120 mg/kg body mass; Pharmanovo, Hannover, Germany) and xylosine (Rompun, 16 mg/kg body mass; Bayer, Leverkusen, Germany). Injection anesthesia was chosen instead of inhalational anesthesia here because of better compatibility with the stereotactic procedure. After incision of the skin at the level of the thoracic/lumbar segment of the spinal column, the spinal cord was exposed after sectioning the paraspinous muscles. The tissue between the processi spinosi of T13 and L1 vertebrae was removed. Glass microcapillaries connected to a vacuum pump were used to inject a total of 1 µl of viral solution bilaterally at the L1 level (total of 3.78 × 109 AAV particles). To prevent any leaking of viral solution, the glass microcapillaries were allowed to remain in place for at least 1 minute after each injection, and were retracted slowly from the spinal cord. Curaspon sponge (CuraMedical, Assendelft, Netherland) was placed over the injection site, and muscles and skin were sutured. Animals were allowed to wake up and recover from the operation under a heating lamp for 1 hour. All animal experiments were approved by the Regierungspräsidium Karlsruhe, Germany.
Sciatic nerve crush injury. Fifteen week old mice were anesthetized by inhalation of 70% N2O, 30% O2, and 1% isoflurane. Before any surgical procedures, depth of anesthesia was controlled by a pain reflex elicited by pinching the skin between the toes, and by the palpebral reflex, elicited by touching the eyelids. Under narcosis, hind limbs were shaved and washed with 70% ethanol. The sciatic nerve was exposed at mid-thigh level and pinched for 30 seconds with fine forceps (S&T JFA-5b, S&T, AG, Neuhasen, Switzerland). Skin was sutured (Ethilon USF 4/0) and animals were allowed to recover under a heating lamp for 1 hour.
Quantification of G-CSF expression. After deep anesthesia, blood samples were collected in heparinized tubes after heart puncture, and the spinal cord was dissected after careful transcardial perfusion with Hank's balanced salt solution. Entire spinal cords were homogenized in lysis buffer (Promega, Mannheim, Germany). G-CSF concentration was measured by an ELISA for mouse G-CSF (Quantikine; R&D Systems, Wiesbaden-Nordenstadt, Germany) from the serum after centrifugation, or from the protein extract.
Hematology. After deep anesthesia, blood samples were collected in heparinized tubes after heart puncture. WBC count was performed by an automatic cell-counting system (Cell-Dyn 4000, Hematology analyzer; Abbott, Weisbaden, Germany). The system uses optical flow cytometric technology to obtain the WBC count and analyze sub-populations, such as neutrophils.
Assessment of disease progression and survival. One week before vector injection (at 64 days of age) mice were trained for all motor-behavior exercises. All tests were done weekly. Rotarod sessions lasted 480 seconds and a constant accelerating mode from 3 to 30 r.p.m. was used (Rotarod, Ugo Basile, Comerio, Italy). Between each session, the mice were allowed to rest for 480 seconds. Mean of three tests was recorded. Muscular strength was measured by grip strength measurements (Ugo Basile). Mean of three tests was recorded. Weight evolution and clinical symptoms were assessed weekly. Clinical end stage of the disease was defined as the inability of the animal to right itself over a period of 30 seconds. Animals were sacrificed at this point.
Immunohistochemistry
Counting of motoneurons: After deep anesthesia, mice were transcardially perfused with Hank's balanced salt solution followed by 4% paraformaldehyde, spinal cords were dissected and embedded in paraffin. Coronal paraffin sections 10 µm thick from the lumbar or cervical spinal cord were stained for choline acetyltransferase using the avidin–biotin complex technique with 3,30-diaminobenzidine hydrochloride as chromogen (DakoCytomation, Via Real Carpiteria, California). Nuclei were stained with hemalaun solution. All neurons in the ventral horn that had a clearly identifiable nucleolus, were >400 mm2 in size, and were ChAT+ were counted (see ref. 8). Ten sections per mouse lumbar spinal cord that were 100 mm apart over a length of 1 mm were counted. Measurements were done on a total of 18 mice from the AAV control (n = 9) and AAV G-CSF groups (n = 9).
Counting of microglia: Coronal paraffin sections 10 µm thick from the spinal cord were stained for ionized calcium binding adaptor molecule 1 (IBA-1, 19741; WAKO, Osaka, Japan rabbit, 1:200). All microglia were counted in ten spinal cord sections per mouse that were 100 mm apart over a length of 1 mm. Measurements were done on a total of 12 mice from the AAV- eGFP (n = 4), AAV G-CSF SOD-1 (G93A) (n = 4) groups, and wild-type littermates (n = 4).
