Abstract
Diabetes ultimately results from an inadequate number of functional beta cells in the islets of Langerhans. Enhancing proliferation of functional endogenous beta cells to treat diabetes remains underexplored. Here, we report that excision of the Men1 gene, whose loss-of-function mutation leads to inherited multiple endocrine neoplasia type 1 (MEN1), rendered resistant to streptozotocin-induced hyperglycemia in a tamoxifen-inducible and temporally controlled Men1 excision mouse model as well as in a tissue-specific Men1 excision mouse model. Men1 excision prevented mice from streptozotocin-induced hyperglycemia mainly through increasing the number of functional beta cells. BrdU incorporation by beta cells, islet size, and circulating insulin levels were significantly increased in Men1-excised mice. Membrane localization of glucose transporter 2 was largely preserved in Men1-excised beta cells, but not in Men1-expressing beta cells. Our findings suggest that repression of menin, a protein encoded by the Men1 gene, might be a valuable means to maintain or increase the number of functional endogenous beta cells to prevent or ameliorate diabetes.
1. Introduction
Pancreatic beta cells are important for glucose sensing, insulin production and secretion, and therefore, are crucial for maintenance of blood glucose levels within the normal range [1, 2]. Both type 1 diabetes (T1D) and type 2 diabetes (T2D) ultimately result from an inadequate number of functional insulin-producing beta cells in the islets of Langerhans. T1D arises from dramatic autoimmune-induced beta-cell damage [1], while T2D develops due to insulin resistance as well as a relatively insufficient number of beta cells [3]. Insulin production is eventually insufficient for maintaining normal blood glucose levels in T1D or T2D, leading to hyperglycemia and secondary complications, including cardiovascular disease, kidney dysfunction, and blindness [1, 3, 4]. While therapies through immunomodulation or improving insulin sensitivity have shown promising effects on preventing or reversing hyperglycemia [5–8], how to promote proliferation or regeneration of functional endogenous beta cells to boost the number of functional beta cells needs to be further investigated. Only a handful of genes have been shown to affect development of hyperglycemia in diabetes mouse models. For example, overexpression of Igf-1 in pancreatic beta cells prevents streptozotocin- (STZ-) induced diabetes [9]. Ablation of p27cip1/kip1, a cyclin-dependent kinase inhibitor, increases the number of beta cells and prevents development of hyperglycemia in db/db mice [10]. Deletion of Lkb1, a tumor suppressor activating AMP-activated protein kinase, promotes beta-cell proliferation and prevents glucose intolerance in high-fat diet-fed mice [11]. However, these genes have been shown to regulate proliferation of multiple types of cells [12, 13]. It is desirable to assess genes that preferentially affect beta-cell proliferation/regeneration in order to prevent or treat diabetes.
Menin is a nuclear protein encoded by the Men1 gene, which is mutated in familial multiple endocrine neoplasia type 1 (MEN1) patients [14]. Menin has been shown to preferentially repress cell proliferation in endocrine tissues including pancreatic beta cells [15, 16]. Although beta-cell proliferation and blood insulin levels are increased a long time after Men1 is excised in beta cells [15, 17, 18], little is known as to whether Men1 excision within a short time can prevent development of hyperglycemia in diabetes mouse models and its underlying mechanisms.
STZ is a glucose analog, and it selectively binds to glucose transporter 2 (GLUT2), the protein localized in the beta cell membrane, and is transported into beta cells [19, 20]. STZ induces beta cell damage, which mimics the loss of functional beta cells observed in T1D and T2D [20–23]. In the present study, we used a multiple low-dose streptozotocin- (MLD-STZ-) induced diabetes mouse model and determined the impact of Men1 excision on STZ-induced hyperglycemia [19, 22]. We found that Men1 excision prevented STZ-induced hyperglycemia, at least partly through promoting beta-cell proliferation, and preserving the number of functional beta cells and circulating insulin levels. These findings suggest that menin is crucial for regulating beta-cell proliferation in vivo and may perhaps be targeted for the prevention or treatment of diabetes.
2. Materials and Methods
2.1. Mice
Me n1l/l ; Cre-ER mice were generated by crossing mice with the floxed Men1 (Men1l/l, FVB/129Sv) to mice harboring the Ubc9 promoter-driven Cre-ERT2 (129Sv/C57BL6J, a gift from Dr. Eric Brown) [17, 24]. Men1l/l mice, with exons 3 to 8 of Men1 flanked by two loxP sites, were kindly provided by Dr. Francis Collins. Men1l/l ; RIP-Cre mice were generated by crossing floxed Men1 mice with mice expressing Cre driven by the rat insulin-2 promoter (RIP-Cre, TgN(ins2-cre)25Mgn, Jackson Laboratory) [15]. Only male mice were used for the following experiments. The genotyping of mice was performed by PCR using mouse tail DNA as templates. For Men1: P1, 5′-CCCACATCCAGTCCCTCTTCAGCT-3′; P2, 5′-AAGGTACAGCAGAGGTCACAGAG-3′; P3, 5′-GACAGGATTGGGAATTCTCTTTT-3′. For Cre-ERT2: forward (F), 5′-ACACCAAAATTTGCCTGCATTACCGG-3′; reverse (R), 5′-TTTCCATGAGTGAACGAACCTGGT-3′. For Rip-Cre: F, 5′-GCGGTCTGGCAGTAA AAACTATC-3′; R, 5′-GTGAAACAGCATTGCTGTCACTT-3′. All mouse experiments were approved by the University Laboratory Animal Resource and the University of Pennsylvania Committee on Animal Care. The animal care was performed in accordance with institutional guidelines.
2.2. Excision of the Floxed Men1 Locus Using Tamoxifen (TAM)
Me n1l/l; Cre-ER and their littermate controls were fed TAM (MP Biomedicals) at 200 mg/kg of body weight per day for two consecutive days, followed by one day off and then for another two consecutive days [18].
2.3. STZ-Induced Hyperglycemia
Hyperglycemia was induced by daily intraperitoneal (i.p.) injection of streptozotocin (Sigma) at 40 mg/kg of body weight per day for 5 consecutive days [22]. STZ working solution was freshly prepared by dissolving STZ in 25 mM sodium citrate solution (pH 4.0) and immediately injected after each preparation. Blood glucose levels were monitored until 4 weeks after the last dose of STZ injections.
