Abstract
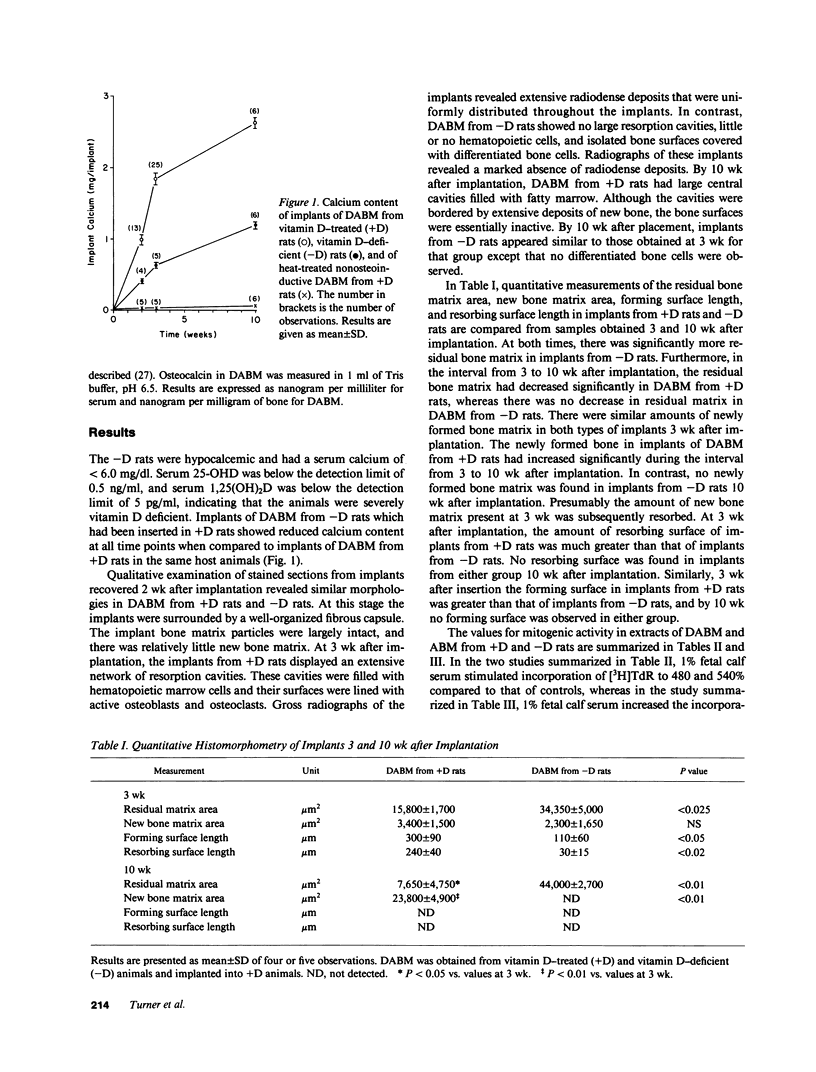
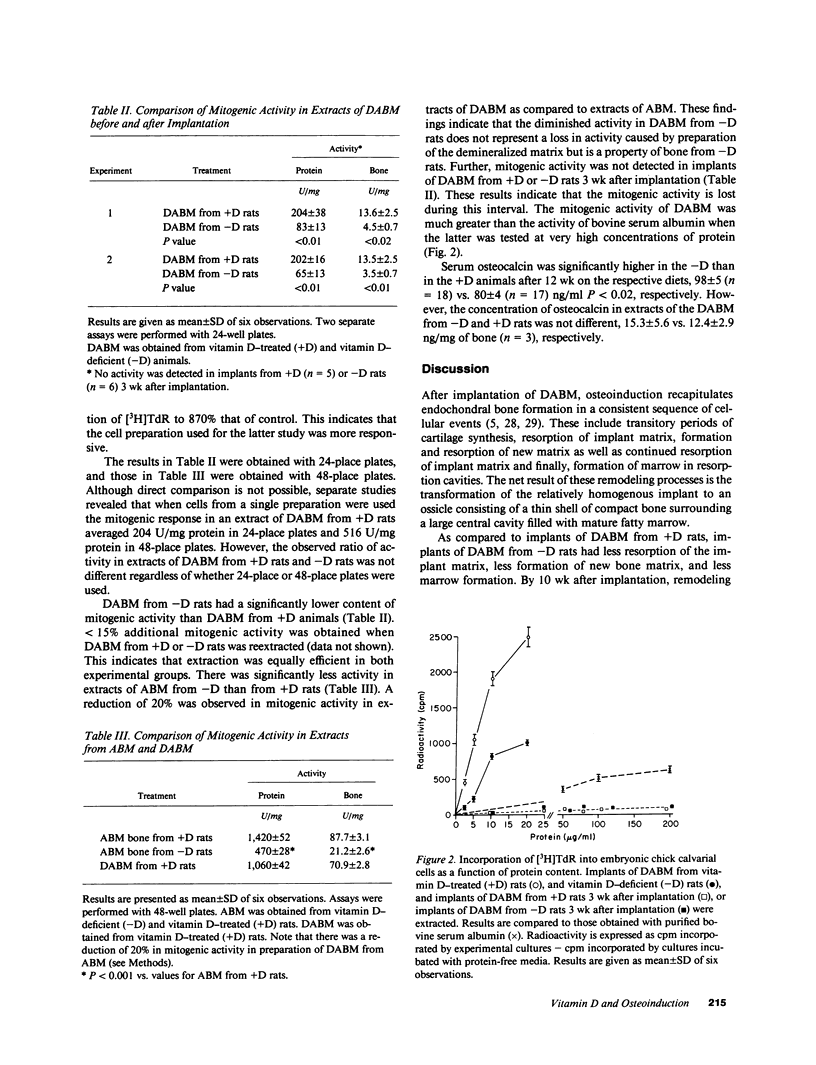
Osteoinduction is the formation of ectopic bone that follows implantation of demineralized allogeneic bone matrix (DABM) and is believed to be secondary to the release of associated inductive factors from bone matrix. To clarify the role of vitamin D in osteoinduction, we implanted DABM from vitamin D-deficient rats (-D rats) into normal rats (+D rats). Because mitogens and osteocalcin might be involved in osteoinduction, these were measured. Mitogenic activity in extracts from mineralized allogeneic bone matrix (ABM) and DABM from both +D and -D rats was determined with an assay that utilizes monolayer cultures of embryonic chick calvarial cells. Osteocalcin in serum and DABM was measured by radioimmunoassay. DABM from -D rats did not promote osteoinduction as effectively as DABM from +D rats. Resorption of implant matrix from -D rats was diminished compared with resorption of matrix from +D rats (P less than 0.01), and the decrease was attributed to a corresponding decrease in the number of osteoclasts in the implants (P less than 0.02). Bone formation (P less than 0.01) and total implant mineralization (P less than 0.001) were significantly reduced in implants from -D rats, and the reductions corresponded with a decline in the number of osteoblasts (P less than 0.05). Mitogenic activity in DABM from +D rats was only slightly decreased as compared with activity in ABM, but DABM from -D rats contained significantly less activity (P less than 0.001). No mitogenic activity was identified in implants of DABM from either +D or -D rats 3 wk after implantation. Serum osteocalcin was significantly higher in -D as compared with +D animals. In contrast, the concentrations of osteocalcin in DABM from the two groups of animals were not significantly different from each other. These findings indicate that the diminished osteoinductive activity of DABM from -D rats results from deficiency of one or more mitogenic factors that are essential for inducing the proliferation and differentiation of bone cells at the implant site and that osteocalcin does not play a role in this regard.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amitani K., Nakata Y. Studies on a factor responsible for new bone formation from osteosarcoma in mice. Calcif Tissue Res. 1975;17(2):139–150. doi: 10.1007/BF02547286. