Abstract
Introduction. Young adults are likely to differ from old patients concerning cerebral infarction. Methods. We compared characteristics of patients aged under and above 50 years, admitted to the Department of Neurology with cerebral infarction between 2006 and 2009, based on prospective registration. Investigation followed one common protocol for both groups. Results and Discussion. One hundred patients (8.2%) were <50 years old, and the proportion of males was higher in this group (72% versus 55.8%, P = .002). Young stroke patients are more often current smokers (44.1% versus 23.6%, P < .001). Common causes for stroke in the young were cervical artery dissection (18% versus 0.6%, P < .001) and cardiac embolism due to disorders other than atrial arrhythmias (18% versus 5.5%, P < .001). Among the old, atrial fibrillation and flutter dominated (29.1% versus 5%, P < .001). Stroke severity and location did not differ. Old patients more often suffered from pneumonia (10.6% versus 2%, P < .003) and urinary tract infection (14.6% versus 2%, P = .001). Conclusions. Males dominate, and current smoking is more common in the young. Cervical artery dissection and nonarrhythmic heart disorders are frequent causes among young patients, while traditional risk factors dominate the old. Stroke severity is similar, but old patients seem more exposed for infectious complications.
1. Introduction
Cerebral infarction may have serious consequences for patients in their prime of life and influence on choice of education, vocation, and family planning. More knowledge regarding pathophysiological mechanisms and prognosis is urgently needed. Several studies have shown that risk factors and etiology differ between young and old patients. Migraine is frequently reported among young adults [1–5] whereas traditional risk factors such as hypertension and dyslipidemia are usually less frequent. Large-artery atherosclerosis is rare [3, 6] whereas cervical artery dissection is a common cause of cerebral infarction among young adults [2, 4, 6, 7]. Cardioembolic stroke is in the majority of cases caused by cardiac conditions with low to uncertain embolic risk, such as patent foramen ovale and atrial septal aneurysm [4, 8]. Methodological differences may obscure comparison between different centres. There has not been many comparisons between young and old patients treated and investigated in a single centre.
The aim of this study was to compare characteristics of cerebral infarction between young and old patients undergoing treatment and investigations according to one common protocol in a single centre.
2. Methods
2.1. Patients
All consecutive patients with acute cerebral infarction (the index stroke) admitted to the Stroke Unit, Department of Neurology, Haukeland University Hospital, Bergen, Norway, between February 2006 and March 2009, were prospectively registered in a database (The Bergen Stroke Registry). Cerebral infarction was defined in accordance with the Baltimore-Washington Cooperative Young Stroke Study Criteria comprising neurological deficits lasting more than 24 hours because of ischemic lesions or transient ischemic attacks where CT or MRI showed infarctions related to the clinical findings [9]. The patients were dichotomized into two groups: <50 years (young patients) and ≥50 years (old patients).
All patients had CT or MRI. Isolated acute ischemic lesions on CT or MRI were defined as lacunar infarctions (LI) if <1.5 cm and located as subcortical or in the brainstem. All other acute ischemic lesions were defined as nonlacunar infarction (NLI). NLI comprised subcortical and brainstem infarction ≥1.5 cm, cortical infarction, mixed cortical and subcortical infarction, and cerebellar infarction. Leukoaraiosis was defined as the presence of hypodense periventricular abnormalities on MRI (T2).
The National Institute of Health Stroke Scale (NIHSS) was used to assess stroke severity. NIHSS measurements were performed on admittance and 7 days after stroke onset or earlier if the patient was discharged earlier (NIHSS7). Likewise, modified Rankin Scale (mRS) score and Barthel Index (BI) were obtained 7 days after stroke onset or earlier if the patient was discharged earlier. Blood pressure, body temperature, and serum glucose on admittance were registered. Diagnostic workup included ECG, Holter monitoring, echocardiography, and duplex sonography of neck vessels. Holter monitoring was performed among patients with embolic stroke and no known atrial fibrillation.
