Abstract
In different behavioral paradigms including the elevated plus maze (EPM), it was observed previously that deletion of the neuropeptide Y Y2 receptor subtype results in potent suppression of anxiety-related and stress-related behaviors. To identify neurobiological correlates underlying this behavioral reactivtiy, expression of c-Fos, an established early marker of neuronal activation, was examined in Y2 receptor knockout (Y2−/−) vs. wildtype (WT) mice. Mice were placed on the open arm (OA) or closed arm (CA) of the EPM for 10 min and the effect on regional c-Fos expression in the brain was investigated. The number of c-Fos positive neurons was significantly increased in both WT and Y2−/− lines after OA and CA exposure in 51 of 54 regions quantified. These regions included various cortical, limbic, thalamic, hypothalamic, and hindbrain regions. Genotype influenced c-Fos responses to arm exposures in 6 of the 51 activated regions: the cingulate cortex, barrel field of the primary somatosensory cortex, nucleus accumbens, dorsal lateral septum, amygdala and lateral periaqueductal gray. These differences in neuronal activity responses to the novel environments were more pronounced after OA than after CA exposure. Mice lacking Y2 receptors exhibited reduced neuronal activation when compared to WT animals in response to the emotional stressors. Reduced neuronal excitability in the identified brain areas relevant to the processing of motivated, explorative as well as anxiety-related behaviors is suggested to contribute to the reduced anxiety-related behavior observed in Y2−/− mice.
Keywords: anxiety, c-Fos mapping, elevated plus maze, neuropeptide Y, Y2 receptor knockout mice
INTRODUCTION
Neuropeptide Y (NPY), a highly conserved 36 amino acid peptide, is abundantly expressed in the central and peripheral nervous systems (Tatemoto et al., 1982). The biological actions of NPY are mediated by the activation of at least five receptors known as the Y1, Y2, Y4, Y5, and Y6 receptor (for review, see Michel et al., 1998; Pedrazzini et al., 2003). In the brain Y1 and Y2 receptors are the most abundant, with high expression of Y1 receptors in the cortex, hippocampus, and thalamic nuclei and of Y2 receptors in the hippocampal formation, lateral septum, amygdala, and locus coeruleus (Dumont et al., 1996, 1998; Kopp et al., 2002; Stanic et al., 2006). However, while Y1 receptors are mainly localized on somata and dentrites, Y2 receptors mostly appear to function as presynaptic receptors (Kopp et al., 2002; Stanic et al., 2006; Wahlestedt et al., 1986, 1993; for review, see Pedrazzini et al., 2003).
NPY has been shown to serve important roles in a number of physiological functions including ingestive behavior, energy homeostasis, cardiovascular regulation, stress, memory, and seizures (Michalkiewicz et al., 2001; Pedrazzini et al., 1998; Sainsbury et al., 2002; Sweerts et al., 2001; for review, see Pedrazzini et al., 2003). Furthermore, NPY has a critical role in the regulation of anxiety-like responses as results from behavioral experiments indicate that central administration of NPY induces anxiolytic-like effects in various animal paradigms including the elevated plus maze (EPM), light/dark test, Montgomery’s and Vogel’s conflict tests (Heilig et al., 1989, 1993; Karlsson et al., 2005; Kask et al., 1998; Nakajima et al., 1998; Pich et al., 1993). Accumulating evidence indicates that the anxiolytic-like response of NPY is mediated via Y1 receptors. For example, intracerebroventricular injection of antisense oligodeoxynucleotide targeting the Y1 receptor mRNA, resulting in decreased density of Y1 receptors, is associated with a reduction of anxiolytic effects of intra-amygdalar injection of NPY (Heilig, 1995). Additionally, injection of the Y1 receptor antagonist BIBO3304 into the basolateral amygdala results in the blockade of the anxiolytic-like effects of NPY in the social interaction test (Sajdyk et al., 1999). Interestingly, the genetic deletion of the Y1 receptor in mice induces an anxiogenic-like effect in the light/dark test box, but an anxiolytic-like effect in the open field and EPM, which is explained by the influence of additional factors such as circadian rhythm (Karl et al., 2006; Karlsson et al., 2008).
On the other hand, there is also growing evidence for an involvement of Y2 receptors in anxiety. The administration of Y2 receptor agonists has demonstrated anxiogenic effects in the social interaction test and in the EPM (Nakajima et al., 1998; Sajdyk et al., 2002), whereas a selective Y2 receptor antagonist has demonstrated an anxiolytic-like profile in the EPM (Bacchi et al., 2006). Studies on Y2 knockout (Y2−/−) mice have confirmed that the deletion of Y2 receptors suppresses anxiety-related behaviors in the EPM, light/dark test, and open field paradigms (Painsipp et al., 2008; Redrobe et al., 2003; Tschenett et al., 2003), which may contribute to their enhanced impulsivity (Greco and Carli, 2006).
Until now, no studies have been conducted in order to investigate neuronal activation patterns associated with the altered behavioral reactivity to novelty of Y2−/− mice. Indeed, the anxiolytic effect of Y2 gene disruption on the EPM as revealed in independent studies (Painsipp et al., 2008; Redrobe et al., 2003; Tschenett et al., 2003) offers an opportunity to investigate neuronal correlates underlying their behavioral responses by using c-Fos immunohistochemistry as a marker of neuronal activation (for review, see Hoffman and Lyo, 2002; Singewald, 2007). Based on the results of the EPM studies (see above), we hypothesized that Y2−/− mice perceive the open arm (OA) of an EPM as less anxiogenic than wildtype (WT) mice. Consequently, less neuronal activation in critical anxiety-related brain regions should indicate neuronal populations contributing to or mediating the altered neurobehavioral reactivity to novelty of Y2−/− mice vs. WT mice.
MATERIALS AND METHODS
Animals
Naïve age-matched (22-weeks-old), male mice were used in all experiments. The generation of germline Y2−/− mice was described previously (Sainsbury et al., 2002). Noninduced conditional Y2lox/lox mice that do not differ from WT mice in terms of Y2 receptor binding, body weight, or plasma corticosterone levels (Sainsbury et al., 2002) were used as controls and are termed hereafter as WT mice. Germline Y2−/− and conditional Y2lox/lox mice were generated from the same founders on a 129Sv × C57BL/6 background (Sainsbury et al., 2002). All mice were housed in groups of 3–5 mice per cage on a 12:12 h light/dark schedule (lights on at 07.00) with food and water available ad libitum. Forty-eight hours before the experiment, animals were single housed, taken in their home cages from the animal facility to the experimental rooms, and allowed to habituate. Behavioral experiments were carried out during the light phase of the cycle from 9.00 to 14.00. The experimental studies described here were designed to minimize animal suffering and number of animals used. They were approved by the Ethical Committee on Animal Care of the Austrian Ministry for Education, Science and Art and are in compliance with international laws and policies.
Experiment 1: c-Fos induction in response to open arm exposure
Mice (WT: n = 9; Y2−/−: n = 9) were placed in the middle part of the OA (30 × 5 cm, rims 2 mm, elevation 73 cm) of an EPM for 10 min, where the access to the neutral zone and the closed arms (CAs) of the maze was prevented by a bar (Salome et al., 2004, 2006). The light intensity on the OA was about 300 lux. During the 10-min arm exposure, the distance the animals traveled on the OA was tracked by an automated system (videomot, TSE, Bad Homburg, Germany). Immediately after OA exposure, animals were returned to their home cages. The OA was cleaned thoroughly with water before each trial. Animals assigned to the basal group (WT: n = 5; Y2−/−: n = 5) remained undisturbed in their home cages.
