Abstract
A total of 200 milk samples from cattle (n = 86) and buffalo (n = 114) were evaluated using milk ring test (MRT) and indirect enzyme-linked immunosorbent assay (i-ELISA). The overall prevalence was found to be 3% and 8.5% in cattle and buffaloes using MRT and i-ELISA, respectively. The prevalence was 4.6% and 1.7% in cattle and buffalo using MRT, respectively, while i-ELISA exhibited 20% and 0% in cattle and buffalo, respectively. The prevalence was higher in government dairy farm, compared to privately owned dairy farm. This paper points out an alarming situation in the target area with respect to the public health significance.
1. Introduction
The livestock sector has emerged as a leading subsector of the agriculture over the years in Pakistan. Livestock production is one of the major activities, as about 30–35 million people of rural areas of Pakistan are engaged in raising livestock and deriving 30–40 percent of their income. It accounts for 53.2% of the agriculture value added and 11.4% of the national GDP [1].
Brucellosis is a highly contagious, zoonotic, and economically important bacterial disease of animals worldwide [2]. It causes significant economic losses including abortion, loss in milk production, low fertility rates, and cost of replacement of animals [3].
It is the second most important zoonotic disease in the world after rabies [4]. The importance of this highly contagious disease is due to its severe hazards to human health, through either direct contact with infected animals or through consumption of contaminated milk and dairy products, and its economic impact on the animal industry, causing an adverse effect on animal health.
It is a significant public health problem in an agricultural country like Pakistan, where the vast majority of the population is involved in land cultivation and livestock farming.
The incidence of brucellosis in Pakistan is increasing particularly in large dairy herds. Earlier studies indicated lower prevalence, that is, 0.33% to 0.65% [5]. Whereas much higher prevalence is reported in some recent studies, that is, 21% to 26% [6]. The incidence is higher in animals kept at organized farms rather than small holdings [7].
It is a global problem of wild and domestic animals, especially cattle, sheep, and goats causing a decrease in reproductive efficacy and an increase in abortion rate [8]. It has also been reported in most of the developing countries, like Nigeria, where it prevails up to 13.5% indicating higher threat to human and animal health [9].
This study was designed to assess the prevalence of this infectious zoonotic disease in the target area and to create awareness among the inhabitants of the area.
2. Materials and Methods
A total of 200 bovine milk samples were collected from government and privately owned organized dairy farms in Quetta City and subjected to milk ring test (MRT) and indirect ELISA for the detection of antibrucella antibodies.
2.1. Milk Ring Test (MRT)
A drop (30 μl) of stained brucella antigen was added to 01 ml of whole milk that has been kept at 4°C using overnight refrigeration. The test result was read after incubation for 1 hour at 37°C. A positive reaction was indicated by a stained cream layer over white column of milk [6].
2.2. Enzyme-Linked Immunosorbent Assay (i-ELISA)
The indirect ELISA (i-ELISA) kit for the detection of antibrucella antibodies was obtained from M/S Svanova, Sweden, and the procedure was followed as per manufacturer's procedure.
All the milk samples were centrifuged at 5000 rpm for 5 minutes to remove the cream layer before use. About 100 μl of sample dilution buffer was added to the control wells and 4 μl of positive control serum and 4 μl of negative control serum were added, respectively, to selected precoated antigen wells. Later, 100 μl of milk samples were added to selected wells. The plate was shaken thoroughly, sealed, and incubated at 37°C for one hour. The plate was rinsed 3 times with PBS-Tween buffer followed by addition of 100 μl of horse reddish peroxidase (HRP) conjugate (prepared in 11.5 ml PBS-Tween buffer diluted in distilled water) to each well and incubated at 37°C for one hour. The plates were again rinsed thrice, and 100 μl substrate solutions (tetramethylbenzidine) were added to all the wells. Microtitration plate was incubated for 10–15 minutes at room temperature. Finally, 50 μl of stopping solution was added to each well. The optical densities (OD) of the control and test-sample wells were adjusted at 405 nm wavelength using an ELISA reader (Thermo Electron, Finland).