Risk factors including hypertension, smoking, diabetes mellitus, myocardial infarction, angina pectoris, peripheral artery disease, and atrial fibrillation were registered on admittance. Hypertension was defined as prior use of antihypertensive medication. Current smoking was defined as smoking at least one cigarette per day. Diabetes mellitus was considered present if the patient was on glucose-lowering diet or medication. Angina pectoris, myocardial infarction, and peripheral artery disease were considered present if diagnosed by a physician any time before stroke onset. Atrial fibrillation required ECG confirmation any time prior to stroke onset. A history of prior cerebral infarction was registered. Old infarctions on CT or MRI were registered, including both clinically silent and symptomatic infarctions. Etiology was determined by the Trial of Org 10172 in Acute Stroke Treatment classification (TOAST) [10], performed by a neurologist (HN). Clinical classification was based on the Oxfordshire Community Stroke Project (OCSP) scale which includes lacunar syndrome (LACS), partial anterior circulation syndrome (PACS), total anterior circulation syndrome (TACS), and posterior circulation syndrome (POCS) [11].
ICA stenosis was defined as a percentage of area reduction in neurosonology, graded from 30–49%, 50–69%, 70–99%, to occlusion (Table 5). Calculation was performed by Phillips software, integrated in IU 22.
Table 5.
Investigations.
| Young patients (n = 100) | Old patients (n = 1117) | P | |
|---|---|---|---|
| ECG on admission | |||
| Total | 82 (82) | 1057 (94.6) | |
| Atrial fibrillation | 2 (2.4) | 181 (17.1) | <.001 |
| Left bundle branch block | 0 (0) | 38 (3.6) | .11 |
| Left ventricle hypertrophy | 6 (7.3) | 73 (6.9) | .82 |
| Unspecific ST depression | 7 (8.5) | 232 (21.9) | .003 |
| Acute anterior myocardial infarction | 0 (0) | 3 (.3) | 1.00 |
| Old anterior myocardial infarction | 2 (2.4) | 52 (4.9) | .42 |
| Acute inferior myocardial infarction | 0 (0) | 2 (.2) | 1.00 |
| Old inferior myocardial infarction | 2 (2.4) | 59 (5.6) | .31 |
| Echocardiography | |||
| Total | 63 (63) | 357 (32.0) | |
| TTE | 28 (44.4) | 284 (79.6) | |
| TEE | 35 (55.6) | 73 (20.4) | |
| Left ventricle hypertrophy | 7 (11.1) | 119 (33.3) | <.001 |
| Patent foramen ovale | 10 (15.9) | 14 (3.9) | .001 |
| Sequelae anterior myocardial infarction | 2 (3.2) | 19 (5.3) | .75 |
| Sequelae inferior myocardial infarction | 0 (0) | 16 (4.5) | .15 |
| Holter monitoring | |||
| Total | 57 (57) | 434 (38.9) | |
| Paroxysmal atrial fibrillation | 1 (1.8) | 78 (18.0) | .001 |
| Duplex of cervical arteries | |||
| Total | 86 (86) | 893 (79.9) | |
| ICA stenosis1 | 11 (12.8) | 356 (39.9) | .000 |
| Symptomatic ICA stenosis ≤49%1* | 0 (0) | 83 (13.9) | .002 |
| Symptomatic ICA stenosis 50–69%1* | 0 (0) | 55 (9.2) | |
| Symptomatic ICA stenosis 70%–99%1* | 2 (3.9) | 34 (5.7) | |
| Symptomatic occlusion1* | 5 (9.8) | 29 (4.9) | |
| No ICA stenosis1* | 44 (86.3) | 397 (66.4) | |
Data are expressed as mean or n (%).
ECG, electrocardiography; ICA, internal carotid artery.
¹Area reduction measured by neurosonology.
*Among patients with ipsilateral infarction in the middle cerebral artery territory.
Complications including pneumonia, urinary tract infection, and seizures were registered.
2.2. Statistics
Chi-square test, Fisher's exact test, and student's t-test were performed when appropriate. Logistic regression was performed to analyse the effect of the two age groups (young or old patients) on outcome day 7 adjusting for sex and NIHSS score on admission. mRS score 0–2 versus 3–6 was used as dependent variable. STATA 11.0 was used for analysis.
3. Results
In total, 1217 patients were included. One hundred (8.2%) were <50 years (range: 18–49 years) and 1117 (91.2%) were ≥50 years (range: 50–98 years). The proportion of males was higher among young patients: 72% versus 55.8% (Table 1).
Table 1.