Experiment 2: c-Fos induction in response to open vs. closed arm exposure
Stimulated by studies showing that rodents show stronger emotional responses to a forced OA vs. a CA exposure of an EPM (Holmes and Rodgers, 1999; Pellow et al., 1985), we decided to include another control condition. Therefore, mice were subjected either to the OA (WT: n = 4; Y2−/−: n = 4) or the CA (WT: n = 7; Y2−/−: n = 6). The CA was identical to the OA, but was enclosed by 14-cm high, nontransparent walls. Settings and procedure for both OA and CA exposure were as in Experiment 1.
c-Fos immunohistochemistry
Given that stress-induced c-Fos protein expression has been shown to reach its maximum after 2 h (e.g., Zangenehpour and Chaudhuri, 2002), we used this time frame also in the present study. Accordingly, 2 h after onset of OA or CA exposure, animals were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 20 mL of 0.9% saline followed by 20 mL of 4% paraformaldehyde in 0.1 mol/L phosphate buffered solution (PBS, pH 7.4). Animals not exposed to the OA were treated identically immediately after removal from their home cages. Brains were then removed and postfixed in 4% paraformaldehyde in PBS at 4°C overnight. Coronal sections (100 μm) were cut with a vibratome (Ted Pella, Redding, CA) and collected freely floating in PBS.
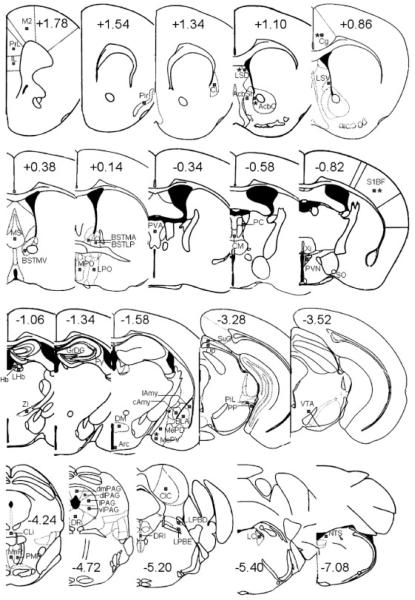
The sections were processed for c-Fos immunoreactivity as described previously (Singewald et al., 2003). They were incubated for 48 h in a polyclonal primary antibody (s.c.-52, Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:20,000 in immunobuffer (pH 7.4) comprising 0.1 mol/L NaCl, 5 mmol/L KCl, 8 mmol/L Na2HPO4, 15 mmol/L NaH2PO4, 10 mmol/L Tris-HCl, 0.3% Triton X-100, and 0.04% thimerosal. The rabbit primary antibody was raised against a peptide in the amino terminus of human c-Fos p62 identical to the corresponding mouse sequence and does not cross-react with FosB, Fra-1, or Fra-2. The sections were then rinsed and incubated with a biotinylated goat antirabbit secondary antibody (Vector Laboratories, Burlingame, CA) for 24 h. An avidin-biotin-horseradish peroxidase procedure with 3,3′-diaminobenzidine as the chromogen was used to visualize the immunoreactivity. Cells containing a nuclear brown-black reaction product were considered positive for c-Fos-immunoreactivity and are referred to hereafter as c-Fos-positive cells. The anatomical localization of c-Fos-positive cells was aided by using the illustrations in a stereotaxic atlas (Franklin and Paxinos, 1997). For quantitative analysis, the number of c-Fos-positive cells was counted bilaterally in a tissue area of 0.01 mm2 in 54 areas with the exception of specific cortical areas where tissue areas of 0.04 mm2 were evaluated (Fig. 1).
Fig. 1.
Schematic diagrams adapted from the mouse brain atlas (Franklin and Paxinos, 1997), showing the 54 brain regions in which c-Fos expression was evaluated. The squares indicate the placement of grids for counting of c-Fos positive cells. Asterisks indicate the regions in which genotype (Y2−/− vs. WT mice) influenced c-Fos induction to open or closed arm exposure in Experiments 1 and 2. The number in each slide indicates the Bregma level of brain section. Abbreviations in alphabetical order: AcbC, accumbens nucleus, core; AcbSh, accumbens nucleus, shell; Arc, arcuate hypothalamic nucleus; BLA, basolateral amygdaloid nucleus; BSTLP, bed nucleus of the stria terminalis, lateral division, posterior part; BSTMA: bed nucleus of the stria terminalis, medial division, anterior part; BSTMV, bed nucleus of the stria terminalis, medial division, ventral part; cAmy, central nucleus of the amydala; Cg, cingulate cortex; CIC, central nucleus of the inferior colliculus; Cl, claustrum; Cli, caudal linear nucleus of the raphe; CM, central medial thalamic nucleus; dlPAG, dorsolateral periaqueductal gray; DM, dorsomedial hypothalamic nucleus; dmPAG, dorsomedial periaqueductal gray; DR, dorsal raphe nucleus; DRI, dorsal raphe nucleus, inferior part; GrDG, granular layer of the dentate gyrus; IL, infralimbic cortex; lAmy, lateral amygdaloid nucleus; LC, locus coeruleus; LHb, lateral habenular nucleus; lPAG, lateral periaqueductal gray; LPBD, lateral parabrachial nucleus, dorsal part; LPBE, lateral parabrachial nucleus, external part; LPO, lateral preoptic area; LSD, lateral septal nucleus, dorsal part; LSV, lateral septal nucleus, ventral part; M2, secondary motor cortex; MePD, medial amygdaloid nucleus, posterodorsal part; MePV, medial amygdaloid nucleus, posteroventral part; MHb, medial habenular nucleus; MnR, median raphe nucleus; MPO, medial preoptic nucleus; MS, medial septal nucleus; NTS, nucleus of the solitary tract; Op, optic nerve layer of the superior colliculus; PC, paracentral thalamic nucleus; PIL, posterior intralaminar thalamic nucleus; Pir, piriform cortex; PMR, paramedian raphe nucleus; PP, peripeduncular nucleus; PrL, prelimbic cortex; PVA, paraventricular thalamic nucleus, anterior part; PVN, paraventricular hypothalamic nucleus; Py, pyramidal cell layer of the hippocampus; S1BF, primary somatosensory cortex, barrel field; SO, supraoptic nucleus; SuG, superficial gray layer of the superior colliculus; vlPAG, ventrolateral periaqueductal gray; VTA, ventral tegmental area; Xi, xiphoid thalamic nucleus; ZI, zona incerta.
Data and statistical analysis
Results are expressed as means ± SEM. Data analysis of the number of c-Fos-positive cells per brain region examined as well as of the distance traveled on the respective arms was performed using a two-way ANOVA for genotype and stressor or arm in Experiments 1 and 2, respectively. Two group comparisons were made by Bonferroni test or by independent t-test. P values less than 0.05 were considered to be significant, while P values less than 0.09 were used to indicate a trend toward significance.
RESULTS
Locomotion of WT and Y2−/− mice during OA and CA exposure
When placed on a particular arm of an EPM, mice irrespective of genotype explored the OA and the CA with similar interest as the distance traveled did not differ between the two arm types. During the 10-min arm exposure, locomotor activity was comparable between WT and Y2−/− mice in Experiment 1 as well as in Experiment 2 (Table I).
TABLE I.