The interpretation of results was performed accordingly.
| (1) |
For milk samples, a PP value equal or more than 10 was taken as positive.
3. Results and Discussion
The data collected revealed high prevalence (8.5%) of the disease in the area; this prevalence is higher than previously reported (3.97%) positive cases in cattle and buffalo using Rose Bengal plate test (RBPT) and serum agglutination test (SAT) by FAQIR [10] who also reported the presence of brucellosis in cattle and buffalo in the same area after screening 680 animals.
The prevalence was recorded much higher in cattle than buffaloes, as, out of 200 milk samples, 17 samples were found positive, all from cattle. These findings are in agreement with those of Abbas and Aldeewan [11] who also reported a higher prevalence of brucellosis in cattle (10.5%) than that in buffalo (1.9%). The higher prevalence in cattle may be attributed to the specie specificity or possibly the higher fat percentage in buffalo milk may be the obstacle in the detection of positive cases.
On farm comparison basis, the prevalence at governmentdairy farm (GDF) in Quetta was 14.8%, as, out of 74 samples, 11 were found positive while the private dairy Farm (PDF) exhibited 4.76% prevalence, as, out of 126 milk samples, only 6 samples were positive (Table 2). This result is in agreement with the observations of Pederson [12] who reported higher prevalence among official herds than that of privately owned dairy farms. However, this result contradicts that of Naeem et al. [13] who reported higher prevalence of bovine brucellosis among privately owned animals.
Table 2.
Comparison of prevalence of brucellosis in government and privately owned dairy farms in Quetta city, Pakistan.
| Animal species | GDF n = 74 | PDF n = 126 | ||
|---|---|---|---|---|
| Positive | Positive | |||
| MRT | i-ELISA | MRT | i-ELISA | |
| Cattle n = 114 | 0/74 | 11 | 4/12 | 6 |
| Buffalo n = 86 | — | — | 2/114 | 0 |
| Total = 200 | 0% | 14.86% | 4.76% | 4.76% |
PDF: private dairy farm.
GDF: government dairy farm.
MRT: milk ring test.
i-ELISA: indirect enzyme linked immunosorbent assay.
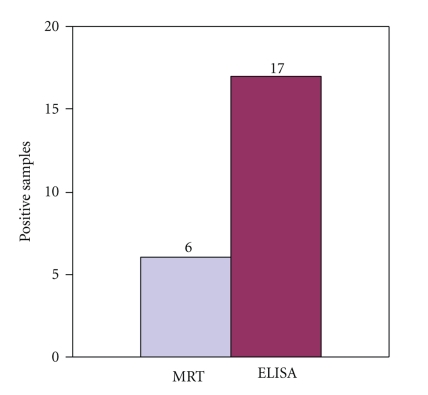
The overall prevalence in organized dairy farms was 3% by MRT, as 6 out of 200 samples were found positive while i-ELISA showed 8.5% prevalence, as 17 out of 200 milk samples were found positive (Table 1).
Table 1.
Prevalence of bovine brucellosis in milk samples using MRT and i-ELISA in District Quetta, Balochistan, Pakistan.
| Animal | Positive samples | |
|---|---|---|
| MRT | i-ELISA | |
| Cattle n = 86 | 4 (4.6%) | 17 (20%) |
| Buffalo n = 114 | 2 (1.7%) | — |
| Percentage n = 200 | 6/200 (3%) | 17/200 (8.5%) |
MRT: milk ring test.
i-ELISA: indirect enzyme-linked immunosorbent assay.
The indirect enzyme linked immunosorbent assay (i-ELISA) detected seventeen (17) milk samples whereas only 6 milk samples were detected positive by MRT indicating high sensitivity and specificity of i-ELISA than those of milk ring test. In the present study, the milk ELISA had detected more positive cases than MRT (Figure 1). These findings corroborate with Kerkhofs et al. [14], Vanzini et al. [15], and Kang' ethe et al. [16], who also reported milk ELISA as more sensitive than MRT.
Figure 1.
Graphical comparison of MRT with i-ELISA for detection of antibrucella antibodies in Milk Samples.