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Bone collagen metabolism in vitamin D deficiency. Biochem J. 1973 Jan;132(1):113–115. doi: 10.1042/bj1320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canalis E., McCarthy T., Centrella M. A bone-derived growth factor isolated from rat calvariae is beta 2 microglobulin. Endocrinology. 1987 Sep;121(3):1198–1200. doi: 10.1210/endo-121-3-1198. [DOI] [PubMed] [Google Scholar]
- Canalis E., Peck W. A., Raisz L. G. Stimulation of DNA and collagen synthesis by autologous growth factor in cultured fetal rat calvaria. Science. 1980 Nov 28;210(4473):1021–1023. doi: 10.1126/science.7434011. [DOI] [PubMed] [Google Scholar]
- Centrella M., Canalis E. Isolation of EGF-dependent transforming growth factor (TGF beta-like) activity from culture medium conditioned by fetal rat calvariae. J Bone Miner Res. 1987 Feb;2(1):29–36. doi: 10.1002/jbmr.5650020106. [DOI] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D. Vitamin D and endochondral ossification in the rat as indicated by the use of sulfur-35 and phosphorus-32. J Exp Med. 1954 Jul 1;100(1):25–32. doi: 10.1084/jem.100.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss W. P., Jr, Hern D. L. Bone matrix studies. Influences of parathyroid extract, calcitonin, and cholecalciferol and of rickets and its treatment. Biochim Biophys Acta. 1979 May 1;584(2):311–326. doi: 10.1016/0304-4165(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Farley J. R., Masuda T., Wergedal J. E., Baylink D. J. Human skeletal growth factor: characterization of the mitogenic effect on bone cells in vitro. Biochemistry. 1982 Jul 6;21(14):3508–3513. doi: 10.1021/bi00257a038. [DOI] [PubMed] [Google Scholar]
- Jowell P. S., Epstein S., Fallon M. D., Reinhardt T. A., Ismail F. 1,25-Dihydroxyvitamin D3 modulates glucocorticoid-induced alteration in serum bone Gla protein and bone histomorphometry. Endocrinology. 1987 Feb;120(2):531–536. doi: 10.1210/endo-120-2-531. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Carnes D. L., Glimcher M. J. Bone and serum concentrations of osteocalcin as a function of 1,25-dihydroxyvitamin D3 circulating levels in bone disorders in rats. Endocrinology. 1987 May;120(5):2123–2130. doi: 10.1210/endo-120-5-2123. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Glimcher M. J., Roufosse A. H., Hauschka P. V., Gallop P. M., Cohen-Solal L., Reit B. Alterations of the gamma-carboxyglutamic acid and osteocalcin concentrations in vitamin D-deficient chick bone. J Biol Chem. 1982 May 10;257(9):4999–5003. [PubMed] [Google Scholar]