Locomotor activity of wildtype (WT) and Y2 knockout (Y2−/−) mice during 10-min exposure to either the open (WT: n = 4 and 9; Y2−/−: n = 4 and 9) or closed arm (WT: n = 5; Y2−/−: n = 6) of an elevated plus maze
| Experiment | Distance traveled (cm) |
||
|---|---|---|---|
| WT | Y2−/− | ||
| Experiment 1 | |||
| Open arm | 354 ± 19 | 379 ± 54 | P = 0.667 |
| Experiment 2 | |||
| Open arm | 390 ± 103 | 562 ± 54 | F1,17 = 2.49, P = 0.137 (genotype) |
| Closed arm | 546 ± 75 | 701 ± 112 | F1,17 = 2.04, P = 0.175 (arm) |
The distance travelled is given in cm as mean ± SEM. Statistical analysis was performed using a t-test in Experiment 1 and a two-way ANOVA in Experiment 2, respectively.
Experiment 1: c-Fos induction in response to open arm exposure
Mean numbers ± SEM of cells expressing c-Fos in all quantified brain regions are shown in Table II. Two-way ANOVA (stress × genotype) revealed that exposure to the OA for 10 min induced increased c-Fos expression in 51 of the 54 investigated brain areas, including parts of the medial prefrontal cortex (cingulate, prelimbic, infralimbic cortex), piriform cortex, bed nucleus of the stria terminalis, lateral septum, habenula, various amygdaloid and hypothalamic nuclei, the parts of the periaqueductal gray and locus coeruleus. Genotype significantly affected the number of c-Fos-positive cells induced by OA exposure in the prelimbic cortex (F1,27 = 9.14; P = 0.006; Figs. 1 and 2), barrel field of the primary somatosensory cortex (F1,27 = 16.34; P < 0.001; Figs. 1, 2, and 3A), nucleus accumbens core region (F1,26 = 7.49; P = 0.012; Figs. 1, 2, and 3A), dorsal part of the lateral septum (F1,26 = 6.14; P = 0.021; Figs. 1, 2, and 3A), granular layer of the dentate gyrus (F1,27 = 21.13; P < 0.001), CA3 pyramidal cell layer of the hippocampus (F1,27 = 6.22; P = 0.020), posteroventral part of the medial amygdala (F1,26 = 4.53; P = 0.044; Figs. 1, 2, and 3A), lateral periaqueductal gray (F1,27 = 5.38; P = 0.029), and central nucleus of the inferior colliculus (F1,27 = 7.47; P = 0.012). Furthermore, a trend toward a significant genotype effect was found in the cingulate cortex (F1,27 = 3.68; P = 0.067; Figs. 1, 2, and 3A), claustrum (F1,27 = 3.72; P = 0.066), zona incerta (F1,27 = 3.77; P = 0.064), and ventral part of the lateral septum (F1,26 = 4.25; P = 0.051). Stress × genotype interactions were detected in the prelimbic cortex (F1,27 = 6.01; P = 0.022), barrel field of the somatosensory cortex (F1,27 = 12.38; P = 0.002), nucleus accumbens core region (F1,22 = 7.14; P = 0.014), granular layer of the dentate gyrus (F1,27 = 7.66; P = 0.011), and CA3 pyramidal cell layer of the hippocampus (F1,27 = 5.50; P = 0.028), and there was a trend toward significance in the piriform cortex (F1,27 = 3.15; P = 0.088), posteroventral part of the medial amygdala (F1,26 = 3.69; P = 0.067), and the locus coeruelus (F1,27 = 3.41; P = 0.076). Specifically, while basal c-Fos expression was generally low and comparable between the two genotypes, Y2−/− relative to WT mice displayed attenuated stress-induced c-Fos expression in the prelimbic, cingulate and primary somatosensory (barrel field) cortices, nucleus accumbens core region, dorsal and ventral lateral septum, granular layer of the dentate gyrus, CA3 pyramidal cell layer of the hippocampus, posteroventral part of the medial amygdala, and locus coeruleus (Table II; Fig. 3A).
TABLE II.
c-Fos expression in wildtype (WT) and Y2 knockout (Y2−/−) mice in different brain areas under basal conditions (WT: n = 5; Y2−/−: n = 5) or after open arm exposure (WT: n = 9; Y2−/−: n = 9)
| Brain area | Basal |
Open arm exposure |
Stress |
|||
|---|---|---|---|---|---|---|
| WT | Y2−/− | WT | Y2−/− | F | P | |
| Forebrain | ||||||
| Cortical areas | ||||||
| Infralimbic cortex (§) | 13.8 ± 1.7 | 10.9 ± 1.7 | 48.9 ± 2.4 | 48.5 ± 2.7 | 188.69 | <0.001 |
| Prelimbic cortex (§) | 7.7 ± 1.6 | 6.1 ± 1.5 | 54.2 ± 2.3 | 38.8 ± 3.2••• | 199.95 | <0.001 |
| Motor cortex (§) | 0.1 ± 0.1 | 0.2 ± 0.2 | 6.8 ± 0.7 | 6.1 ± 1.0 | 57.43 | <0.001 |
| Piriform cortex | 1.9 ± 0.6 | 2.9 ± 0.9 | 28.6 ± 1.4 | 24.6 ± 1.6 | 255.90 | <0.001 |
| Claustrum | 3.0 ± 1.4 | 2.0 ± 0.5 | 13.7 ± 0.9 | 10.6 ± 1.0 | 82.18 | <0.001 |
| Cingulate cortex (§) | 7.1 ± 1.9 | 6.4 ± 2.2 | 53.8 ± 1.3 | 40.9 ± 4.8• | 130.24 | <0.001 |
| Somatosensory 1, barrel field (§) | 1.8 ± 1.4 | 0.6 ± 0.4 | 30.6 ± 2.9 | 13.3 ± 1.6••• | 81.87 | <0.001 |
| Nucleus accumbens, core | 1.9 ± 0.6 | 1.8 ± 0.4 | 16.9 ± 0.7 | 10.6 ± 1.4• | 82.91 | <0.001 |
| Nucleus accumbens, shell | 1.7 ± 0.4 | 1.7 ± 0.3 | 9.8 ± 0.5 | 8.8 ± 1.0 | 86.52 | <0.001 |
| Lateral septal nucleus, dorsal | 4.1 ± 1.4 | 2.2 ± 0.5 | 17.0 ± 0.6 | 13.4 ± 1.3b | 115.95 | <0.001 |
