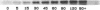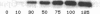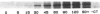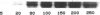Abstract
Human heart failure is associated with a diminished contractile response to beta-adrenergic agonists. We hypothesized that alterations in the activity of a guanine nucleotide-binding regulatory protein (G protein) might be partially responsible for this abnormality. We therefore measured the activity of G proteins in failing human myocardium utilizing bacterial toxin-catalyzed ADP ribosylation. The activity of a 40,000-mol wt pertussis toxin substrate (alpha G40) was increased by 36% in failing human hearts when compared with nonfailing controls. In contrast, there was no change in the level of the stimulatory regulatory subunit (Gs). The increased activity in alpha G40 was associated with a 30% decrease in basal as well as 5'-guanylyl imidodiphosphate-stimulated adenylate cyclase activity. These data suggest that increased alpha G40 activity is a new marker for failing myocardium and may account at least in part for the diminished responsiveness to beta 1-adrenergic agonists in the failing human heart.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science. 1975 Feb 28;187(4178):750–752. doi: 10.1126/science.163487. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Minobe W., Cubicciotti R. S., Sageman W. S., Lurie K., Billingham M. E., Harrison D. C., Stinson E. B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982 Jul 22;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Strosberg A., Montgomery W., Minobe W. Pharmacology and inotropic potential of forskolin in the human heart. J Clin Invest. 1984 Jul;74(1):212–223. doi: 10.1172/JCI111404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Umans V., Fowler M., Minobe W., Rasmussen R., Zera P., Menlove R., Shah P., Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986 Sep;59(3):297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. Peptide mapping in one dimension by limited proteolysis of sodium dodecyl sulfate-solubilized proteins. Methods Enzymol. 1983;96:222–229. doi: 10.1016/s0076-6879(83)96020-2. [DOI] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Birnbaumer L., Sekura R. D. Effects of guanine nucleotides and Mg on human erythrocyte Ni and Ns, the regulatory components of adenylyl cyclase. J Biol Chem. 1984 Sep 25;259(18):11408–11418. [PubMed] [Google Scholar]
- Codina J., Hildebrandt J., Iyengar R., Birnbaumer L., Sekura R. D., Manclark C. R. Pertussis toxin substrate, the putative Ni component of adenylyl cyclases, is an alpha beta heterodimer regulated by guanine nucleotide and magnesium. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4276–4280. doi: 10.1073/pnas.80.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- Downs R. W., Jr, Spiegel A. M., Singer M., Reen S., Aurbach G. D. Fluoride stimulation of adenylate cyclase is dependent on the guanine nucleotide regulatory protein. J Biol Chem. 1980 Feb 10;255(3):949–954. [PubMed] [Google Scholar]
- Downs R. W., Sekura R. D., Levine M. A., Spiegel A. M. The inhibitory adenylate cyclase coupling protein in pseudohypoparathyroidism. J Clin Endocrinol Metab. 1985 Aug;61(2):351–354. doi: 10.1210/jcem-61-2-351. [DOI] [PubMed] [Google Scholar]
- Enomoto K., Asakawa T. Inhibition of catalytic unit of adenylate cyclase and activation of GTPase of Ni protein by beta gamma-subunits of GTP-binding proteins. FEBS Lett. 1986 Jun 23;202(1):63–68. doi: 10.1016/0014-5793(86)80650-0. [DOI] [PubMed] [Google Scholar]
- Farfel Z., Brickman A. S., Kaslow H. R., Brothers V. M., Bourne H. R. Defect of receptor-cyclase coupling protein in psudohypoparathyroidism. N Engl J Med. 1980 Jul 31;303(5):237–242. doi: 10.1056/NEJM198007313030501. [DOI] [PubMed] [Google Scholar]
- Farfel Z., Brothers V. M., Brickman A. S., Conte F., Neer R., Bourne H. R. Pseudohypoparathyroidism: inheritance of deficient receptor-cyclase coupling activity. Proc Natl Acad Sci U S A. 1981 May;78(5):3098–3102. doi: 10.1073/pnas.78.5.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman A. M., Levine M. A., Baughman K. L., Van Dop C. NAD+-mediated stimulation of adenylate cyclase in cardiac membranes. Biochem Biophys Res Commun. 1987 Feb 13;142(3):631–637. doi: 10.1016/0006-291x(87)91461-6. [DOI] [PubMed] [Google Scholar]
