Results from a clinical gene transfer trial for the X-linked hematological disorder Wiskott–Aldrich syndrome (WAS), carried out by Klein and colleagues at Hannover Medical School in Germany, were reported in the 11 November 2010 issue of the New England Journal of Medicine.1 The article described the clinical outcome and three-year follow-up of two young boys treated with autologous CD34+ cells transduced with a γ-retroviral vector encoding the WAS protein. Following submyeloablative conditioning, long-term engraftment levels of vector-modified CD34+ cells of 9% and 20% were observed in the bone marrow of the two patients, with similar or higher levels of corrected cells found in multiple peripheral blood hematopoietic lineages. Correction of immune abnormalities as well as resolution of autoimmunity and thrombocytopenia were observed in both patients. Although this was a remarkable success, almost as important was the announcement by the investigators in a press release,2 but absent from the publication, that one of what is now a total of 10 treated patients has developed an acute T-cell leukemia related to a vector insertion. Of note, in the New England Journal of Medicine article, significant clonal imbalances were reported for both patients, with clones observed with increased frequency having insertions in proto-oncogenes such as LMO2, MDS/EVI1, PRDM16, and CCND2. This WAS trial thus combines the insertional risk profile reported for the induction of myelodysplastic syndromes (connected with MDS/EVI1) and acute lymphoblastic leukemia (LMO2, CCND2) in previous clinical trials to correct chronic granulomatous disease and X-linked severe combined immunodeficiency (X-SCID).3,4
Preliminary data indicate that the development of an acute T-cell leukemia in the WAS trial was associated with an insertional event upstream of LMO2 and additional chromosomal aberrations (C. Klein, Hannover Medical School, personal communication, 29 November 2010), highly reminiscent of the leukemic adverse events observed in the Paris and London SCID-X1 trials. Again, this highlights the now well-recognized insertional gene activation activity of first-generation γ-retroviral vectors containing transcriptionally active long terminal repeats (LTRs) based on various forms of murine leukemia virus. With this event, there have now been leukemias or pre-leukemias reported in four different trials using LTR-driven γ-retroviral vectors to target CD34+ hematopoietic cells (X-SCID, Paris and London trials; chronic granulomatous disease, Germany; WAS, Germany).3,4,5,6 In response to the new adverse events, principal investigators in Germany have put on hold two phase I clinical trials using LTR-driven γ-retroviral vectors for gene transfer into hematopoietic stem or progenitor cells (HPSCs): the WAS trial and another trial exploring the safety of a novel anti-HIV principle in the context of AIDS-related lymphoma (C. Klein, Hannover Medical School, and B. Fehse, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, personal communication, 29 November 2010).
Still puzzling, and without adequate explanation, is the observation that approximately 20 patients with adenosine deaminase (ADA)-SCID treated with γ-retroviral vector gene transfer in the Milan and London trials have done exceedingly well, with no patient developing leukemia.7 Although a trend to clonal skewing was observed in many patients, no clonal outgrowth has been observed.8 Similarly, an ongoing ADA trial in Los Angeles utilizing an MLV-based γ-retroviral vector has reported no adverse events due to vector insertion (D. Kohn, University of California, Los Angeles, personal communication, 24 November 2010). Furthermore, numerous clinical trials targeting mature T cells utilizing LTR-driven γ-retroviral vectors have not yielded evidence for insertional adverse events despite long-term persistence of transduced cells. These data suggest that disease background factors and cell-intrinsic mechanisms may modify the risk of insertional mutagenesis. To complicate matters further, an ongoing trial for adrenoleukodystrophy utilizes a self-inactivating (SIN) lentiviral vector containing the MND viral LTR as an internal promoter. In the two patients reported on last year, no clonal imbalances were observed.9 Although results from more patients are needed, this suggests that the lentiviral vector system may mitigate the deleterious effects of viral LTR enhancers, perhaps through integration-site preferences.