Counting of NMJs: After deep anesthesia, mice were transcardially perfused with Hank's balanced salt solution followed by 4% paraformaldehyde, muscles were dissected and cryoprotected for 1 hour in 30% sucrose solution, frozen on dry ice, and stored at −80 °C. 40-µm thick cryosections were stained for presynaptic structures (axons) with α-neurofilament-L (AB9568; Chemicon, Billerica, Massachusetts rabbit, 1:200) and for the nicotinic acetylcholine receptor with α-bungarotoxin-TRITC (T1175; Invitrogen, Eugene, Oregon; 1:200). Five sections per animals were counted, corresponding to ~200 nicotinic acetylcholine receptors per animal.
Statistics. Experiments were performed in a randomized and blinded manner, including computer-generated probe randomizations and probe labeling, blindness of all experimenters to treatment identities until the end of the experiment, and separation of data analyses from experiment conduction. Animals were age- and litter-matched. Group or pairwise parametric or nonparametric comparisons were done using NCSS software (NCSS, Kaysville, UT) or JMP 8.01 (SAS Institute, Cary, North Carolina). Survival and onset data were analyzed using the log-rank test. A P value <0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. AAV gene in the spinal cord after intramuscular injection. RT-PCR for viral genome in the lumbar spinal cord of SOD-1 (G93A) mice after intramuscular injection of AAV G-CSF. (+), AAV plasmid. Figure S2. Complete recovery of mice after intraspinal injection of AAV. (A) One week after surgery, motor functions of operated mice are similar to their previous level for the overall performance on rotarod test (p=0.80; n=12) and for the muscle strength measured with the grip strength test (p=0.25; n=12). (B) One week after surgery, body mass of operated mice is maintained and shows a trend for increase (p< 0.12; n=12). Figure S3. Intramuscular injection of AAV G-CSF enhances survival of SOD-1 (G93A) mice. Kaplan-Meyer graphs showing time to clinical endstage of the disease. Survival of SOD-1 (G93A) mice is increased by 7% after intramuscular AAV G-CSF treatment (p<0.05, n=12). Figure S4. Size distribution of ChAT+ cells in the spinal cord. Shown is the histogram distribution of ChAT-positive cells in C3/C4 and L3/L4 spinal segments for the SOD-1 (G93A) mice treated with G-CSF, the SOD-1 (G93A) mice injected with the control vector and the C57 B/6 wild type mice. Figure S5. Microglia cells in the spinal cord of SOD-1 (G93A) mice. Quantification of microglia number in the lumbar spinal of SOD-1 (G93A) and wild type mice at 15 weeks of age. G-CSF has no influence on the number of microglia in the spinal cord of SOD-1 (G93A) mice when compared to the control group of SOD-1 (G93A) mice (p<0,29, n=4). SOD-1 (G93A) mice have however more microglia in the spinal cord when compared to wild type (p<0.05, n=4).
Acknowledgments
We thank Gisela Eisenhardt, Ulrike Bolz, Simone Hoppe, Gerhard Rimner, and Paul Ruf for excellent support during this work. A.S. is inventor on patent applications claiming the use of G-CSF for the treatment of neurodegenerative conditions.
Supplementary Material
AAV gene in the spinal cord after intramuscular injection. RT-PCR for viral genome in the lumbar spinal cord of SOD-1 (G93A) mice after intramuscular injection of AAV G-CSF. (+), AAV plasmid.
Complete recovery of mice after intraspinal injection of AAV. (A) One week after surgery, motor functions of operated mice are similar to their previous level for the overall performance on rotarod test (p=0.80; n=12) and for the muscle strength measured with the grip strength test (p=0.25; n=12). (B) One week after surgery, body mass of operated mice is maintained and shows a trend for increase (p< 0.12; n=12).
Intramuscular injection of AAV G-CSF enhances survival of SOD-1 (G93A) mice. Kaplan-Meyer graphs showing time to clinical endstage of the disease. Survival of SOD-1 (G93A) mice is increased by 7% after intramuscular AAV G-CSF treatment (p<0.05, n=12).
Size distribution of ChAT+ cells in the spinal cord. Shown is the histogram distribution of ChAT-positive cells in C3/C4 and L3/L4 spinal segments for the SOD-1 (G93A) mice treated with G-CSF, the SOD-1 (G93A) mice injected with the control vector and the C57 B/6 wild type mice.