- Linkhart T. A., Jennings J. C., Mohan S., Wakley G. K., Baylink D. J. Characterization of mitogenic activities extracted from bovine bone matrix. Bone. 1986;7(6):479–487. doi: 10.1016/8756-3282(86)90007-4. [DOI] [PubMed] [Google Scholar]
- Mechanic G. L., Toverud S. U., Ramp W. K., Gonnerman W. A. The effect of vitamin D on the structural crosslinks and maturation of chick bone collagen. Biochim Biophys Acta. 1975 Jun 26;393(2):419–425. doi: 10.1016/0005-2795(75)90070-7. [DOI] [PubMed] [Google Scholar]
- Mechanic G. L., Toverud S. U., Ramp W. K. Quantitative changes of bone collagen crosslinks and precursors in vitamin D deficiency. Biochem Biophys Res Commun. 1972 May 26;47(4):760–765. doi: 10.1016/0006-291x(72)90557-8. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Poser J. W. Chemotactic activity of the gamma-carboxyglutamic acid containing protein in bone. Calcif Tissue Int. 1983;35(2):164–168. doi: 10.1007/BF02405025. [DOI] [PubMed] [Google Scholar]
- Price P. A., Baukol S. A. 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J Biol Chem. 1980 Dec 25;255(24):11660–11663. [PubMed] [Google Scholar]
- Price P. A., Baukol S. A. 1,25-dihydroxyvitamin D3 increases serum levels of the vitamin K-dependent bone protein. Biochem Biophys Res Commun. 1981 Apr 15;99(3):928–935. doi: 10.1016/0006-291x(81)91252-3. [DOI] [PubMed] [Google Scholar]
- Price P. A., Lothringer J. W., Nishimoto S. K. Absence of the vitamin K-dependent bone protein in fetal rat mineral. Evidence for another gamma-carboxyglutamic acid-containing component in bone. J Biol Chem. 1980 Apr 10;255(7):2938–2942. [PubMed] [Google Scholar]
- Price P. A., Williamson M. K. Effects of warfarin on bone. Studies on the vitamin K-dependent protein of rat bone. J Biol Chem. 1981 Dec 25;256(24):12754–12759. [PubMed] [Google Scholar]
- Puzas J. E., Drivdahl R. H., Howard G. A., Baylink D. J. Endogenous inhibitor of bone cell proliferation. Proc Soc Exp Biol Med. 1981 Jan;166(1):113–122. doi: 10.3181/00379727-166-41032. [DOI] [PubMed] [Google Scholar]