- Feldman M. D., Copelas L., Gwathmey J. K., Phillips P., Warren S. E., Schoen F. J., Grossman W., Morgan J. P. Deficient production of cyclic AMP: pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation. 1987 Feb;75(2):331–339. doi: 10.1161/01.cir.75.2.331. [DOI] [PubMed] [Google Scholar]
- Fowler M. B., Laser J. A., Hopkins G. L., Minobe W., Bristow M. R. Assessment of the beta-adrenergic receptor pathway in the intact failing human heart: progressive receptor down-regulation and subsensitivity to agonist response. Circulation. 1986 Dec;74(6):1290–1302. doi: 10.1161/01.cir.74.6.1290. [DOI] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Hudson T. H., Johnson G. L. Peptide mapping of adenylate cyclase regulatory proteins that are cholera toxin substrates. J Biol Chem. 1980 Aug 10;255(15):7480–7486. [PubMed] [Google Scholar]
- Huff R. M., Neer E. J. Subunit interactions of native and ADP-ribosylated alpha 39 and alpha 41, two guanine nucleotide-binding proteins from bovine cerebral cortex. J Biol Chem. 1986 Jan 25;261(3):1105–1110. [PubMed] [Google Scholar]
- Johnson G. L., Bourne H. R. Influence of cholera toxin on the regulation of adenylate cyclase by GTP. Biochem Biophys Res Commun. 1977 Sep 23;78(2):792–798. doi: 10.1016/0006-291x(77)90249-2. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kantrowitz N. E., Bristow M. R., Minobe W. A., Billingham M. E., Harrison D. C. Histamine-mediated myocardial damage in rabbits. J Mol Cell Cardiol. 1982 Sep;14(9):551–555. doi: 10.1016/0022-2828(82)90216-4. [DOI] [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. A., Eil C., Downs R. W., Jr, Spiegel A. M. Deficient guanine nucleotide regulatory unit activity in cultured fibroblast membranes from patients with pseudohypoparathyroidism type I. a cause of impaired synthesis of 3',5'-cyclic AMP by intact and broken cells. J Clin Invest. 1983 Jul;72(1):316–324. doi: 10.1172/JCI110971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A. Regulation of adenylate cyclase by hormones and G-proteins. FEBS Lett. 1987 Jan 26;211(2):113–118. doi: 10.1016/0014-5793(87)81419-9. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Mangano T. J., Watkins D. C. Heart contains two substrates (Mr = 40,000 and 41,000) for pertussis toxin-catalyzed ADP-ribosylation that co-purify with Ns. Biochem Biophys Res Commun. 1985 Apr 30;128(2):809–815. doi: 10.1016/0006-291x(85)90119-6. [DOI] [PubMed] [Google Scholar]
- Moss J., Burns D. L., Hsia J. A., Hewlett E. L., Guerrant R. L., Vaughan M. NIH conference. Cyclic nucleotides: mediators of bacterial toxin action in disease. Ann Intern Med. 1984 Nov;101(5):653–666. doi: 10.7326/0003-4819-101-5-653. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Nielsen T. B., Lad P. M., Preston M. S., Rodbell M. Characteristics of the guanine nucleotide regulatory component of adenylate cyclase in human erythrocyte membranes. Biochim Biophys Acta. 1980 Apr 17;629(1):143–155. doi: 10.1016/0304-4165(80)90273-1. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution, activity, and properties of the 35,000-dalton (beta) subunit. J Biol Chem. 1983 Sep 25;258(18):11361–11368. [PubMed] [Google Scholar]
- Robishaw J. D., Smigel M. D., Gilman A. G. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem. 1986 Jul 25;261(21):9587–9590. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Skorecki K. L., Verkman A. S., Ausiello D. A. Cross talk between stimulatory and inhibitory guanosine 5'-triphosphate binding proteins: role in activation and desensitization of the adenylate cyclase response to vasopressin. Biochemistry. 1987 Jan 27;26(2):639–645. doi: 10.1021/bi00376a040. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Gierschik P., Levine M. A., Downs R. W., Jr Clinical implications of guanine nucleotide-binding proteins as receptor-effector couplers. N Engl J Med. 1985 Jan 3;312(1):26–33. doi: 10.1056/NEJM198501033120106. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Unnerstall J. R., Niehoff D. L., Kuhar M. J., Palacios J. M. Quantitative receptor autoradiography using [3H]ultrofilm: application to multiple benzodiazepine receptors. J Neurosci Methods. 1982 Jul;6(1-2):59–73. doi: 10.1016/0165-0270(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]