| Lateral septal nucleus, ventral | 5.8 ± 2.5 | 3.9 ± 1.3 | 39.6 ± 1.8 | 33.2 ± 1.7b | 249.76 | <0.001 |
| Medial septal nucleus | 0.1 ± 0.1 | 0.5 ± 0.3 | 3.6 ± 0.4 | 2.8 ± 0.4 | 59.71 | <0.001 |
| Bed nucleus, stria terminalis, ventral | 2.0 ± 0.2 | 1.7 ± 0.3 | 9.3 ± 1.3 | 7.6 ± 0.6 | 46.76 | <0.001 |
| Bed nucleus, stria terminalis, anterior | 2.3 ± 0.5 | 2.3 ± 0.4 | 9.6 ± 0.7 | 9.8 ± 0.8 | 104.30 | <0.001 |
| Bed nucleus, stria terminalis, lateral division, posterior |
2.3 ± 0.6 | 3.2 ± 0.5 | 6.7 ± 0.7 | 7.6 ± 0.9 | 29.40 | <0.001 |
| Lateral preoptic area | 4.6 ± 0.6 | 4.4 ± 0.4 | 10.4 ± 0.8 | 9.2 ± 0.7 | 48.07 | <0.001 |
| Medial preoptic area | 2.9 ± 0.6 | 2.7 ± 0.3 | 19.0 ± 1.6 | 18.7 ± 1.7 | 101.31 | <0.001 |
| Hippocampal formation | ||||||
| Granular layer, dentate gyrus | 6.9 ± 1.3 | 5.3 ± 1.0 | 17.2 ± 0.4 | 10.8 ± 0.9••• | 81.53 | <0.001 |
| Pyramidal cell layer, CA3 | 1.0 ± 0.3 | 0.9 ± 0.2 | 7.7 ± 0.4 | 4.4 ± 0.9•• | 58.78 | <0.001 |
| Amygdala | ||||||
| Lateral nucleus | 2.0 ± 0.5 | 2.7 ± 0.7 | 7.7 ± 0.5 | 7.4 ± 0.5 | 87.46 | <0.001 |
| Central nucleus | 3.0 ± 1.4 | 3.5 ± 0.4 | 4.2 ± 0.7 | 7.7 ± 1.6 | 4.00 | 0.058 |
| Medial nucleus, posterodorsal | 2.7 ± 0.8 | 2.4 ± 1.1 | 15.6 ± 2.0 | 12.2 ± 1.4 | 45.31 | <0.001 |
| Medial nucleus, posteroventral | 1.7 ± 0.3 | 1.5 ± 0.4 | 14.8 ± 0.7 | 10.8 ± 1.2• | 133.89 | <0.001 |
| Basolateral nucleus | 2.4 ± 1.1 | 2.9 ± 0.9 | 14.4 ± 0.8 | 12.6 ± 0.9 | 131.55 | <0.001 |
| Diencephalon | ||||||
| Thalamus | ||||||
| Paraventricular nucleus, anterior | 13.4 ± 1.1 | 12.0 ± 1.3 | 24.2 ± 1.9 | 25.7 ± 2.1 | 37.56 | <0.001 |
| Paracentral nucleus | 5.6 ± 1.3 | 5.8 ± 1.8 | 19.8 ± 2.1 | 20.1 ± 3.0 | 29.17 | <0.001 |
| Central medial nucleus | 6.0 ± 1.4 | 6.1 ± 0.9 | 16.8 ± 1.8 | 18.7 ± 1.3 | 55.49 | <0.001 |
| Xiphoid nucleus | 4.8 ± 2.6 | 3.4 ± 1.3 | 17.2 ± 1.9 | 13.2 ± 1.3 | 334.26 | <0.001 |
| Lateral habenular nucleus | 1.2 ± 0.8 | 3.1 ± 0.4 | 14.3 ± 2.1 | 11.6 ± 1.6 | 38.69 | <0.001 |
| Medial habenular nucleus | 0.0 ± 0.0 | 0.60 ± 0.5 | 0.75 ± 0.4 | 0.89 ± 0.5 | 1.50 | 0.234 |
| Zona incerta | 14.6 ± 1.7 | 12.3 ± 1.3 | 25.8 ± 2.2 | 19.6 ± 2.1 | 17.65 | <0.001 |
| Posterior intralaminar nucleus | 4.3 ± 0.4 | 4.5 ± 0.8 | 11.6 ± 0.7 | 9.8 ± 0.9 | 59.94 | <0.001 |
| Peripeduncular nucleus | 3.6 ± 0.8 | 4.2 ± 0.8 | 8.4 ± 0.9 | 7.7 ± 1.3 | 12.29 | 0.003 |
| Hypothalamus | ||||||
| Dorsomedial nucleus | 6.4 ± 1.8 | 6.9 ± 2.6 | 29.4 ± 2.0 | 23.0 ± 2.3 | 67.84 | <0.001 |
| Paraventricular nucleus | 2.5 ± 1.0 | 3.1 ± 1.0 | 22.8 ± 3.6 | 29.7 ± 6.2 | 22.87 | <0.001 |
| Supraoptic nucleus | 2.2 ± 0.5 | 2.4 ± 1.1 | 8.7 ± 0.9 | 7.1 ± 0.7 | 38.13 | <0.001 |
| Arcuate nucleus | 1.6 ± 0.6 | 1.2 ± 0.5 | 7.0 ± 1.1 | 7.3 ± 1.0 | 31.27 | <0.001 |
| Midbrain, pons, hindbrain | ||||||
| Superficial gray, superior colliculus | 9.4 ± 1.6 | 6.7 ± 1.7 | 23.0 ± 1.5 | 18.4 ± 2.6 | 32.94 | <0.001 |
| Optic nerve layer of the superior colliculus | 8.4 ± 1.9 | 7.7 ± 2.5 | 20.4 ± 1.0 | 17.3 ± 1.3 | 46.56 | <0.001 |
| Ventral tegmental area | 0.1 ± 0.1 | 0.5 ± 0.4 | 2.2 ± 0.4 | 1.9 ± 0.2 | 28.96 | <0.001 |
| Raphe nuclei | ||||||
| Caudal linear nucleus | 1.9 ± 0.9 | 2.9 ± 0.7 | 8.8 ± 1.5 | 7.0 ± 0.9 | 19.05 | <0.001 |
| Paramedian nucleus | 0.9 ± 0.3 | 1.2 ± 0.1 | 6.9 ± 0.8 | 5.1 ± 0.5 | 54.09 | <0.001 |
| Median nucleus | 1.3 ± 0.5 | 1.3 ± 0.4 | 19.2 ± 1.0 | 17.2 ± 2.0 | 122.15 | <0.001 |
| Dorsal | 2.0 ± 0.6 | 2.3 ± 0.4 | 8.9 ± 0.7 | 9.7 ± 0.6 | 101.90 | <0.001 |
| Dorsal nucleus, inferior | 2.3 ± 0.7 | 1.9 ± 0.8 | 12.2 ± 2.1 | 13.1 ± 1.0 | 43.18 | <0.001 |
| Periaqueductal gray | ||||||
| Lateral | 4.3 ± 1.1 | 2.6 ± 0.4 | 10.8 ± 0.6 | 9.3 ± 0.5 | 88.51 | <0.001 |
| Ventrolateral | 2.6 ± 0.6 | 3.5 ± 0.3 | 10.4 ± 0.4 | 9.9 ± 0.7 | 145.21 | <0.001 |
| Dorsolateral | 2.2 ± 0.6 | 2.7 ± 0.9 | 8.9 ± 0.6 | 7.6 ± 0.7 | 63.39 | <0.001 |
| Dorsomedial | 2.7 ± 0.5 | 3.4 ± 0.8 | 8.9 ± 0.4 | 7.2 ± 0.8 | 46.97 | <0.001 |
| Lateral parabrachial nucleus, dorsal | 6.2 ± 1.3 | 5.7 ± 1.5 | 14.2 ± 1.2 | 13.1 ± 2.1 | 18.07 | <0.001 |
| Lateral parabrachial nucleus, external | 3.1 ± 0.6 | 2.1 ± 0.4 | 8.8 ± 1.2 | 7.2 ± 1.0 | 25.34 | <0.001 |
| Central nucleus, inferior colliculus | 4.8 ± 0.3 | 3.6 ± 0.6 | 5.5 ± 0.2 | 4.5 ± 0.4 | 3.95 | 0.058 |
| Locus coeruleus | 3.6 ± 0.4 | 3.8 ± 0.5 | 19.2 ± 1.4 | 14.6 ± 1.2• | 105.71 | <0.001 |
| Nucleus of the solitary tract | 1.4 ± 0.6 | 0.8 ± 0.4 | 5.6 ± 1.4 | 8.3 ± 1.3 | 18.43 | <0.001 |
The number of c-Fos-positive cells/0.01 mm2 and /0.04 mm2 (regions marked with §), respectively, is given as mean ± SEM. Brain areas where a significant difference between WT and Y2−/− was observed are highlighted by bold letters. Statistical analysis was performed using two-way ANOVA and post Bonferroni test:
P < 0.09
P < 0.05
P < 0.01
P < 0.001 vs. respective WT group.