2.4. Physiological Measurements
Blood glucose levels were assayed from tail vein blood by a glucose meter (OneTouch, Lifescan). Blood serum insulin levels were measured by ELISA using a mouse insulin kit (Crystal Chem). Serum glucagon levels were determined by a RIA Kit (Linco).
2.5. Immunostaining
BrdU at 100 mg/kg of body weight was i.p. injected into mice 16 to 24 hours before they were sacrificed. Two to three paraffin-embedded pancreas sections (5 μm, at least 100 μm apart) of each mouse and genotype were stained with affinity-purified antibodies against menin (1 : 500) [25], insulin (Abcam, 1 : 100), glucagon (Abcam, 1 : 100), BrdU (Accurate Chemical & Scientific, 1 : 150), Pdx1 (Millipore, 1 : 500), and GLUT2 (Millipore, 1 : 200). Secondary antibodies used were FITC (Abcam, 1 : 200) and Alexa Fluor 546 (Invitrogen, 1 : 200). Images were captured by a Nikon Eclipse E800 fluorescence microscope with a CCD digital camera. Total insulin staining area was quantified by MetaMorph software (Molecular Devices).
2.6. Statistical Analysis
Results are expressed as mean ± SEM. For a two-group comparison, unpaired Student's t-test or rank sum test was used. P values less than .05 were considered statistically significant.
3. Results
3.1. Men1 Ablation Renders Mice Resistant to STZ-Induced Hyperglycemia
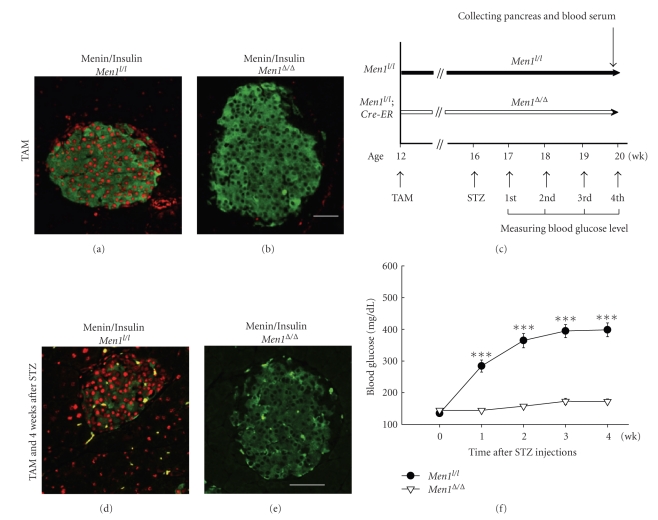
To determine the impact of Men1 excision on the development of diabetes, we evaluated whether Men1 excision can prevent streptozotocin- (STZ-) induced hyperglycemia using a conditional Men1 knockout mouse model. To generate Men1l/l; Cre-ER mice, mice harboring floxed Men1 were crossed with mice harboring an Ubc9 promoter-driven Cre-ERT2 transgene (Cre-ER) [17, 24]. The resulting Men1l/+; Cre-ER mice and Men1l/+ mice were bred, and Men1l/l; Cre-ER mice and their littermates (Men1l/l) were used for the experiments. To check the efficiency of Men1 excision in Men1l/l; Cre-ER mice, Men1l/l and Men1l/l; Cre-ER mice were fed TAM, and menin levels in pancreatic islets were determined by immunofluorescence staining 4 weeks after TAM feeding. Immunostaining showed that in the control Men1l/l mice, menin was preferentially expressed in the nucleus of islet cells as compared with acinar cells (Figure 1(a)). Menin was barely detectable in both islets and acinar cells of the Men1l/l; Cre-ER mice, indicating that the Men1 gene was effectively excised in Men1l/l; Cre-ER mice (Figure 1(b)).
Figure 1.
Ablation of floxed Men1 prevents development of streptozotocin- (STZ-) induced hyperglycemia. (a) and (b) Immunostaining for menin (red) and insulin (green), respectively, in pancreatic islets in mice fed TAM and without STZ treatment. Control Men1l/l and Men1l/l; Cre-ER mice (n = 8 mice) were fed tamoxifen (TAM) at the age of 12 weeks at 200 mg/kg of body weight per day for two consecutive days, followed by one day off and then for another two consecutive days, as described in Section 2. Four weeks after the last dose of TAM feeding, mice were sacrificed and pancreas sections of Men1l/l (a) or Men1l/l; Cre-ER mice (b) were immunostaed for menin and insulin. (c) A schematic of experimental design. Control Men1l/l (n = 18 mice) and Men1l/l; Cre-ER mice (n = 21 mice) were fed TAM at the age of 12 weeks. Four weeks after the last dose of TAM feeding, STZ was i.p. injected at 40 mg/kg of body weight per day for 5 consecutive days. Blood glucose levels were monitored till 4 weeks after STZ injections. (d) and (e) Immunostaining for menin (red) and insulin (green) in islets in control Men1l/l (d) and Men1l/l; Cre-ER mice (e) that were treated with TAM and followed by STZ injections, as described in Figure 1(c). The pancreata were collected 4 weeks after STZ treatment. (f) Non-fasting blood glucose levels before and until 4 weeks after STZ injections (n = 18 to 21 mice). Scale bar, 25 μm. ***, P < .001.
We then administered multiple low-dose STZ (MLD-STZ) to the control and Men1-excised mice to induce beta cell damage and diabetes four weeks after Men1 excision (Figure 1(c)) [19, 22]. Immunofluorescence staining showed that Men1 was effectively excised in the islets of Men1l/l; Cre-ER mice as compared with control mice 4 weeks after STZ treatment (Figures 1(d) and 1(e)). In the control mice, nonfasting blood glucose levels started to increase as early as one week after STZ injections and ultimately reached 400 mg/dL 4 weeks after STZ injections (Figure 1(f)). Men1-excised mice, on the other hand, did not develop hyperglycemia during the whole period of study (Figure 1(f)), demonstrating that Men1 ablation prevents STZ-induced hyperglycemia.