Demography of young and old patients with cerebral infarction, based on patient history recorded on admission.
| Young patients (n = 100) | Old patients (n = 1117) | P | |
|---|---|---|---|
| Age (mean) | 40.8 (SD 7.6) | 73.4 (SD 11.8) | |
| Females | 28 (28.0) | 494 (44.2) | .002 |
| Males | 72 (72.0) | 623 (55.8) | |
| Married | 62 (62.6) | 631 (57.8) | .40 |
| Employed | 81 (85.3) | 236 (22.0) | <.001 |
| Prior cerebral infarction | 4 (4.0) | 179 (16.2) | <.001 |
| Myocardial infarction | 4 (4.0) | 155 (13.9) | .003 |
| Angina pectoris | 4 (4.0) | 160 (14.4) | .002 |
| Mechanic aortic valve | 5 (5.0) | 21 (1.9) | .05 |
| Peripheral artery disease | 3 (3.0) | 89 (8.1) | .08 |
| Hypertension | 27 (27.0) | 598 (53.8) | <.001 |
| Paroxysmal atrial fibrillation | 2 (2.0) | 104 (9.4) | .009 |
| Chronic atrial fibrillation | 0 (0.0) | 105 (9.46) | <.001 |
| Diabetes mellitus | 10 (10.0) | 163 (14.8) | .23 |
| Migraine | 14 (17.7) | 149 (19.4) | .88 |
| Prior depression | 15 (18.3) | 185 (22.8) | .41 |
| Current smoking | 41 (44.1) | 249 (23.6) | <.001 |
| Never smoking | 38 (40.9) | 439 (41.6) | |
| Quitted smoking | 14 (15.1) | 368 (34.9) |
Data are expressed as mean or n (%). SD: standard deviation.
The following risk factors were more frequent among old patients: myocardial infarction, angina pectoris, hypertension, atrial fibrillation, and prior cerebral infarction. Mechanic aortic valves and current smoking were more frequent among young patients (Table 1).
There was no difference concerning NIHSS score on admittance or OCSP classification. Systolic blood pressure was lower among young patients on admittance: 155 mmHg versus 168 mmHg (Table 2).
Table 2.
Characteristics of cerebral infarction in young and old patients.
| Young patients (n = 100) | Old patients (n = 1117) | P | |
|---|---|---|---|
| Classification | |||
| LACS | 20 (20.2) | 281 (25.2) | .33 |
| TACS | 17 (17.2) | 184 (16.5) | |
| PACS | 38 (38.4) | 458 (41.0) | |
| POCS | 24 (24.2) | 193 (17.3) | |
| Parameters on admission | |||
| Systolic blood pressure (mmHg) | 155 | 168 | <.001 |
| Body temperature (centigrade) | 36.8 | 36.6 | .47 |
| Serum glucose (mmol/L) | 6.5 | 6.8 | .26 |
|
Scores On admission |
|||
| NIHSS | 5.7 | 6.3 | .45 |
| At day 7 | |||
| NIHSS | 4.4 | 4.9 | .50 |
| mRS 0–2 | 70 (70.0) | 677 (60.6) | .11 |
| mRS 3–5 | 26 (26.0) | 408 (36.5) | |
| mRS 6 | 4 (4.0) | 32 (2.9) | |
| Barthel Index (mean) | 86.9 | 78.1 | .01 |
| Complications | |||
| Nasogastric feeding | 6 (6.0) | 132 (11.8) | .10 |
| Pneumonia | 2 (2.0) | 118 (10.6) | .003 |
| Urinary tract infection | 2 (2.0) | 163 (14.6) | <.001 |
| Seizures | 4 (4.0) | 40 (3.6) | .78 |
| Etiology | |||
| Large-artery atherosclerosis | 3 (3.0) | 139 (12.4) | .003 |
| Cardiac embolism | 21 (21.0) | 328 (29.4) | .08 |
| Small vessel disease | 14 (14.0) | 170 (15.2) | .88 |
| Other causes | 23 (23.0) | 10 (0.9) | <.001 |
| Unknown | 39 (39.0) | 468 (41.9) | .4 |
Data are expressed as mean or n (%).
NIHSS, The National Institute of Health Stroke Scale; LACS, lacunar stroke syndrome; TACS, total anterior circulation stroke syndrome; PACS, partial anterior circulation stroke syndrome; POCS, posterior circulation stroke syndrome; mRS, modified Rankin Scale.