Fig. 2.
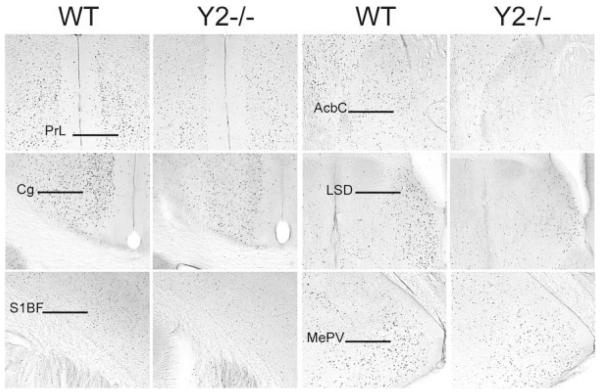
Representative bright-field photomicrographs showing decreased Fos expression in Y2 knockout (Y2−/−) compared to wildtype (WT) mice in response to open arm exposure. Cortical areas: prelimbic cortex (PrL), cingulate cortex (Cg), primary somatosensory cortex (S1BF); nucleus accumbens core (AcbC); dorsal lateral septum (LSD) and medial amygdala, posteroventral part (MePV). Scale bar = 200 μm.
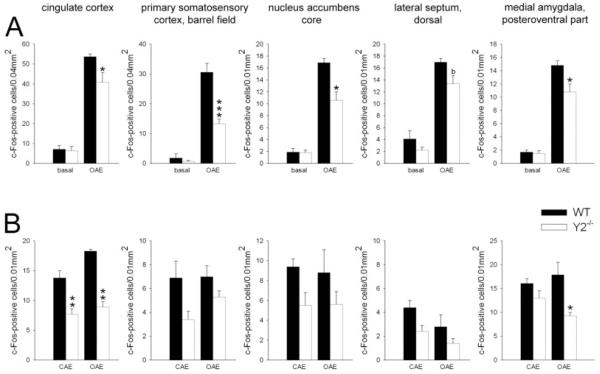
Fig. 3.
Quantitative analysis of c-Fos-like immunoreactivity in Y2 knockout (Y2−/−) and wildtype (WT) mice under basal conditions (A), after open arm (OAE; A and B) and closed arm (CAE; B) exposure. Only those brain areas are graphically presented in which the difference in stress-induced c-Fos expression between the two genotypes was statistically significant and consistent in both Experiments 1 (A) and 2 (B). Each column indicates the mean ± SEM number of c-Fos-positive cells in a tissue area of 0.01 or 0.04 mm2. n = 4–9 per experimental group. *P < 0.05, **P < 0.01, and ***P < 0.001 Y2−/− vs. WT mice.
Experiment 2: c-Fos induction in response to open vs. closed arm exposure
Two-way ANOVA (genotype × arm) revealed a significant effect of genotype in c-Fos responses to arm exposure in the cingulate cortex (Fig. 3B), motor cortex, barrel field of the primary somatosensory cortex (Fig. 3B), nucleus accumbens core region (Fig. 3B), dorsal part of the lateral septum (Fig. 3B), and post-eroventral part of the medial amygdala (Fig. 3B) which are summarized in Table III. Genotype additionally affected c-Fos induction in the lateral periaqueductal gray (F1,19 = 4.82; P = 0.043), ventral (F1,19 = 11.37; P = 0.004), and inferior (F1,20 = 5.65; P = 0.030) dorsal raphe and external lateral parabrachial nucleus (F1,26 = 4.79; P = 0.043). Trends toward a significant genotype effect were detected in the dorsomedial periaqueductal gray (F1,19 = 3.50; P = 0.080), median raphe (F1,19 = 4.16; P = 0.058), and dorsal lateral parabrachial nucleus (F1,26 = 3.28; P = 0.089). Moreover, an effect of arm type was observed in the cingulate cortex (F1,17 = 4.97; P = 0.043), ventral dorsal raphe (F1,19 = 4.89; P = 0.042), and lateral periaqueductal gray (F1,19 = 6.14; P = 0.025) as well as a trend toward significance in the dorsal part of the lateral septum (F1,19 = 4.42; P = 0.052). There was a significant genotype × arm interaction in the lateral periaqueductal gray (F1,19 = 6.14; P = 0.025) with WT mice displaying reduced c-Fos expression after CA vs. OA exposure (P = 0.018), and in the paraventricular hypothalamic nucleus (magnucellular part) (F1,19 = 5.67; P = 0.030). Further, trends toward a significant genotype × arm interaction were detected in the central amygdala (F1,19 = 3.86; P = 0.067) and xiphoid nucleus (F1,17 = 4.11; P = 0.082).
TABLE III.
c-Fos expression in wildtype (WT) and Y2 knockout (Y2−/−) mice in different brain areas after open arm exposure (WT: n = 4; Y2−/−: n = 4) and closed arm exposure (WT: n = 7; Y2−/−: n = 6)
| Brain area | Open arm exposure |
Closed arm exposure |
Genotype |
|||
|---|---|---|---|---|---|---|
| WT | Y2−/− | WT | Y2−/− | F | P | |
| Prelimbic cortex | 5.4 ± 1.4 | 4.1 ± 1.1 | 5.0 ± 0.3 | 3.5 ± 0.6 | 2.71 | 0.121 |
| Cingulate cortex | 18.3 ± 0.3 | 8.9 ± 0.9•• | 13.8 ± 1.2 | 7.7 ± 0.9•• | 37.32 | <0.001 |
| Secondary motor cortex | 9.5 ± 2.0 | 5.4 ± 0.8 | 9.2 ± 0.5 | 4.8 ± 0.9• | 18.70 | <0.001 |
| Somatosensory 1, barrel field | 7.0 ± 0.9 | 5.3 ± 0.5 | 6.9 ± 1.4 | 3.4 ± 0.7 | 4.87 | 0.042 |
| Nucleus accumbens, core | 8.8 ± 2.3 | 5.6 ± 1.3 | 9.4 ± 0.8 | 5.5 ± 1.3 | 8.92 | 0.008 |
| Lateral septal nucleus, dorsal | 2.8 ± 1.0 | 1.4 ± 0.4 | 4.4 ± 0.6 | 2.4 ± 0.5 | 6.87 | 0.019 |
| Lateral septal nucleus, ventral | 15.0 ± 1.4 | 15.1 ± 1.4 | 19.4 ± 2.5 | 15.5 ± 1.1 | 0.83 | 0.376 |
| Granular layer, dentate gyrus | 10.3 ± 1.6 | 11.3 ± 1.6 | 10.0 ± 1.2 | 10.2 ± 0.7 | 0.21 | 0.656 |
| Pyramidal cell layer, CA3 | 5.4 ± 0.9 | 3.6 ± 0.6 | 5.4 ± 0.6 | 4.8 ± 0.7 | 2.86 | 0.109 |
| Medial amygdala, posteroventral | 17.9 ± 2.6 | 9.3 ± 0.7• | 16.1 ± 1.0 | 13.0 ± 1.5 | 12.20 | 0.003 |
| Locus coeruleus | 19.0 ± 4.3 | 16.6 ± 2.8 | 17.0 ± 1.1 | 18.6 ± 2.6 | 0.02 | 0.881 |
The number of Fos-positive cells/0.01 mm2 is given as mean ± SEM. Statistical analysis was performed using two-way ANOVA and post Bonferroni test:
P < 0.05,
P < 0.01 vs. respective WT group.