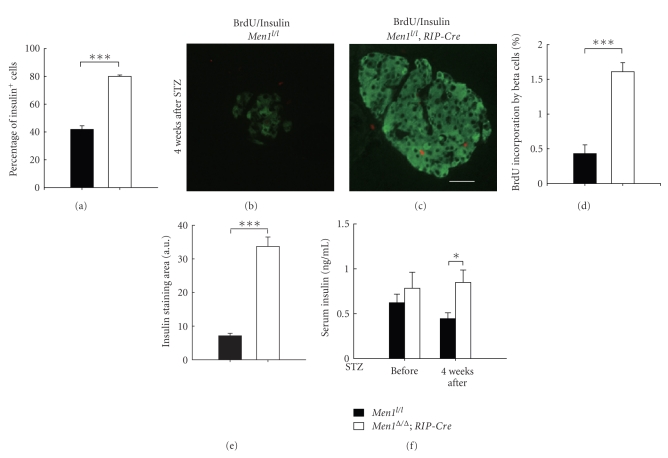
3.2. Men1 Ablation Promotes Beta-Cell Proliferation and Increases the Number of Functional Beta Cells
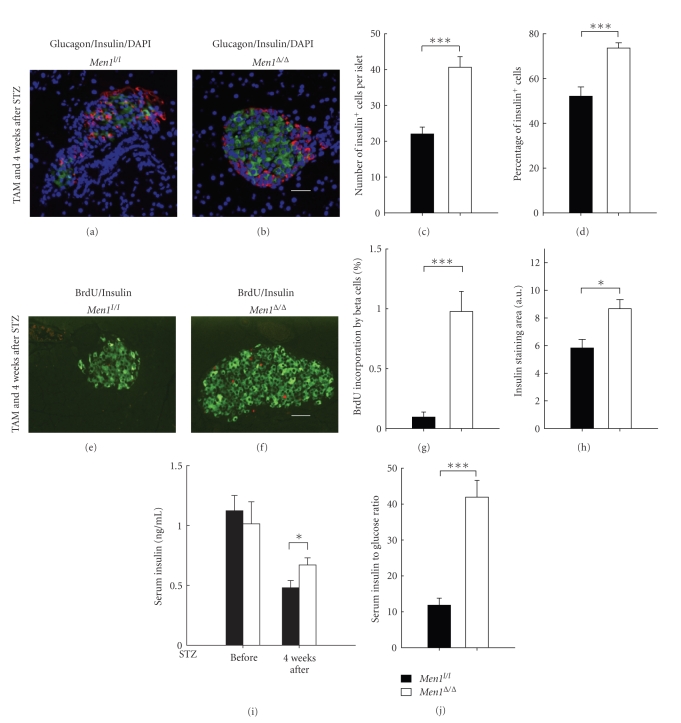
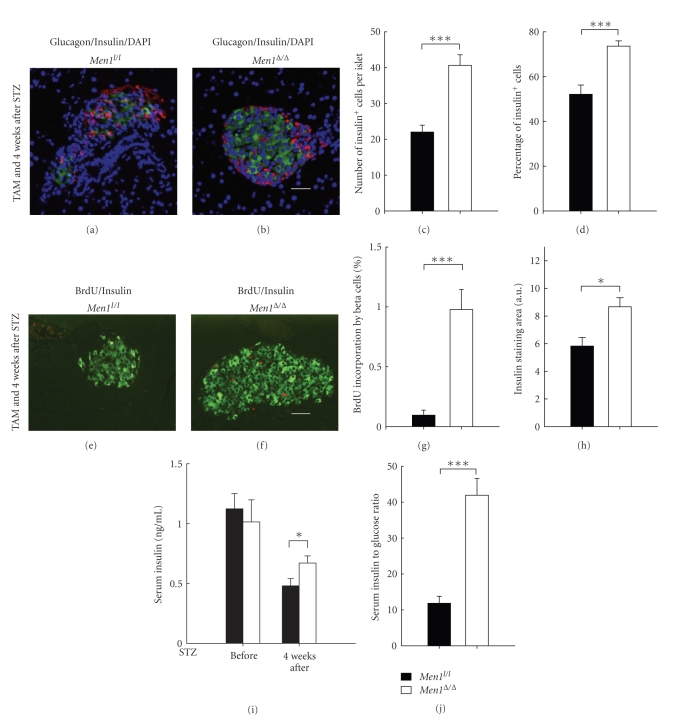
To determine the underlying mechanisms whereby Men1 ablation prevents STZ-induced hyperglycemia, we determined cell composition in islets, BrdU incorporation by beta cells, islet size, and circulating insulin levels in control and Men1-excised mice 4 weeks after STZ injections.
Immunofluorescence staining showed that both the number and the percentage of insulin-producing beta cells were significantly reduced in islets of the control mice (Figures 2(a), 2(c)-2(d)), as compared with the Men1-excised mice (Figures 2(b)–2(d)). These findings suggest that Men1 excision either protected beta cells from STZ-induced beta cell damage or increased the number of functional beta cells. BrdU incorporation by beta cells, a marker for cell proliferation, was significantly higher in the Men1-excised mice than the control mice (Figures 2(e)-2(g)), indicating that Men1 excision increased the number of beta cells at least partly through promoting beta-cell proliferation. Consistent with an increase in the number of proliferating beta cells after Men1 excision, insulin staining area was significantly larger in the Men1-excised mice (Figure 2(h)). Random blood insulin levels, which were similar between the control and Men1-excised mice before STZ treatment, dropped in both groups after STZ injections (Figure 2(i)). However, it is noteworthy that blood insulin level was reduced by ∼57% in the control Men1-expressing mice (before, 1.12 ng/mL versus 4 wk after, 0.48 ng/mL, Figure 2(i)), while it was only reduced by ∼34% in the Men1-excised mice (before, 1.01 ng/mL versus 4 wk after, 0.67 ng/mL, Figure 2(i)), suggesting that there was less STZ-induced beta cell damage and deficit in insulin production and/or secretion in the Men1-exicsed mice. Moreover, the blood insulin level was significantly higher in the Men1-excised mice than the control mice after STZ injections (P < .05, Figure 2(i)).