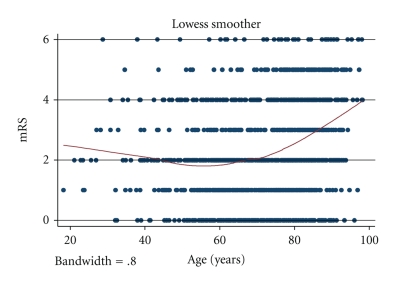
Outcome on day 7 (or on discharge if discharged earlier) was similar regarding mRS score and NIHSS score, whereas mean Barthel Index was higher among young patients: 86.9 versus 78.1. Figure 1 shows mRS scores according to age. The mortality rates did not differ significantly on day 7, respectively, on discharge (P = .5). Logistic regression showed that mRS score 0–2 versus 3–6 was associated with NIHSS score on admittance (odds ratio (OR) 1.29 (95% confidence interval (CI) 1.25–1.34), P < .001), but not with sex (OR .76 (95%CI .57–1.01), P = .064) or young versus old patients (OR .69 (95%CI .40–1.20), P = .19). Subanalysis for patients >45 years and <45 years, traditionally regarded as “young” in stroke literature, did not change the results concerning stroke severity on admission (NIHSS): 6.9 in the young versus 6.2 in the old group, P = .6, neither was there a difference regarding short-term outcome at day 7: mRS 2.3 versus 2.3, P = .81.
Figure 1.
mRS scores, at day 7 or at discharge (if before 7 days), among patients with cerebral infarction according to age. Solid line shows mean mRS. mRS, modified Rankin Scale.
Pneumonia and urinary tract infections were less frequent among young patients. Seizures were seen in about 4% in both groups (Table 2).
Cardiac embolism was found in 21% of the young patients versus 29.4% of the old patients and included most frequently in the young with patent foramen ovale (in 2 cases combined with atrial septal aneurysm), mechanical heart valve and paroxysmal atrial fibrillation, or combinations of these conditions. Other causes were found in 23% of young patients versus 0.9% of the old patients, and cervical artery dissection was the most frequent one (18%). More rare conditions included pseudoaneurysm of the ICA, giant aneurysm of the MCA, prothrombotic disorders, and Moya moya. Large-artery atherosclerosis was less frequent among young patients: 3% versus 12.4% (Tables 2, 3, and 4).
Table 3.
Heart disorders associated with cardiac embolism.
| Young patients (n = 21) | Old patients (n = 328) | P | |
|---|---|---|---|
| Patent foramen ovale | 4 | 9 | — |
| Patent foramen ovale and atrial septal aneurysm | 2 | 0 | — |
| Patent foramen ovale and paroxysmal atrial fibrillation | 1 | 0 | — |
| Atrial fibrillation (paroxysmal and chronic) | 3 | 261 | <.01 |
| Atrial flutter | 0 | 6 | .54 |
| Atrial septal defect | 1 | 0 | — |
| Atrial septal defect and paroxysmal atrial fibrillation | 1 | 0 | — |
| Atrial septal aneurysm | 0 | 2 | — |
| Ventricular septal defect | 1 | 0 | — |
| Anterior myocardial infarction/akinesia | 2 | 6 | — |
| Heart valve dysfunction | 0 | 15 | — |
| Mechanical heart valve | 4 | 10 | — |
| Mechanical heart valve and prothrombotic disorder | 1 | 0 | — |
| Ventricular thrombus | 0 | 2 | — |
| Papillary fibroelastoma | 1 | 0 | — |
| Cardiomyopathy | 0 | 2 | — |
| Severe heart failure | 0 | 3 | .66 |
| Other | 0 | 12 | — |
| Cardiac embolism due to atrial fibrillation/atrial flutter* | 5 (5) | 267 (29.1) | <.001 |
| Cardiac embolism due to disorders other than atrial fibrillation/atrial flutter* | 18 (18) | 61 (5.5) | <.001 |
P value is given only for diagnoses where equal investigation methods were used for both groups.
*in relation to all 100 young and 1117 old patients included in the study.
Table 4.