Differences in the numbers of c-Fos-positive cells between WT and Y2−/− mice were observed in several brain areas. Compared to WT mice, Y2−/− had lower numbers of c-Fos-positive cells in the cingulate cortex after both OA (P = 0.003) and CA (P = 0.005) exposure (Fig. 3B). Furthermore, a significantly reduced neuronal activation was observed in knockout vs. WT animals in the posteroventral part of the medial amygdala (P = 0.035; Fig. 3B) and lateral periaqueductal gray (P = 0.049) after OA exposure as well as in the motor cortex (P = 0.018) following CA arm exposure.
DISCUSSION
Our present experiments demonstrate (1) a pronounced over-expression of the neuronal activity marker c-Fos in brain areas related to the processing of emotions in response to novel environments with proposed high and low anxiogenic potentials, respectively (Pellow et al., 1985), and (2) that genotype significantly affects stress-induced c-Fos expression in a specific subset of brain areas, namely the cingulate cortex, barrel field of the somatosensory cortex, nucleus accumbens core region, dorsal lateral septum, posteroventral part of the medial amygdala, and lateral periaqueductal gray. Specifically, this effect is attenuated in mice lacking the Y2 receptor that display an anxiolytic phenotype also as neurobehavioral response to novelty (Painsipp et al., 2008; Redrobe et al., 2003; Tschenett et al., 2003), and it seems to be more pronounced after OA then CA exposure.
Enhanced c-Fos expression by OA and CA exposure
In the present study, c-Fos expression was triggered in the majority of the analyzed regions following OA exposure (Tables II and III). This activation pattern is in accordance with previous studies using OA exposure as a challenge (Nguyen et al., 2006; Salome et al., 2004; Viltart et al., 2006). Similarly, the CA also induced a pronounced c-Fos expression which did not differ from that after OA exposure in all of the brain areas investigated with the exception of the cingulate cortex, dorsal lateral septum, ventral dorsal raphe, and lateral periaqueductal gray. So far, only one study (Mairesse et al., 2007) compared neuronal activity in limbic regions in response to OA vs. CA exposure and reported reduced effects after CA exposure in the infralimbic cortex, paraventricular hypothalamus, hippocampus, and amygdala which are not supported by the present findings. These discrepancies in OA- vs. CA-induced neuronal activity patterns between the present and previous study may be due to some methodological differences including exposure times (5 vs. 10 min arm exposure).
Here, we show that exposure to either the OA or CA of an EPM that both elicit behavioral activity evokes c-Fos expression in numerous brain regions. Of these, the motor, cingulate and piriform cortices, nucleus accumbens, and locus coeruleus are associated with the elaboration of motivated behaviors induced by novelty (Stone et al., 2006) (for review, see Sewards and Sewards, 2003) or exploration of a novel environment (Handa et al., 1993; Staiger et al., 2000; Uslaner et al., 2001, 2003). Many of these brain areas express motoric α1-adrenoreceptors (Stone et al., 2004), suggesting that they could be activated in the murine brain upon traveling in the arms. However, both the OA and CA are thought to be associated with very low locomotor activation. Interestingly, in the EPM, which the OA test is related to, motor activity is only one factor, with the strongest measure being anxiety (File, 2001). In line with that, many of the activated regions such as amygdala, bed nucleus of the stria terminalis, paraventricular hypothalamic nucleus, lateral septum, and locus coeruleus are thought to be also involved in anxiety-related processing (Charney et al., 1998). Given the influence of arm type on c-Fos responses in the cingulate cortex, dorsal lateral septum, ventral dorsal raphe, and lateral periaqueductal gray, it is suggested that these identified brain areas primarily mediate the more anxiogenic potential of the OA vs. the CA (Pellow et al., 1985). Thus, our results provide further evidence that OA exposure represents a valuable challenge to study central mechanisms underlying behavioral reactivity to novelty including anxiety-related behaviors.
Effect of genotype on challenge-induced c-Fos responses
The genetic deletion of Y2 receptors reproducibly influenced neuronal activity in response to a novel environment in the cingulate cortex, barrel field of the somatosensory cortex, nucleus accumbens, dorsal lateral septum, medial amygdala, and lateral periaqueductal gray. Specifically, the c-Fos response in Y2−/− mice was attenuated in these aforementioned brain regions after OA exposure, while in the remaining areas similar activation was observed in Y2−/− and WT Mice.
All of the above brain areas are thought to mediate motivational behavior during novelty (Handa et al., 1993; Staiger et al., 2000; Stone et al., 2006; Uslaner et al., 2001, 2003; for review, see Sewards and Sewards, 2003), as well as stress-associated responses and to be part of proposed fear/anxiety circuitries (Charney et al., 1998). Accordingly, the administration of anxiogenic drugs has been shown to elevate c-Fos expression in the medial prefrontal cortex, amygdala, periaqueductal gray and the lateral septum (Singewald and Sharp, 2000; Singewald et al., 2003). Conversely, administration of anxiolytic drugs such as benzodiazepines or NK1 receptor antagonists reduces stressor-induced c-Fos expression in prelimbic cortex, cingulate cortex, medial amygdala, and nucleus accumbens as well as the dorsomedial hypothalamic nucleus, hippocampus, and locus coeruleus (Beck and Fibiger, 1995; Hahn and Bannon, 1999; McGregor et al., 2004). Hence, the effect of genotype and, in particular, the attenuation of the OA- and CA-induced c-Fos expression in these same regions observed in Y2−/− as compared to WT mice is suggested to contribute to the altered neurobehavioral reactivity to novelty of these animals, possibly resulting in their anxiolytic phenotype (Painsipp et al., 2008; Redrobe et al., 2003; Tschenett et al., 2003). However, it is unlikely that the differences in the activation patterns between Y2−/− and WT mice are due to altered explorative behavior, given that the two genotypes did not differ in locomotion in both the OA and the CA of the EPM.
Possible mechanism(s) underlying the altered, challenge-induced neuronal excitability of Y2−/− mice
In situ hybridization techniques (Naveilhan et al., 1998; Parker and Herzog, 1999), binding studies (Dumont et al., 1996, 1998; Gackenheimer et al., 2001), and immunohistochemical studies (Fetissov et al., 2004; Stanic et al., 2006) confirm the localization of Y2 receptors in each of the six identified areas showing altered challenge-induced neuronal activation in Y2−/− mice.
Y2 receptors are thought to be mainly presynaptic receptors mediating an inhibition of transmitter release by inhibiting Ca2+ influx through N-type channels as demonstrated in the hippocampus in vitro and in vivo (El Bahh et al., 2005). Depending on the nerve terminal on which they are located, they may reduce GABA, NPY, glutamate, or noradrenaline release (Chen and van den Pol, 1996; Greber et al., 1994; Klapstein and Colmers, 1992; Martire et al., 1993; Sun et al., 2001). The anxiolytic phenotype of Y2−/− mice, thus, could be mediated by augmented release of NPY and/or of GABA, for example, in the amygdala due to loss of presynaptic Y2-mediated inhibition.