Figure 2.
Ablation of floxed Men1 promotes beta-cell proliferation and increases beta cell number in STZ-treated mice. Study design was described in Figure 1(c). Control Men1l/l (n = 18 mice) and Men1l/l; Cre-ER mice (n = 21 mice) were fed TAM at age of 12 weeks. Four weeks after the last dose of TAM feeding, STZ was i.p. injected at 40 mg/kg of body weight per day for 5 consecutive days. Pancreata and blood serum were collected from mice for further analysis, 4 weeks after STZ injections. (a-b) Immunostaining for insulin (green) and glucagon (red) in islets in control Men1l/l (a) and Men1l/l; Cre-ER mice (b). Nuclei were counterstained using DABI (blue). (c) Quantitation of number of insulin-secreting beta cells in islets (n = 8 mice). (d) Quantitation of percentage of beta cells in islets (n = 8 mice). (e) and (f) Immunostaining for BrdU (red) and insulin (green) in islets in control Men1l/l (e) and Men1l/l; Cre-ER mice (f). (g) Quantitation of BrdU incorporation by beta cells (n = 8 mice). (h) Quantitation of insulin staining area (n = 8 mice). (i) Non-fasting serum insulin levels before and 4 weeks after STZ injections (n = 18 to 21 mice). (j) Ratio of serum insulin (ng/mL) to blood glucose levels (mg/dL), multiplied by 10000. Scale bar, 25 μm. *, P < .05; ***, P < .001.
It is also noteworthy that the control mice, even with severe hyperglycemia (∼400 mg/dL, Figure 1(f)), did not secrete more insulin to reduce high blood glucose levels (4 wk after STZ, 0.48 ng/mL, Figure 2(i)), indicating a very obvious deficit in insulin production and/or secretion in the control mice after STZ treatment. On the contrary, even though the blood insulin level in the Men1-excised mice was lower four weeks after than before STZ injections (before STZ, 1.01 ng/mL; 4 wk after STZ, 0.67 ng/mL, Figure 2(i)), the Men1-excised mice were normoglycemic (∼150 mg/dL, Figure 1(f)). These results indicate that insulin produced/secreted by the Men1-excised mice was adequate to control their blood glucose levels within a normal range and the nearly normal blood glucose levels did not trigger further insulin secretion/production. Notably, we also found that the ratio of blood insulin to glucose levels was significantly higher in the Men1-excised mice than the control Men1-expressing mice (Figure 2(j)), suggesting that, after STZ treatment, islet function and/or total insulin secretion by islets in response to blood glucose stimulation was better preserved in the Men1-excised mice, and that beta cells in the Men1-excised mice were able to secrete more insulin into blood circulation and keep blood glucose levels within a relatively normal range. Taken together, these findings strongly suggest that Men1 ablation prevents STZ-induced hyperglycemia at least in part through promoting beta-cell proliferation, resulting in the maintenance of a larger number of functional beta cells and higher circulating insulin concentrations.
It has been reported that the number of glucagon-producing alpha cells is increased in diabetic state, and knockdown of glucagon-receptor normalizes high glucose levels in diabetic mice [26, 27]. Recent studies have also shown that Men1-excised glucagon-producing alpha cells can transdifferentiate to insulin-producing beta cells in normal mice, and alpha cells transdifferentiate into beta cells more rapidly when beta cells are under severe stress [28, 29]. Therefore, to determine whether Men1 ablation prevents STZ-induced hyperglycemia through reducing the number of alpha cells, the average number and percentage of alpha cells in islets and circulating glucagon levels were determined. We found that the number of glucagon-secreting alpha cells per islet was similar in the control and Men1-excised mice (Figure 3(a)). Although the percentage of alpha cells was higher in control islets than Men1-excised islets after STZ treatment (Figure 3(b)), which was likely due to a low number of beta cells and consequently a lower total number of cells in islets in the control mice, the circulating glucagon levels were similar between the control and Men1-excised mice (Figure 3(c)). This result suggests that transdifferentiation of alpha cells into beta cells was not substantially affected by Men1 excision 4 weeks after STZ injections. Therefore, Men1 ablation likely prevents STZ-induced hyperglycemia mainly through its impact on beta-cell proliferation and increasing the number of functional beta cells.
Figure 3.
Ablation of floxed Men1 does not affect the number of glucagon-secreting alpha cells and serum glucagon levels in STZ-treated mice. Study design was described in Figure 1(c). Control Men1l/l (n = 18 mice) and Men1l/l; Cre-ER mice (n = 21 mice) were fed TAM at age of 12 weeks. Four weeks after the last dose of TAM feeding, STZ was i.p. injected at 40 mg/kg of body weight per day for 5 consecutive days. Pancreata and blood serum were collected from mice for further analysis, 4 weeks after STZ injections. (a) Quantitation of number of alpha cells in islets (n = 8 mice). (b) Quantitation of the percentage of alpha cells in islets (n = 8 mice). (c) Non-fasting serum glucagon levels before and 4 weeks after STZ injections (n = 18 to 21 mice). *, P < .05.