Other causes of cerebral infarction.
| Young patients (n = 23) | Old patients (n = 10) | P | |
|---|---|---|---|
| Cervical artery dissection | 18 | 7 | <.001 |
| Giant aneurysm MCA | 1 | 0 | .001 |
| Pseudoaneurysm ICA | 1 | 0 | .001 |
| Moya moya | 1 | 0 | .001 |
| Prothrombotic disorder | 1 | 1 | .03 |
| Pulmonary shunt | 1 | 0 | .001 |
| Migraine | 0 | 1 | .76 |
| CADASIL | 0 | 1 | .76 |
MCA, middle cerebral artery; ICA, internal carotid artery.
The frequency of atrial fibrillation on ECG on admittance was low among young patients compared to old patients: 2.4% versus 17.0%. Likewise the frequency of atrial fibrillation disclosed on Holter monitoring was low among young patients: 1.8% versus 17.7% (Table 5).
Based on MRI findings, there were no differences concerning location of cerebral infarction. Fewer young patients showed leukoaraiosis (7.8% versus 50.4%) or had sequels after old infarctions on MRI (10% versus 21.3%) (Table 6).
Table 6.
MRI findings among young and old patients with cerebral infarction.
| Young patients | Old patients | P | |
|---|---|---|---|
| MRI | 89 (89) | 848 (76.0) | .003 |
| DWI positive | 84 (93.3) | 815 (96.7) | .13 |
| Anterior circulation | 68 (68) | 812 (72.7) | .35 |
| Posterior circulation | 30 (30) | 297 (26.6) | .48 |
| Middle cerebral artery | 66 (66) | 790 (70.7) | .36 |
| Anterior cerebral artery | 3 (3) | 37 (3.3) | 1.00 |
| Occipital | 8 (8) | 102 (9.1) | .86 |
| Thalamus | 3 (3) | 79 (7.1) | .15 |
| Mesencephalon | 3 (3) | 20 (1.8) | .43 |
| Pons | 2 (2) | 64 (5.7) | .16 |
| Medulla oblongata | 5 (5) | 24 (2.2) | .08 |
| Cerebellum | 11 (11) | 87 (7.8) | .25 |
| More than one artery domain | 6 (6) | 57 (5.1) | .54 |
| Anterior and posterior circulation | 2 (2) | 35 (3.1) | .83 |
| Bilateral middle cerebral arteries | 4 (4) | 22 (2) | .19 |
| Leukoaraiosis (MRI) | 7 (7.8) | 424 (50.4) | <.001 |
| Old infarctions (MRI)* | 10 (10) | 238 (21.3) | .006 |
| Embolic infarction (MRI) | 66 (79) | 594 (73) | .30 |
| Lacunar infarction (MRI) | 18 (21) | 223 (27) | .25 |
Data are expressed as mean or n (%).
MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging.
*Including both silent and symptomatic infarctions.
4. Discussion
The proportion of males was larger among the young patients than among the old patients. The proportion of males was also higher compared to other studies of cerebral infarction among young adults [7, 12]. Accumulation of traditional risk factors probably starts earlier in males than in females. Women have a longer life expectancy, which may play a role for the relatively larger proportion of female stroke patients in the older group. On the other hand, it is possible that a change in risk factors or life style has reduced the frequency of stroke among young females in recent years. Smoking has decreased among young women [13], and there has been a change regarding the use of oral contraceptives [14]. Another possible reason is better diagnostic methods of cerebral infarction because of high use of DWI. Psychogenic neurological symptoms are, for example, more frequent among females [15, 16] and may sometimes be mistaken for stroke but are easily distinguishable by DWI. Other studies showed migraine as a cause of stroke in up to 20% in the early 1990s [17], while newer studies find this in only few patients [4, 7, 18–21]. Complex migraine might have been misdiagnosed as cerebral infarction in the pre-DWI era. It is unlikely that this mistake was performed in this study because there was no difference regarding the frequency of migraine among young and old patients. The diagnosis of migraine was based on an interview by a neurologist during the hospital stay strengthening our findings. Thus, our result indicates that migraine is not particularly related to cerebral infarction among young patients compared to old patients.
Most traditional risk factors were less frequent among young patients. However, the fact of smoking made an exception. It has previously been shown that smoking is more frequent among young patients with cerebral infarction compared to matched controls [6]. In our study, the proportion of current smoking was clearly higher among the young compared to the old, and the proportion of past smoking was lower in the young patients group. The frequency of diabetes mellitus did not differ between young and old ischemic stroke patients.