During stressful conditions, the release of NPY and GABA is enhanced (Cook, 2004; Husum et al., 2002) presumably resulting in an attenuation of the stress response by stimulating postsynaptic, inhibitory GABAA, and Y1 receptors (Heilig et al., 1989; Wahlestedt et al., 1993), respectively. GABAA receptors mediate a hyperpolarization of postsynaptic neurons by opening their Cl−-channels and a Cl− influx. Stimulation of Y1 receptors by NPY also causes inhibition of postsynaptic cells by voltage-dependent inhibition of Ca2+ currents and/or activation of inwardly rectifying K+ currents (McQuiston et al., 1996; Sun and Miller, 1999). At the same time, presynaptically located Y2 receptors may physiologically dampen down the release of NPY and GABA enhanced by stress. Since this presynaptic regulatory mechanism is impaired in Y2−/− mice, a disinhibited release of NPY and GABA is suggested to result in a higher stimulation of postsynaptic inhibitory Y1 (Sun et al., 2001) and GABA receptors, respectively, both of which may then attenuate stress-induced c-Fos expression in the postsynaptic cells. Indeed, it has been shown that pharmacological blockade of Y2 receptors enhances stress-induced NPY release (King et al., 2000).
Interestingly, we did not observe any differences in c-Fos expression in the arcuate nucleus of the hypothalamus in Y2−/− mice compared to WT following OA and CA exposure. From the arcuate nucleus of the hypothalamus, neurons expressing NPY mRNA send projections to various regions such as the nucleus accumbens, lateral septum (dorsal and ventral), and amygdala (for review, see Chronwall, 1985; Heilig, 2004). In all these mentioned target areas, altered neuronal activity has been observed in Y2−/− mice indicating that Y2 autoreceptors on nerve terminals primarily determine the putatively enhanced NPY release in Y2−/− mice. In addition to a local effect of enhanced NPY on c-Fos expression, effects may also be mediated indirectly via the well-known interconnections within the fear-anxiety circuitry (for review, see Gray and McNaughton, 2000).
The hypothesis that enhanced NPY release in specific anxiety-related regions may be involved in the anxiolytic phenotype of Y2−/− mice is consistent with previous observations. For example, it has been shown that intracerebroventricular application of NPY elicits an anxiolytic effect (Heilig et al., 1989; Karlsson et al., 2005; Nakajima et al., 1998). Moreover, reduced anxiety-related behavior was found in the EPM in rats with upregulated NPY levels in the amygdala (by injecting viral vector encoding NPY), as compared to rats with downregulated NPY release (by injecting NPY antisense) (Primeaux et al., 2005). Hence, it is likely that attenuated neuronal activation in the amygdala observed in the present study is crucially implicated in the anxiolytic phenotype of Y2−/− mice. Indeed, it has been most recently demonstrated that NPY in the amygdala induces resilience to stress-induced reductions in social responses (Sajdyk et al., 2008). Whether local modulation of NPY transmission in (dorsal) lateral septum can also alter anxiety-related behavior remains to be shown.
In summary, exposure to either the OA or CA of an EPM was used as stimuli with putatively different anxiogenic potentials for activating neurons in regions. By evaluating subsequent c-Fos expression patterns, we found that Y2−/− mice show altered neuronal activity (hyperexcitability) in defined regions, namely the cingulate cortex, amygdala, nucleus accumbens, dorsal lateral septum, barrel field of the primary somatosensory cortex and lateral periaqueductal gray. These brain regions are well known to be associated with diverse behavioral reactivity to novelty including motivated and explorative as well as anxiety-related responses. Therefore, our results suggest that the altered neuronal activation patterns in anxiety-relevant substrates may mediate or contribute to the anxiolytic-like phenotype observed in Y2−/− mice.
Acknowledgments
Contract grant sponsors: Österreichische Nationalbank (ÖNB), FWF (Nationale Forschungsnetzwerke S102).
Footnotes
N.K.N. and S.B.S. contributed equally to this work.
REFERENCES
- Bacchi F, Mathe AA, Jimenez P, Stasi L, Arban R, Gerrard P, Caberlotto L. Anxiolytic-like effect of the selective neuropeptide Y Y2 receptor antagonist BIIE0246 in the elevated plus-maze. Peptides. 2006;27:3202–3207. doi: 10.1016/j.peptides.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: With and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Grillon C, Bremner DJ. The neurobiological basis of anxiety and fear: Circuits, mechanisms, and neurochemical interactions (part I) Neuroscientist. 1998;4:35–44. [Google Scholar]
- Chen G, van den Pol AN. Multiple NPY receptors coexist in pre- and postsynaptic sites: Inhibition of GABA release in isolated self-innervating SCN neurons. J Neurosci. 1996;16:7711–7724. doi: 10.1523/JNEUROSCI.16-23-07711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6(Suppl 2):1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Fournier A, St-Pierre S, Quirion R. Autoradiographic distribution of Leu31, Pro34] PYY and [125I] PYY3-36 binding sites in the rat brain evaluated with two newly developed Y1 and Y2 receptor radioligands. Synapse. 1996;22:139–158. doi: 10.1002/(SICI)1098-2396(199602)22:2<139::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- El Bahh B, Balosso S, Hamilton T, Herzog H, Beck-Sickinger AG, Sperk G, Gehlert DR, Vezzani A, Colmers WF. The anti-epileptic actions of neuropeptide Y in the hippocampus are mediated by Y and not Y receptors. Eur J Neurosci. 2005;22:1417–1430. doi: 10.1111/j.1460-9568.2005.04338.x. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Byrne LC, Hassani H, Ernfors P, Hokfelt T. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. J Comp Neurol. 2004;470:256–265. doi: 10.1002/cne.11047. [DOI] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Gackenheimer SL, Schober DA, Gehlert DR. Characterization of neuropeptide Y Y1-like and Y2-like receptor subtypes in the mouse brain. Peptides. 2001;22:335–341. doi: 10.1016/s0196-9781(01)00335-7. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. 2nd ed. Oxford University Press; Oxford: 2000. [Google Scholar]
- Greber S, Schwarzer C, Sperk G. Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2 receptors in rat hippocampal slices in vitro. Br J Pharmacol. 1994;113:737–740. doi: 10.1111/j.1476-5381.1994.tb17055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Carli M. Reduced attention and increased impulsivity in mice lacking NPY Y2 receptors: Relation to anxiolytic-like phenotype. Behav Brain Res. 2006;169:325–334. doi: 10.1016/j.bbr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Bannon MJ. Stress-induced C-fos expression in the rat locus coeruleus is dependent on neurokinin 1 receptor activation. Neuroscience. 1999;94:1183–1188. doi: 10.1016/s0306-4522(99)00319-x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Bollnow MR. Induction of c-fos mRNA in the brain and anterior pituitary gland by a novel environment. Neuroreport. 1993;4:1079–1082. [PubMed] [Google Scholar]
- Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and paradoxically increases feeding. Regul Pept. 1995;59:201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: Mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: Are we all ‘fos-ed out’? J Neuroendocrinol. 2002;14:259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ. Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci Biobehav Rev. 1999;23:971–980. doi: 10.1016/s0149-7634(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Husum H, Gruber SH, Bolwig TG, Mathe AA. Extracellular levels of NPY in the dorsal hippocampus of freely moving rats are markedly elevated following a single electroconvulsive stimulation, irrespective of anticonvulsive Y1 receptor blockade. Neuropeptides. 2002;36:363–369. doi: 10.1016/s0143-4179(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Karl T, Burne TH, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav Brain Res. 2006;167:87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Holmes A, Heilig M, Crawley JN. Anxiolyticlike actions of centrally-administered neuropeptide Y, but not galanin, in C57BL/6J mice. Pharmacol Biochem Behav. 2005;80:427–436. doi: 10.1016/j.pbb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M. The neuropeptide Y Y1 receptor sub-type is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology. 2008;195:547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Kask A, Rago L, Harro J. Anxiolytic-like effect of neuropeptide Y (NPY) and NPY13-36 microinjected into vicinity of locus coeruleus in rats. Brain Res. 1998;788:345–348. doi: 10.1016/s0006-8993(98)00076-6. [DOI] [PubMed] [Google Scholar]