3.3. Men1 Ablation Specifically in Pancreatic Beta Cells Prevents Development of STZ-Induced Hyperglycemia
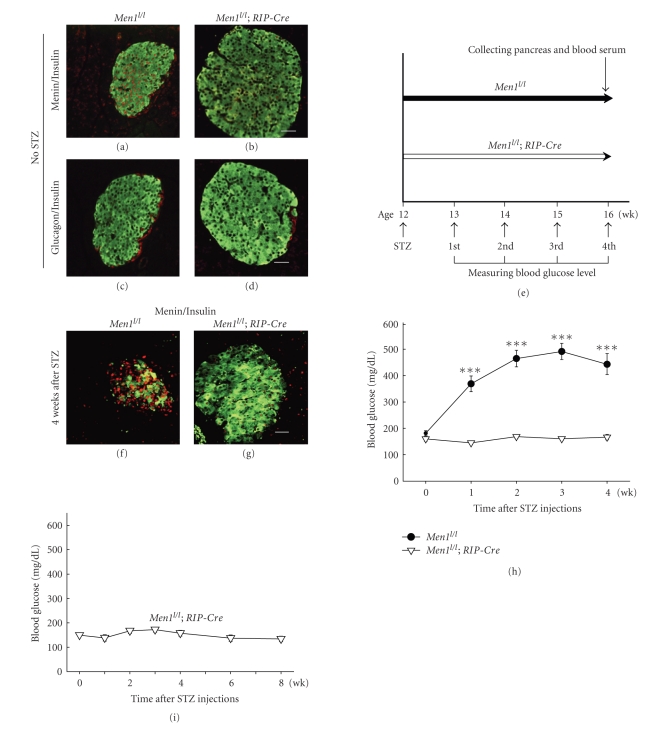
To determine whether Men1 excision, specifically in beta cells, contributes to prevention of STZ-induced hyperglycemia, Men1l/l; RIP-Cre mice were generated where Men1 was preferentially excised in beta cells by the rat insulin promoter-driven Cre recombinase [15]. Immunostaining showed that menin protein level was significantly reduced exclusively in beta cells but not in adjacent acinar cells in the Men1l/l; RIP-Cre mice at 12 weeks of age (Figure 4(b)), as compared with the control Men1l/l mice (Figure 4(a)). These findings suggest that Men1 was excised specifically in beta cells in the Men1l/l; RIP-Cre mice. Islet size was increased in the Men1l/l; RIP-Cre mice as compared with the control Men1l/l mice (Figures 4(a) and 4(b)), which was consistent with the observations in the previous study [15]. On the other hand, composition of beta cells and alpha cells in islets was similar between the control and Men1l/l; RIP-Cre mice (Figures 4(c) and 4(d)).
Figure 4.
Tissue specific ablation of floxed Men1 in beta cells increases resistance to STZ-induced hyperglycemia. (a–d) Control Men1l/l and Men1l/l; RIP-Cre mice at the age of 12 weeks (n = 2 to 3 mice) without STZ treatment were sacrificed, and pancreata sections were immunostainined for menin, insulin, or glucagon. (a-b) Immunostaining for menin (red) and insulin (green) in islets in control Men1l/l (a) and Men1l/l; RIP-Cre mice (b). (c-d) Immunostaining for insulin (green) and glucagon (red) in islets in Men1l/l (c) and Men1l/l; RIP-Cre mice (d). (e) A schematic of experimental design. Control Men1l/l and Men1l/l; RIP-Cre mice (n = 15 mice) at the age of 12 weeks were injected with STZ at 40 mg/kg of body weight per day for 5 consecutive days. Blood glucose levels were monitored, and mice were sacrificed 4 weeks after STZ injections. (f-g) Immunostaining for menin (red) and insulin (green) in islets in Men1l/l (f) and Men1l/l; RIP-Cre mice (g) after STZ injections, as described in Figure 4(e). The pancreata were collected 4 weeks after STZ treatment. (h) Non-fasting blood glucose levels before and 4 weeks after STZ injections (n = 12 to 15 mice). (i) Men1l/l; RIP-Cre mice at the age of 12 weeks were injected with STZ at 40 mg/kg of body weight per day for 5 consecutive days. Blood glucose levels in Men1l/l; RIP-Cre mice before and until 8 weeks after STZ injections (n = 6 mice).
Me n1l/l and Men1l/l; RIP-Cre mice at 12 weeks of age were then injected with MLD-STZ, and blood glucose levels were monitored up to 4 weeks after STZ injections (Figure 4(e)). Men1 was effectively excised in islets of the Men1l/l; RIP-Cre mice as compared with the control mice (Figures 4(f) and 4(g)). Similar to what was observed in the TAM-treated Men1l/l; Cre-ER mice, specific Men1 excision in beta cells in the Men1l/l; RIP-Cre mice also rendered mice resistant to STZ-induced beta cell damage and hyperglycemia. Non-fasting blood glucose levels in the control mice started to increase as early as one week after STZ injections and ultimately reached 450 mg/dL 4 weeks after STZ injections (Figure 4(h)). In contrast, Men1l/l; RIP-Cre mice did not develop hyperglycemia during the whole study (Figure 4(h)). Moreover, blood glucose levels in the Men1l/l; RIP-Cre mice remained normal up to 8 weeks after STZ injections (Figure 4(i)). Consistent with their normal blood glucose levels 4 weeks after STZ injections, the percentage of insulin-secreting beta cells in islets was significantly higher in the Men1l/l; RIP-Cre mice than the control mice (Figure 5(a)), suggesting that there was less beta cell damage and/or more functional beta cells preserved in the Men1l/l; RIP-Cre mice. BrdU incorporation by beta cells was also significantly higher in the Men1; RIP-Cre mice than the control mice (Figures 5(b)–5(d)), further confirming that Men1 excision increased the number of beta cells at least partly through promoting beta-cell proliferation. Consistent with the observation that beta-cell proliferation was increased after Men1 excision (Figures 5(b)–5(d)), the insulin staining area was significantly larger in the Men1l/l; RIP-Cre mice than the control mice (Figure 5(e)). Furthermore, random serum insulin levels in the Men1l/l; RIP-Cre mice was significantly higher than the control mice after STZ injections (Figure 5(f)). Moreover, there was no much difference in blood insulin levels in the Men1l/l; RIP-Cre mice before and 4 weeks after STZ injections (Figure 5(f)), strongly suggesting that there was less beta cell damage and consequently higher circulating insulin levels in the Men1l/l; RIP-Cre mice. These results also support that Men1-specific excision in pancreatic beta cells prevents STZ-induced hyperglycemia.
Figure 5.
Tissue specific ablation of floxed Men1 promotes beta-cell proliferation and increases beta cell number in STZ-treated mice. Study design was described in Figure 4(e). Control Men1l/l and Men1l/l; RIP-Cre mice (n = 15 mice) at the age of 12 weeks were injected with STZ at 40 mg/kg of body weight per day for 5 consecutive days. Pancreata and blood serum were collected from mice for further analysis, 4 weeks after STZ injections. (a) Quantitation of percentage of insulin-secreting beta cells in islets (n = 4 to 7 mice). (b and c) Immunostaining for BrdU (red) and insulin (green) in islets in Men1l/l (b) and Men1l/l; RIP-Cre mice (c). (d) Quantitation of BrdU incorporation by beta cells (n = 4 to 7 mice). (e) Quantitation of insulin staining area (n = 4 to 7 mice). (f) Non-fasting serum insulin levels before and 4 weeks after STZ injections (n = 12 to 15 mice). Scale bar, 25 μm. *, P < .05; ***, P < .001.