Large-artery atherosclerosis was a rare cause of cerebral infarction among the young patients. Its frequency was also lower than among young patients with cerebral infarction in previous studies [6, 7]. This may indicate that symptomatic atherosclerosis has decreased among young people in recent years.
There was no difference concerning small vessel disease among young and old patients, and the frequency was similar to the findings in other studies of cerebral infarction among young adults [6, 7]. This is perhaps surprising because there is much uncertainty regarding the pathophysiological mechanisms of lacunar infarctions [22–24].
The frequency of cardiac embolism was similar between young and old patients (Table 2), and the proportion of cardiac embolism in the young is in line with other findings [3, 7, 19, 20, 25]. However, the specific cardiac sources differed between young and old patients. Atrial fibrillation was the dominating cardiac source among old patients but infrequent among young adults. In young adults the dominating heart disorders were patent foramen ovale with and without atrial septal aneurysm, followed by mechanical heart valves. This matches with the findings in other studies [7, 19], but mechanical heart valves were more frequently found as the cause of infarction in our study.
The proportion of other causes did not differ from most investigations [3, 4, 6, 7, 18, 21, 26]. Cervical artery dissection was with 18% the most common other cause among the young patients. Dissections were mostly located in unilateral ICA, less frequently in unilateral VA, and in a few cases in bilateral ICA.
Neither proportion of patients with unknown etiology was different from other studies, which is 31–62% in young patients [3, 6, 20, 27] and 35% in stroke patients overall in this category [26].
The distribution of infarctions in the anterior and posterior circulation was similar between young and old patients. The frequency of posterior circulation infarction was lower than in some other studies including young patients [7, 12]. We believe that this reflects better diagnostic precision in this study because most patients underwent DWI. Frequent MRI may also explain that we found a higher frequency of leukoaraiosis in old patients compared to recent studies [7, 12]. In our study, 7.8% among the young versus 50.4% among the old patients had leukoaraiosis. Old infarctions on MRI were found in 10% of the young patients versus 21.3% of the old ones. Multiple infarctions were common but less frequently seen in our study compared to recent publications [7, 12], and there was no difference between young and old patients.
There was no difference with respect to severity of neurological deficits on admittance between young and old patients. There was also small difference in the one-week outcome or mortality at day 7. Only Barthel Index was significantly higher among young patients whereas modified Rankin score or NIHSS score did not differ, neither was there any difference concerning the one-week improvement among young and old patients on multivariate analyses. This may indicate that young adults in our investigation do not tackle cerebral ischemia better than old patients concerning short-term outcome, which is in contrast to recent observation made by a Swiss group [28]. Differences in methodology (e.g., stroke unit cohort versus population-based study) may account for this discrepancy. However, subanalyses suggested that patients >80 years may experience less improvement than patients <80 years (analysis not shown).
This is one of the largest studies making a hospital-based direct comparison between ischemic stroke patients <50 years and ≥50 years admitted to a single centre, which we consider to be one of its strengths. All patients underwent investigations and treatment according to one common protocol. Another strength was the frequent use of MRI which promotes high diagnostic precision. However, there are some limitations; using the Baltimore-Washington Cooperative Young Stroke Study Criteria may complicate comparison with other studies using other criteria such as the WHO criteria. However, specificity is high in our study due to the frequent use of MRI. As described in Section 2, certain risk factors were registered as present when diagnosed before stroke onset. We might have missed some patients with untreated hypertension, atrial fibrillation and diabetes here, especially in the young patient group. We did not register outcome at 3 months, which gives an incomplete impression about the patients' outcome in the different groups. Young patients may improve more in long-term outcome compared to old patients. Although investigations were thorough in most patients, not all patients underwent complete workup. We might have missed few patients with, for example, atrial fibrillation or carotid stenosis due to that fact.
In conclusion, there are important differences between young and old patients with respect to risk factors, etiology, and distribution of gender. However, severity of stroke on admittance and short-term outcome is similar among young and old patients.