Microglia cells in the spinal cord of SOD-1 (G93A) mice. Quantification of microglia number in the lumbar spinal of SOD-1 (G93A) and wild type mice at 15 weeks of age. G-CSF has no influence on the number of microglia in the spinal cord of SOD-1 (G93A) mice when compared to the control group of SOD-1 (G93A) mice (p<0,29, n=4). SOD-1 (G93A) mice have however more microglia in the spinal cord when compared to wild type (p<0.05, n=4).
REFERENCES
- Mitchell JD., and, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369:2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez de Aguilar JL, Echaniz-Laguna A, Fergani A, René F, Meininger V, Loeffler JP, et al. Amyotrophic lateral sclerosis: all roads lead to Rome. J Neurochem. 2007;101:1153–1160. doi: 10.1111/j.1471-4159.2006.04408.x. [DOI] [PubMed] [Google Scholar]
- Pasinelli P., and, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65 Suppl 1:S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- Henriques A, Pitzer C., and, Schneider A. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand. Front Neurosci. 2010;4:32. doi: 10.3389/fnins.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K, Platzer E, Gabrilove JL, Lu L, Levi E, Polivka A, et al. Purification to apparent homogeneity and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Haematol Blood Transfus. 1985;29:398–401. doi: 10.1007/978-3-642-70385-0_82. [DOI] [PubMed] [Google Scholar]
- Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer C, Krüger C, Plaas C, Kirsch F, Dittgen T, Müller R, et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 2008;131 Pt 12:3335–3347. doi: 10.1093/brain/awn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer C, Klussmann S, Krüger C, Letellier E, Plaas C, Dittgen T, et al. The hematopoietic factor granulocyte-colony stimulating factor improves outcome in experimental spinal cord injury. J Neurochem. 2010;113:930–942. doi: 10.1111/j.1471-4159.2010.06659.x. [DOI] [PubMed] [Google Scholar]
- Meuer K, Pitzer C, Teismann P, Krüger C, Göricke B, Laage R, et al. Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson's disease. J Neurochem. 2006;97:675–686. doi: 10.1111/j.1471-4159.2006.03727.x. [DOI] [PubMed] [Google Scholar]
- Henriques A, Pitzer C, Dupuis L., and, Schneider A. G-CSF protects motoneurons against axotomy-induced apoptotic death in neonatal mice. BMC Neurosci. 2010;11:25. doi: 10.1186/1471-2202-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison RW, Casto BC., and, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Bouard D, Alazard-Dany D., and, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16:1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar BK, Lladó J, Sherkat N, Rothstein JD., and, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Haenggeli C, Gasmi M, Bishop KM, Bartus RT, Maragakis NJ, et al. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 2007;1185:256–265. doi: 10.1016/j.brainres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Leichtlein CB, Symes CW, Serikawa T, Young D., and, During MJ. Restoration of aspartoacylase activity in CNS neurons does not ameliorate motor deficits and demyelination in a model of Canavan disease. Mol Ther. 2005;11:745–753. doi: 10.1016/j.ymthe.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Neidhart J, Mangalik A, Kohler W, Stidley C, Saiki J, Duncan P, et al. Granulocyte colony-stimulating factor stimulates recovery of granulocytes in patients receiving dose-intensive chemotherapy without bone marrow transplantation. J Clin Oncol. 1989;7:1685–1692. doi: 10.1200/JCO.1989.7.11.1685. [DOI] [PubMed] [Google Scholar]
- Hedrich H., and, Bullock G. The Laboratory Mouse (The Handbook of Experimental Animals). Elsevier Academic Press: London, p. 278; 2004. [Google Scholar]
- Ludolph AC, Bendotti C, Blaugrund E, Chio A, Greensmith L, Loeffler JP, et al. Guidelines for preclinical animal research in ALS/MND: A consensus meeting. Amyotroph Lateral Scler. 2010;11:38–45. doi: 10.3109/17482960903545334. [DOI] [PubMed] [Google Scholar]
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Dupuis L., and, Loeffler JP. Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models. Curr Opin Pharmacol. 2009;9:341–346. doi: 10.1016/j.coph.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Sanes JR., and, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- Pan HC, Wu HT, Cheng FC, Chen CH, Sheu ML., and, Chen CJ. Potentiation of angiogenesis and regeneration by G-CSF after sciatic nerve crush injury. Biochem Biophys Res Commun. 2009;382:177–182. doi: 10.1016/j.bbrc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Pan B, Polley MA, Hoffman PN., and, Farah MH. Measuring nerve regeneration in the mouse. Exp Neurol. 2010;223:60–71. doi: 10.1016/j.expneurol.