3.4. Men1 Ablation Preserves GLUT2 Membrane Localization in Pancreatic Beta Cells in STZ-Treated Mice
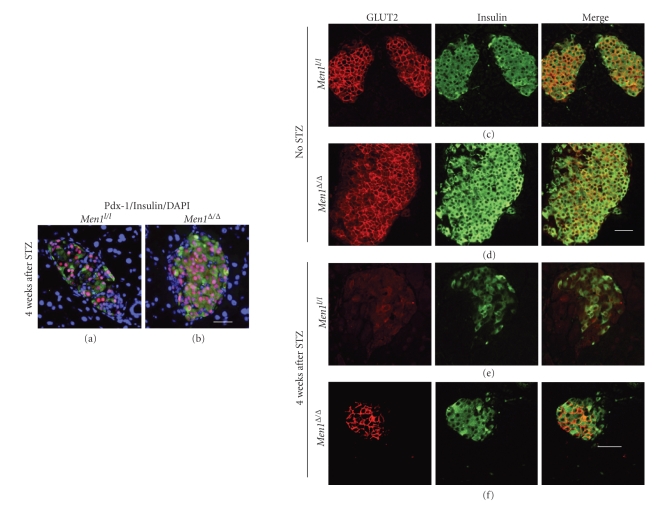
Pdx-1 is a crucial transcription factor for the development and regeneration of beta cells in both normal and diabetic mice [30, 31]. GLUT2 is an important beta cell membrane protein responsible for transporting glucose across beta cell membranes, and it is involved in glucose sensing and glucose-stimulated insulin secretion [32]. STZ binds to GLUT2 in beta cell membranes and is transported into beta cells, and it induces beta cell damage through several mechanisms including damaging genomic DNA [20]. In order to determine whether Men1 ablation prevents STZ-induced hyperglycemia partly through its regulation on Pdx-1 and/or GLUT2, we sought to detect expression or localization of Pdx-1 and GLUT2 in beta cells in STZ-treated control and Men1-excised mice by immunostaining.
We found that the number of Pdx-1 positive cells was higher in Men1-excised islets than control islets (Figures 6(a) and 6(b)). However, given the fact that Pdx-1 protein levels in individual beta cells did not appear substantially different between control and Men1-excised islets after STZ injections, whether Men1 excision directly up-regulates Pdx-1 expression still needs to be determined. Immunofluorescence staining showed that GLUT2 was detectable in both cell membranes and cytoplasm of beta cells in control and Men1-excised mice without STZ treatment (Figures 6(c) and 6(d)). Notably, after STZ injections, GLUT2 expression was largely preserved in beta cell membranes in the Men1-excised mice (Figure 6(f)), while it was barely detectable in beta cell membranes in the control mice (Figure 6(e)). These findings suggest that Men1 excision protects/preserves GLUT2 membrane localization in beta cell membranes and thus preserves the function of beta cells after STZ injections.
Figure 6.
Ablation of floxed Men1 preserves membrane localization of glucose transporter 2 (GLUT2) in beta cells in STZ-treated mice. (a-b) Immunostaining for Pdx-1 (red) and insulin (green) in islets in control Men1l/l (a) and Men1l/l; Cre-ER mice (b) treated with TAM, followed by STZ injections, as described in Figure 1(c). Control Men1l/l (n = 18 mice) and Men1l/l; Cre-ER mice (n = 21 mice) were fed TAM at age of 12 weeks. Four weeks after the last dose of TAM feeding, STZ was i.p. injected at 40 mg/kg of body weight per day for 5 consecutive days. Pancreata were collected from mice 4 weeks after STZ injections. Nuclei were counterstained using DABI (blue). (c-d) Immunostaining for GLUT2 (red) and insulin (green) in islets in control Men1l/l (c) and Men1l/l; Cre-ER mice (d) without STZ treatment, as described in Figures 1(a) and 1(b). Control Men1l/l and Men1l/l; Cre-ER mice (n = 8 mice) were fed tamoxifen (TAM) at the age of 12 weeks at 200 mg/kg of body weight per day for two consecutive days, followed by one day off and then for another two consecutive days. Pancreata were collected 4 weeks after TAM feeding. (e-f) Immunostaining for GLUT2 and insulin in islets in control Men1l/l (e) and Men1l/l; Cre-ER mice (f) treated with TAM and followed by STZ injections, as described in Figure 1(c). Control Men1l/l (n = 18 mice) and Men1l/l; Cre-ER mice (n = 21 mice) were fed TAM at age of 12 weeks. Four weeks after the last dose of TAM feeding, STZ was i.p. injected at 40 mg/kg of body weight per day for 5 consecutive days. Pancreata were collected 4 weeks after STZ injections. Scale bar, 25 μm.
4. Discussions and Conclusions
Previous studies have shown that beta-cell proliferation and circulating insulin levels were increased in mice long after Men1 excision [15, 17]. However, whether relatively acute Men1 excision can prevent STZ-induced hyperglycemia and its underlying mechanisms remains unclear. In the present study, we employed an approach of temporally controlled Men1 excision in multiple tissues as well as beta-cell specific Men1 excision and found that Men1 excision prevented development of hyperglycemia in STZ-treated mice. Our results further showed that Men1 excision prevented development of hyperglycemia mainly through increasing the number of functional beta cells.