- Puzas J. E., Drivdahl R. H., Howard G. A., Baylink D. J. Endogenous inhibitor of bone cell proliferation. Proc Soc Exp Biol Med. 1981 Jan;166(1):113–122. doi: 10.3181/00379727-166-41032. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. B. Formation of bone marrow in fibroblast-transformation ossicles. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2212–2216. doi: 10.1073/pnas.72.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath T. K., Wientroub S., Reddi A. H. Extracellular matrix proteins involved in bone induction are vitamin D dependent. Biochem Biophys Res Commun. 1984 Nov 14;124(3):829–835. doi: 10.1016/0006-291x(84)91032-5. [DOI] [PubMed] [Google Scholar]
- Stauffer M., Baylink D., Wergedal J., Rich C. Decreased bone formation, mineralization, and enhanced resorption in calcium-deficient rats. Am J Physiol. 1973 Aug;225(2):269–276. doi: 10.1152/ajplegacy.1973.225.2.269. [DOI] [PubMed] [Google Scholar]
- Stracke H., Schulz A., Moeller D., Rossol S., Schatz H. Effect of growth hormone on osteoblasts and demonstration of somatomedin-C/IGF I in bone organ culture. Acta Endocrinol (Copenh) 1984 Sep;107(1):16–24. doi: 10.1530/acta.0.1070016. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Kang A. H., Trelstad R. L., Gross J. Collagen heterogeneity within different growth regions of long bones of rachitic and non-rachitic chicks. Biochem J. 1972 May;127(4):715–720. doi: 10.1042/bj1270715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Urist M. R., DeLange R. J., Finerman G. A. Bone cell differentiation and growth factors. Science. 1983 May 13;220(4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Dowell T. A., Hay P. H., Strates B. S. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968 Jul-Aug;59:59–96. [PubMed] [Google Scholar]
- Vandersteenhoven J. J., Spector M. Histological investigation of bone induction by demineralized allogeneic bone matrix: a natural biomaterial for osseous reconstruction. J Biomed Mater Res. 1983 Nov;17(6):1003–1014. doi: 10.1002/jbm.820170610. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Zonal analysis of phospholipids in the epiphyseal cartilage and bone of normal and rachitic chickens and pigs. Calcif Tissue Res. 1971;8(1):36–53. doi: 10.1007/BF02010121. [DOI] [PubMed] [Google Scholar]