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Warita H, Itoyama Y., and, Abe K. Selective impairment of fast anterograde axonal transport in the peripheral nerves of asymptomatic transgenic mice with a G93A mutant SOD1 gene. Brain Res. 1999;819:120–131. doi: 10.1016/s0006-8993(98)01351-1. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Kadoya K, Hirsch M, Samulski RJ., and, Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther. 2008;16:296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Erickson D, Schaffer D, Hinh L, Gage FH., and, Peterson DA. Targeted retrograde gene delivery for neuronal protection. Mol Ther. 2002;5:50–56. doi: 10.1006/mthe.2001.0520. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL., and, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- Franich NR, Fitzsimons HL, Fong DM, Klugmann M, During MJ., and, Young D. AAV vector-mediated RNAi of mutant huntingtin expression is neuroprotective in a novel genetic rat model of Huntington's disease. Mol Ther. 2008;16:947–956. doi: 10.1038/mt.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Duysen EG, Poluektova LY, Murrin LC., and, Lockridge O. Protection from the toxicity of diisopropylfluorophosphate by adeno-associated virus expressing acetylcholinesterase. Toxicol Appl Pharmacol. 2006;214:152–165. doi: 10.1016/j.taap.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Jamshidi P, Lorenzana AO, Lee JK, Gray SJ, Samulski RJ, et al. Transient demyelination increases the efficiency of retrograde AAV transduction. Mol Ther. 2010;18:1496–1500. doi: 10.1038/mt.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Moss RB, Milla C, Colombo J, Accurso F, Zeitlin PL, Clancy JP, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Lee JS, Mulligan RC, Clark KR., and, Mendell JR. Lack of toxicity of alpha-sarcoglycan overexpression supports clinical gene transfer trial in LGMD2D. Neurology. 2008;71:240–247. doi: 10.1212/01.wnl.0000306309.85301.e2. [DOI] [PubMed] [Google Scholar]
- Mandel RJ. CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer's disease. Curr Opin Mol Ther. 2010;12:240–247. [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Kay MA. AAV vectors and tumorigenicity. Nat Biotechnol. 2007;25:1111–1113. doi: 10.1038/nbt1007-1111. [DOI] [PubMed] [Google Scholar]
- Buie LK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51:236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH., and, Olanow CW. Regulatable promoters and gene therapy for Parkinson's disease: is the only thing to fear, fear itself. Exp Neurol. 2008;209:34–40. doi: 10.1016/j.expneurol.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L., and, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Yamasaki R, Tanaka M, Fukunaga M, Tateishi T, Kikuchi H, Motomura K, et al. Restoration of microglial function by granulocyte-colony stimulating factor in ALS model mice. J Neuroimmunol. 2010;229:51–62. doi: 10.1016/j.jneuroim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Gowing G, Philips T, Van Wijmeersch B, Audet JN, Dewil M, Van Den Bosch L, et al. Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J Neurosci. 2008;28:10234–10244. doi: 10.1523/JNEUROSCI.3494-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AAV gene in the spinal cord after intramuscular injection. RT-PCR for viral genome in the lumbar spinal cord of SOD-1 (G93A) mice after intramuscular injection of AAV G-CSF. (+), AAV plasmid.
Complete recovery of mice after intraspinal injection of AAV. (A) One week after surgery, motor functions of operated mice are similar to their previous level for the overall performance on rotarod test (p=0.80; n=12) and for the muscle strength measured with the grip strength test (p=0.25; n=12). (B) One week after surgery, body mass of operated mice is maintained and shows a trend for increase (p< 0.12; n=12).
Intramuscular injection of AAV G-CSF enhances survival of SOD-1 (G93A) mice. Kaplan-Meyer graphs showing time to clinical endstage of the disease. Survival of SOD-1 (G93A) mice is increased by 7% after intramuscular AAV G-CSF treatment (p<0.05, n=12).
Size distribution of ChAT+ cells in the spinal cord. Shown is the histogram distribution of ChAT-positive cells in C3/C4 and L3/L4 spinal segments for the SOD-1 (G93A) mice treated with G-CSF, the SOD-1 (G93A) mice injected with the control vector and the C57 B/6 wild type mice.
Microglia cells in the spinal cord of SOD-1 (G93A) mice. Quantification of microglia number in the lumbar spinal of SOD-1 (G93A) and wild type mice at 15 weeks of age. G-CSF has no influence on the number of microglia in the spinal cord of SOD-1 (G93A) mice when compared to the control group of SOD-1 (G93A) mice (p<0,29, n=4). SOD-1 (G93A) mice have however more microglia in the spinal cord when compared to wild type (p<0.05, n=4).