It was also noticed that, in contrast to the observation that blood insulin level was reduced in the Men1l/l ; Cre-ER mice subjected to STZ treatment 4 weeks after Men1 excision, blood insulin level was not decreased in the STZ-treated Men1l/l ; RIP-Cre mice (Men1l/l ; Cre-ER mice, Figure 2(i); Men1l/l ; RIP-Cre mice, Figure 5(f)). The difference in the time and duration of Men1 excision in these two groups of mice might partially explain the discrepancy. For the Men1l/l ; Cre-ER mice, the Men1 gene was excised at age of 12 weeks through TAM feeding and these mice were then challenged with MLD-STZ 4 weeks after the last dose of TAM feeding (Figure 1(c)). There were only 4 weeks (after the last dose of TAM) for Men1-excised mice to replicate or regenerate more functional beta cells before they were injected with STZ. In contrast, in the Men1l/l ; RIP-Cre mice, the Men1 gene was excised in beta cells during embryonic development, since the rat insulin promoter-driven Cre recombinase is expressed and activated by insulin during embryonic stage. There were about 12 weeks for Men1l/l ; RIP-Cre mice to produce more functional beta cells before they were challenged with STZ. Duration of Men1 excision was much longer in the Men1l/l ; RIP-Cre mice than the TAM-treated Men1l/l ; Cre-ER mice, therefore it is likely that the Men1l/l ; RIP-Cre mice have more functional beta cells than the TAM-treated Men1l/l ; Cre-ER mice before STZ injections and consequently higher number of beta cells after STZ injections. As a result, blood insulin level was not significantly reduced in the Men1l/l ; RIP-Cre mice after STZ injections. In addition, it is also possible that earlier Men1 excision during embryonic stage might lead to some compensation or adaptation of beta cells in the Men1l/l ; RIP-Cre mice, which could also partially contribute to the relative resistance of beta cells to STZ-induced damage and reduction in blood insulin level.
Recent studies have shown that Men1-excised glucagon-producing alpha cells transdifferentiate to insulin-producing beta cells in mice [28]. Moreover, transdifferentiation from alpha cells to beta cells occurs more rapidly when beta cells are under severe stress [29]. However, our results showed that the average number of alpha cells per islet and blood glucagon level were similar between the control and Men1-excised mice, suggesting that Men1 excision did not substantially trigger transdifferentiation of alpha cells to beta cells 4 weeks after STZ injections. However, we cannot rule out that Men1-excised alpha cells or other non-beta cells in the pancreas can reprogram into insulin-secreting beta-cells during the late stage of STZ-induced diabetes.
Our further analysis using immunostaining revealed that there were more Pdx-1 positive cells in Men1-excised mice. Pdx-1 is an important transcription factor in beta-cell development and growth [33, 34]. Our results showed that Pdx-1 protein level in an individual beta-cell does not appear to increase significantly in Men1-excised mice as compared with control mice, suggesting that Pdx-1 expression level might be a marker for functional beta-cells after STZ injections, and a reduced number of Pdx-1 positive cells in the control mice may be a result of reduction in the total number of functional beta-cells in those mice. Immunostaining on pancreas sections also showed that GLUT2 localization in beta-cell membranes after STZ injections were largely preserved in the Men1-excised mice as compared with the control mice (Figures 6(e) and 6(f)). This result may partly explain why Men1-excised mice showed normal blood glucose levels and higher circulating insulin levels, since GLUT2 is involved in glucose sensing and glucose-stimulated insulin secretion [32]. On the other hand, because Men1 excision did not reduce membrane expression of GLUT2 in beta-cells in mice without STZ treatments (Figures 6(c) and 6(d)), it is unlikely that relative resistance of Men1-excised mice to STZ-induced beta-cell damage was due to changes in membrane GLUT2 expression in beta-cells. Alternatively, we could not rule out that Men1 excision improves the overall function of beta-cells, rendering Men1-excised cells more resistant to STZ-induced beta-cell damage.
It is also noteworthy that the RIP-Cre transgene, which we used to specifically excise Men1 in beta-cells in Men1l/l; RIP-Cre mice, has been reported to express in non-beta cells, including the lung, spleen, testis, and brain [35], especially in a group of poorly defined neurons in the hypothalamus (RipCre neurons) [36]. Knockout of insulin receptor substrate 2 in this group of hypothalamic neuronal population causes disruption in energy homeostasis, increases food intake, and leads to obesity at a later stage [36]. Because it has not been reported that tumors develop in the hypothalamus in either MEN1 patients or Men1 knockout mice, it is not clear whether menin represses proliferation of RipCre neurons. Moreover, it is also unclear whether Men1 excision in RipCre neurons affects food intake or other pathways involved in energy homeostasis or glucose metabolism. Therefore, we could not rule out the possibility that Men1 excision in non-beta cells may partly attribute to improvement in glucose homeostasis in STZ-treated mice.
In conclusion, we have found that Men1 excision prevents STZ-induced hyperglycemia mainly through increasing the number of functional beta cells. BrdU incorporation by beta cells, islet size, and circulating insulin levels were significantly increased in Men1-excised mice. Consistent with our findings, menin is physiologically suppressed through the prolactin signaling pathway in pancreatic beta cells in pregnant mice to prevent gestational diabetes [37]. These results suggest that menin might be targeted to promote beta-cell proliferation and ameliorate diabetes. However, since cell hyperplasia, insulinoma, and hyperinsulinemia have been found in Men1 knockout mouse models or MEN1 patients [15, 38, 39], there is a justifiable concern that disruption of menin aiming to promote beta-cell proliferation may lead to insulinoma, and permanent deletion or inhibition of menin itself might not be a viable therapeutic approach to treat diabetes. Instead, it might be possible to promote beta-cell proliferation through reversibly inhibiting menin or blocking the interaction between menin and its partners. These approaches may retain menin's tumor suppressing functions, such as DNA repair [40], but transiently dampen its ability to suppress beta-cell proliferation, thereby increasing the number of functional endogenous beta cells and ameliorating diabetes. In this regard, small molecule compounds that target the interaction between menin and its partners, if developed, could be useful for preventing or treating diabetes.
Conflict of Interests
Relevant to this paper no potential conflict of interests is declared by the authors.