- King PJ, Williams G, Doods H, Widdowson PS. Effect of a selective neuropeptide Y Y(2) receptor antagonist, BIIE0246 on neuropeptide Y release. Eur J Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Colmers WF. 4-Aminopyridine and low Ca2+ differentiate presynaptic inhibition mediated by neuropeptide Y, baclofen and 2-chloroadenosine in rat hippocampal CA1 in vitro. Br J Pharmacol. 1992;105:470–474. doi: 10.1111/j.1476-5381.1992.tb14277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hokfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Viltart O, Salome N, Giuliani A, Catalani A, Casolini P, Morley-Fletcher S, Nicoletti F, Maccari S. Prenatal stress alters the negative correlation between neuronal activation in limbic regions and behavioral responses in rats exposed to high and low anxiogenic environments. Psychoneuroendocrinology. 2007;32:765–776. doi: 10.1016/j.psyneuen.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Martire M, Pistritto G, Mores N, Agnati LF, Fuxe K. Region-specific inhibition of potassium-evoked [3H] noradrenaline release from rat brain synaptosomes by neuropeptide Y-(13-36). Involvement of NPY receptors of the Y2 type. Eur J Pharmacol. 1993;230:231–234. doi: 10.1016/0014-2999(93)90807-t. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: Region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Petrozzino JJ, Connor JA, Colmers WF. Neuropeptide Y1 receptors inhibit N-type calcium currents and reduce transient calcium increases in rat dentate granule cells. J Neurosci. 1996;16:1422–1429. doi: 10.1523/JNEUROSCI.16-04-01422.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalkiewicz M, Michalkiewicz T, Kreulen DL, McDougall SJ. Increased blood pressure responses in neuropeptide Y transgenic rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R417–R426. doi: 10.1152/ajpregu.2001.281.2.R417. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Nakajima M, Inui A, Asakawa A, Momose K, Ueno N, Teranishi A, Baba S, Kasuga M. Neuropeptide Y produces anxiety via Y2-type receptors. Peptides. 1998;19:359–363. doi: 10.1016/s0196-9781(97)00298-2. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Arenas E, Ernfors P. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87:289–302. doi: 10.1016/s0306-4522(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Nguyen NK, Keck ME, Hetzenauer A, Thoeringer CK, Wurst W, Deussing JM, Holsboer F, Muller MB, Singewald N. Conditional CRF receptor 1 knockout mice show altered neuronal activation pattern to mild anxiogenic challenge. Psychopharmacology. 2006;188:374–385. doi: 10.1007/s00213-006-0513-1. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Edelsbrunner ME, Tasan RO, Singewald N, Herzog H, Holzer P. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. 2008;7:532–542. doi: 10.1111/j.1601-183X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor sub-type mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Seydoux J, Kunstner P, Aubert JF, Grouzmann E, Beermann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: The universal soldier. Cell Mol Life Sci. 2003;60:350–377. doi: 10.1007/s000180300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pich EM, Agnati LF, Zini I, Marrama P, Carani C. Neuropeptide Y produces anxiolytic effects in spontaneously hypertensive rats. Peptides. 1993;14:909–912. doi: 10.1016/0196-9781(93)90065-o. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: Evidence from Y2 receptor knockout mice. Behav Brain Res. 2003;141:251–255. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, Cox HM, Sperk G, Hokfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA. 2002;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002;71:419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): Differential Fos expression in HAB and LAB rats. Biol Psychiatry. 2004;55:715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Salome N, Landgraf R, Viltart O. Confinement to the open arm of the elevated-plus maze as anxiety paradigm: Behavioral validation. Behav Neurosci. 2006;120:719–723. doi: 10.1037/0735-7044.120.3.719. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull. 2003;61:25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Singewald N. Altered brain activity processing in high-anxiety rodents revealed by challenge paradigms and functional mapping. Neurosci Biobehav Rev. 2007;31:18–40. doi: 10.1016/j.neubiorev.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K. Exploration of a novel environment leads to the expression of inducible transcription factors in barrel-related columns. Neuroscience. 2000;99:7–16. doi: 10.1016/s0306-4522(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Stanic D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hokfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol. 2006;499:357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Ahsan R, Quartermain D. Gross mapping of α1-adrenoceptors that regulate behavioral activation in the mouse brain. Behav Brain Res. 2004;152:167–175. doi: 10.1016/j.bbr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Stone EA, Yan L, Ahsan MR, Lehmann ML, Yeretsian J, Quartermain D. Role of CNS α1-adrenoceptor activity in central fos responses to novelty. Synapse. 2006;59:299–307. doi: 10.1002/syn.20243. [DOI] [PubMed] [Google Scholar]
- Sun L, Miller RJ. Multiple neuropeptide Y receptors regulate K+ and Ca2+ channels in acutely isolated neurons from the rat arcuate nucleus. J Neurophysiol. 1999;81:1391–1403. doi: 10.1152/jn.1999.81.3.1391. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Akk G, Huguenard JR, Prince DA. Differential regulation of GABA release and neuronal excitability mediated by neuropeptide Y1 and Y2 receptors in rat thalamic neurons. J Physiol. 2001;531:81–94. doi: 10.1111/j.1469-7793.2001.0081j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweerts BW, Jarrott B, Lawrence AJ. The effect of acute and chronic restraint on the central expression of prepro-neuropeptide Y mRNA in normotensive and hypertensive rats. J Neuroendocrinol. 2001;13:608–617. doi: 10.1046/j.1365-2826.2001.00674.x. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y—A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Norton CS, Watson SJ, Akil H, Robinson TE. Amphetamine-induced c-fos mRNA expression in the caudateputamen and subthalamic nucleus: Interactions between dose, environment, and neuronal phenotype. J Neurochem. 2003;85:105–114. doi: 10.1046/j.1471-4159.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- Viltart O, Mairesse J, Darnaudery M, Louvart H, Vanbesien-Mailliot C, Catalani A, Maccari S. Prenatal stress alters Fos protein expression in hippocampus and locus coeruleus stress-related brain structures. Psychoneuroendocrinology. 2006;31:769–780. doi: 10.1016/j.psyneuen.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Yanaihara N, Hakanson R. Evidence for different pre- and post-junctional receptors for neuropeptide Y and related peptides. Regul Pept. 1986;13:307–318. doi: 10.1016/0167-0115(86)90048-0. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Pich EM, Koob GF, Yee F, Heilig M. Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science. 1993;259:528–531. doi: 10.1126/science.8380941. [DOI] [PubMed] [Google Scholar]
- Zangenehpour S, Chaudhuri A. Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res. 2002;109:221–225. doi: 10.1016/s0169-328x(02)00556-9. [DOI] [PubMed] [Google Scholar]