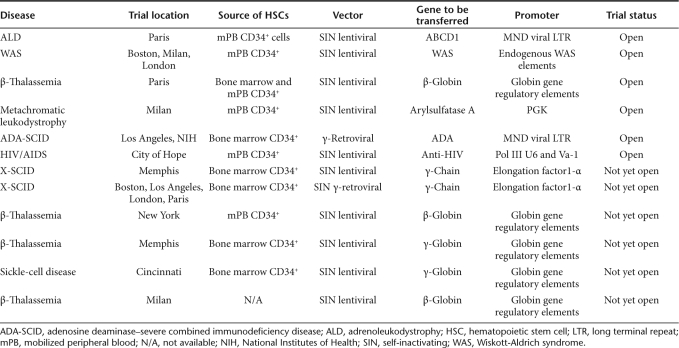
On the basis of these results, a strong argument can be made that γ-retroviral vectors that retain transcriptionally active LTRs containing a battery of strong enhancers should not be used in future clinical gene transfer trials involving genetic modification of HPSCs unless this intervention offers the only reasonable salvage therapy. An exception may be the open trials for ADA-SCID, but even here, new clinical trials should use a potentially safer vector design. This conclusion seems to be supported by the field, as evidenced by the preferential use of alternative vectors (such as SIN lentiviral or γ-retroviral vectors) and designs in recently opened or planned clinical gene transfer trials (Table 1). Specifically, the use of SIN lentiviral and γ-retroviral vectors—and in the future maybe even transposon-based approaches, in which the viral LTR elements have been removed—will be favored. These vectors utilize internal enhancer–promoter elements to drive transgene expression, and investigators have invested significant work to define internal promoters and RNA processing tools that mediate sufficient levels of gene expression while avoiding long-distance enhancer-mediated interactions with cellular promoters. In extensive nonclinical studies that address the insertional risk profile of such redesigned vectors, consistent data were reported from independent investigators using different experimental platforms that SIN vectors with selected promoters have a significantly reduced potential to transform hematopoietic cells by insertional mutagenesis.10,12,13,14 However, so far it remained unclear which insertion profile is safer for a vector with a low risk of enhancer-mediated crosstalk: the tendency of γ-retroviral vectors to integrate close to transcriptional start sites or the preference of lentiviral vectors to integrate into active transcription units. Another approach to improving safety might include the addition of a suicide gene to therapeutic vectors for use in the setting of evolving monoclonality. In the context of gene-modified T cells, this strategy has shown promise in feasibility and efficacy.15,16
Table 1. Open or planned clinical gene transfer trials using hematopoietic cells.
Interestingly, one ongoing trial for β-thalassemia, utilizing a lentiviral vector containing a β-globin gene driven by erythroid enhancer elements, has reported clonal dominance within the gene-modified cell population, but no hematopoietic abnormalities have developed thus far.17 Long-term follow-up and the treatment of additional patients are needed to understand this event. Only through a combination of improved nonclinical assay systems and well-designed human clinical trials will further understanding be gained regarding the relative risk of the new approaches that eliminate inclusion of viral LTRs in the vector design.
REFERENCES
- Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Diez IA, Dewey FA, et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Informationsdienst Wissenschaft. Effective gene therapy for children with Wiskott-Aldrich-syndrome Press release < < https://www.idw-online.de/pages/de/news396307 > ( 11 November 2010).
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- Aiuti A., and, Roncarolo MG. Ten years of gene therapy for primary immune deficiencies. Hematology Am Soc Hematol Educ Program. 2009;2009:682–689. doi: 10.1182/asheducation-2009.1.682. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Cassani B, Andolfi G, Mirolo M, Biasco L, Recchia A, et al. Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy. J Clin Invest. 2007;117:2233–2240. doi: 10.1172/JCI31666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, et al. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
- Thornhill SI, Schambach A, Howe SJ, Ulaganathan M, Grassman E, Williams D, et al. Self-inactivating gammaretroviral vectors for gene therapy of X-linked severe combined immunodeficiency. Mol Ther. 2008;16:590–598. doi: 10.1038/sj.mt.6300393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M, et al. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113:5104–5110. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]