Acknowledgments
This work was supported in part by Grants from the NIH (R01-DK085121, X.H.) and a Grant from the American Diabetes Association (7-07-RA-60, X.H.). The authors thank Dr. Doris Stoffers for her suggestions and stimulating discussion, and Peter Blessington for his excellent technical assistance. We also thank Ms. Shivani Sethi for reading and editing the manuscript.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: pathogenesis and treatment. The Lancet. 2008;371(9631):2153–2156. doi: 10.1016/S0140-6736(08)60932-0. [DOI] [PubMed] [Google Scholar]
- 3.Stumvoll M, Goldstein BJ, van Haeften TW. Pathogenesis of type 2 diabetes. Endocrine Research. 2006;32(1-2):19–37. doi: 10.1080/07435800701743810. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. The Lancet. 2001;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 5.Parker MJ, Xue S, Alexander JJ, et al. Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes. 2009;58(10):2277–2284. doi: 10.2337/db09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begum S, Chen W, Herold KC, Papaioannou VE. Remission of type 1 diabetes after anti-CD3 antibody treatment and transplantation of embryonic pancreatic precursors. Endocrinology. 2009;150(10):4512–4520. doi: 10.1210/en.2009-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45(12):1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 8.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. Journal of Clinical Investigation. 2000;106(4):467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson K, Lu Y, De Jesus K, et al. A general and islet cell-enriched overexpression of IGF-I results in normal islet cell growth, hypoglycemia, and significant resistance to experimental diabetes. American Journal of Physiology. 2008;294(5):E928–E938. doi: 10.1152/ajpendo.00606.2007. [DOI] [PubMed] [Google Scholar]
- 10.Uchida T, Nakamura T, Hashimoto N, et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nature Medicine. 2005;11(2):175–182. doi: 10.1038/nm1187. [DOI] [PubMed] [Google Scholar]
- 11.Fu A, Ng ACH, Depatie C, et al. Loss of Lkb1 in adult β cells increases β cell mass and enhances glucose tolerance in mice. Cell Metabolism. 2009;10(4):285–295. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Tanner FC, Boehm M, Akyürek LM, et al. Differential effects of the cyclin-dependent kinase inhibitors p27Kip1, p21Cip1 and p16Ink4 on vascular smooth muscle cell proliferation. Circulation. 2000;101(17):2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 13.Green AS, Chapuis N, Maciel TT, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116(20):4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 14.Marx SJ, Stratakis CA. Multiple endocrine neoplasia—introduction. Journal of Internal Medicine. 2005;257(1):2–5. doi: 10.1111/j.1365-2796.2004.01419.x. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree JS, Scacheri PC, Ward JM, et al. Of mice and MEN1: insulinomas in a conditional mouse knockout. Molecular and Cellular Biology. 2003;23(17):6075–6085. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scacheri PC, Crabtree JS, Kennedy AL, et al. Homozygous loss of menin is well tolerated in liver, a tissue not affected in MEN1. Mammalian Genome. 2004;15(11):872–877. doi: 10.1007/s00335-004-2395-z. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(3):1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnepp RW, Chen YX, Wang H, et al. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Research. 2006;66(11):5707–5715. doi: 10.1158/0008-5472.CAN-05-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEvoy RC, Andersson J, Sandler S, Hellerstrom C. Multiple low-dose streptozotocin-induced diabetes in the mouse. Evidence for stimulation of a cytotoxic cellular immune response against an insulin-producing beta cell line. Journal of Clinical Investigation. 1984;74(3):715–722. doi: 10.1172/JCI111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Nagafuchi S, Yamaguchi K, Takaki R. The role of thymic immunity and insulitis in the development of streptozocin-induced diabetes in mice. Diabetes. 1984;33(9):894–900. doi: 10.2337/diab.33.9.894. [DOI] [PubMed] [Google Scholar]
- 22.Liadis N, Murakami K, Eweida M, et al. Caspase-3-dependent β-cell apoptosis in the initiation of autoimmune diabetes mellitus. Molecular and Cellular Biology. 2005;25(9):3620–3629. doi: 10.1128/MCB.25.9.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed MJ, Meszaros K, Entes LJ, et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49(11):1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 24.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 25.Jin S, Mao H, Schnepp RW, et al. Menin associates with FANCD2, a protein involved in repair of DNA damage. Cancer Research. 2003;63(14):4204–4210. [PubMed] [Google Scholar]
- 26.Conarello SL, Jiang G, Mu J, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50(1):142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DG, Goebel CU, Hruby VJ. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science. 1982;215(4536):1115–1116. doi: 10.1126/science.6278587. [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Herrera PL, Carreira C, et al. α cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138(5):1954–e8. doi: 10.1053/j.gastro.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kushner JA, Ye J, Schubert M, et al. Pdx1 restores β cell function in Irs2 knockout mice. Journal of Clinical Investigation. 2002;109(9):1193–1201. doi: 10.1172/JCI14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver-Krasinski JM, Kasner MT, Yang J, et al. The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. Journal of Clinical Investigation. 2009;119(7):1888–1898. doi: 10.1172/JCI37028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorens B, Wu YJ, Leahy JL, Weir GC. The loss of GLUT2 expression by glucose-unresponsive β cells of db/db mice is reversible and is induced by the diabetic environment. Journal of Clinical Investigation. 1992;90(1):77–85. doi: 10.1172/JCI115858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes and Development. 2008;22(24):3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holland AM, Góñez LJ, Naselli G, MacDonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of β-cells in the adult pancreas. Diabetes. 2005;54(9):2586–2595. doi: 10.2337/diabetes.54.9.2586. [DOI] [PubMed] [Google Scholar]
- 35.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic β cell carcinogenesis. Cancer Cell. 2002;1(2):193–202. doi: 10.1016/s1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 36.Choudhury AI, Heffron H, Smith MA, et al. The role of insulin receptor substrate 2 in hypothalamic and β cell function. Journal of Clinical Investigation. 2005;115(4):940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318(5851):806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 38.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nature Reviews Cancer. 2005;5(5):367–375. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 39.Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Molecular Endocrinology. 2003;17(9):1880–1892. doi: 10.1210/me.2003-0154. [DOI] [PubMed] [Google Scholar]
- 40.Busygina V, Kottemann MC, Scott KL, Plon SE, Bale AE. Multiple endocrine neoplasia type 1 interacts with forkhead transcription factor CHES1 in DNA damage response. Cancer Research. 2006;66(17):8397–8403. doi: 10.1158/0008-5472.CAN-06-0061. [DOI] [PubMed] [Google Scholar]