4. Conclusion
The present study reflects much higher prevalence (8.5%) of the disease in the target area. This is a serious threat to the public health and also to animal life. Most of the field workers, veterinarians, butchers, and milkmen are more prone to this disease. So, care should be taken while handling the suspected animal cases. Similarly, a large-scale survey should be done in the area to detect the carrier animals and should be treated according to the international rules. A large scale vaccination may also help to lower the prevalence of this disease; therefore, in larger dairy herds, vaccination may be helpful to overcome this problem. A large scale campaign through media may also be helpful to create awareness among people to use boiled or pasteurized milk to lower the risk of this human health problem.
References
- 1.GOP (Government of Pakistan) Economic Survey 2009-2010.
- 2.OIE. Manual of Standards for Diagnostic Tests and Vaccines. 4th edition. Paris, France: OIE; 2000. Bovine brucellosis; pp. 328–345. [Google Scholar]
- 3.McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Veterinary Microbiology. 2002;90(1–4):111–134. doi: 10.1016/s0378-1135(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 4.FAO. Progress in understanding brucellosis. Vet Record. 641, 2003. [PubMed]
- 5.Sheikh SA, Shah MA, Khan SA. Some observations on the incidence of brucellosis in West Pakistan. Pakistan Journal of Science. 1967;19:189–192. [Google Scholar]
- 6.Sharma SN, Adlakha SC. Textbook of Veterinary Microbiology. Vol. 192. New Delhi, India: Vikas Publishing House; 1997. [Google Scholar]
- 7.Ahmad R, Munir MA. Epidemiological investigation of brucellosis in Pakistan. The Pakistan Veterinary Journal. 1995;15:169–172. [Google Scholar]
- 8.Rijpens NP, Jannes G, van Asbroeck M, Rossau R, Herman LMF. Direct detection of Brucella spp. in raw milk by PCR and reverse hybridization with 16S-23S rRNA spacer probes. Applied and Environmental Microbiology. 1996;62(5):1683–1688. doi: 10.1128/aem.62.5.1683-1688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertu WJ, Dapar M, Gusi AM, Ngulukun SS, Leo S, Jwander LD. Prevalence of Brucella antibodies in marketed milk in Jos and environs. African Journal of Food Science. 2010;4(2):062–064. [Google Scholar]
- 10.FAQIR. Seroepidemiological survey of bovine brucellosis associated with reproductive disorders in Quetta district, Balochistan. Lahore, Pakistan: Animal Reproduction, CVS; 1991. M.S. thesis. [Google Scholar]
- 11.Abbas BA, Aldeewan AB. Occurrence and epidemiology of Brucella spp. in raw milk samples at Basrah province, Iraq. Bulgarian Journal of Veterinary Medicine. 2009;12(2):136–142. [Google Scholar]
- 12.Pederson CH. A preliminary report on infertility among cattle and buffaloes in Punjab. Working paper. 1980;(3) Pak.Vet. J.10 (4): 552.
- 13.Naeem K, Akhtar S, Ullah N. The serological survey of bovine brucellosis in Rawalpindi and Islamabad. The Pakistan Veterinary Journal. 1990;10(4):154–156. [Google Scholar]
- 14.Kerkhofs P, Botton Y, Thiange P, Dekeyser P, Limet JN. Diagnosis of bovine brucellosis by enzyme immunoassay of milk. Veterinary Microbiology. 1990;24(1):73–80. doi: 10.1016/0378-1135(90)90052-w. [DOI] [PubMed] [Google Scholar]
- 15.Vanzini VR, Aguirre NP, Valentini BS, et al. Comparison of an indirect ELISA with the Brucella milk ring test for detection of antibodies to Brucella abortus in bulk milk samples. Veterinary Microbiology. 2001;82(1):55–60. doi: 10.1016/s0378-1135(01)00370-4. [DOI] [PubMed] [Google Scholar]
- 16.Kang’ethe EK, Arimi SM, Omore AO, et al. The prevalence of antibodies to Brucella abortus in marketed milk in Kenya and its public health implications. In: Proceedings of the the 3rd All Africa Conference on Animal Agriculture; November 2000. [Google Scholar]