References
- 1.Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation collaborative study of cardiovascular disease and steroid hormone contraception. British Medical Journal. 1999;318(7175):13–18. doi: 10.1136/bmj.318.7175.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevan H, Sharma K, Bradley W. Stroke in young adults. Stroke. 1990;21(3):382–386. doi: 10.1161/01.str.21.3.382. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr., Kappelle LJ, Biller J, et al. Ischemic stroke in young adults: experience in 329 patients enrolled in the Iowa Registry of Stroke in young adults. Archives of Neurology. 1995;52(5):491–495. doi: 10.1001/archneur.1995.00540290081021. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen B, Malm J, Carlberg B, et al. Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in Northern Sweden. Stroke. 1997;28(9):1702–1709. doi: 10.1161/01.str.28.9.1702. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Butler MJ, Biller J, Toffol GJ. Nonhemorrhagic cerebral infarction in young adults. Archives of Neurology. 1986;43(8):793–796. doi: 10.1001/archneur.1986.00520080041017. [DOI] [PubMed] [Google Scholar]
- 6.Naess H, Nyland HI, Thomassen L, Aarseth J, Myhr KM. Etiology of and risk factors for cerebral infarction in young adults in western Norway: a population-based case-control study. European Journal of Neurology. 2004;11(1):25–30. doi: 10.1046/j.1351-5101.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- 7.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke the Helsinki young stroke registry. Stroke. 2009;40(4):1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 8.Cerrato P, Grasso M, Imperiale D, et al. Stroke in young patients: etiopathogenesis and risk factors in different age classes. Cerebrovascular Diseases. 2004;18(2):154–159. doi: 10.1159/000079735. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CJ, Kittner SJ, McCarter RJ, et al. Interrater reliability of an etiologic classification of ischemic stroke. Stroke. 1995;26(1):46–51. doi: 10.1161/01.str.26.1.46. [DOI] [PubMed] [Google Scholar]
- 10.Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Sandercock PAG, Warlow CP, Price SM. Incidence of stroke in Oxfordshire: first year’s experience of a community stroke register. Oxfordshire community stroke project. British Medical Journal. 1983;287(6394):713–717. doi: 10.1136/bmj.287.6394.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naess H, Nyland HI, Thomassen L, Aarseth J, Nyland G, Myhr KM. Incidence and short-term outcome of cerebral infarction in young adults in Western Norway. Stroke. 2002;33(8):2105–2108. doi: 10.1161/01.str.0000023888.43488.10. [DOI] [PubMed] [Google Scholar]
- 13.SSB. Nordmenns røykevaner. 2010, http://www.ssb.no/emner/03/01/royk/index.html.
- 14.Schwartz SM, Siscovick DS, Longstreth WT, et al. Use of low-dose oral contraceptives and stroke in young women. Annals of Internal Medicine. 1997;127(8 I):596–603. doi: 10.7326/0003-4819-127-8_part_1-199710150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosomatic Medicine. 1998;60(2):150–155. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Reuber M, Howlett S, Khan A, Grünewald RA. Non-epileptic seizures and other functional neurological symptoms: predisposing, precipitating, and perpetuating factors. Psychosomatics. 2007;48(3):230–238. doi: 10.1176/appi.psy.48.3.230. [DOI] [PubMed] [Google Scholar]
- 17.Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurologic Clinics. 1992;10(1):113–124. [PubMed] [Google Scholar]
- 18.Nedeltchev K, Der Maur TA, Georgiadis D, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(2):191–195. doi: 10.1136/jnnp.2004.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TH, Hsu WC, Chen CJ, Chen ST. Etiologic study of young ischemic stroke in Taiwan. Stroke. 2002;33(8):1950–1955. doi: 10.1161/01.str.0000021409.16314.16. [DOI] [PubMed] [Google Scholar]
- 20.Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington cooperative young stroke study. Neurology. 1998;50(4):890–894. doi: 10.1212/wnl.50.4.890. [DOI] [PubMed] [Google Scholar]
- 21.Rasura M, Spalloni A, Ferrari M, et al. A case series of young stroke in Rome. European Journal of Neurology. 2006;13(2):146–152. doi: 10.1111/j.1468-1331.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- 22.Futrell N. Lacumar infarction: embolism is the key. Stroke. 2004;35(7):1778–1779. doi: 10.1161/01.STR.0000131930.41057.48. [DOI] [PubMed] [Google Scholar]
- 23.Norrving B. Lacunar infarction: embolism is the key: against. Stroke. 2004;35(7):1779–1780. doi: 10.1161/01.STR.0000131931.84333.c0. [DOI] [PubMed] [Google Scholar]
- 24.Davis SM, Donnan GA. Why lacunar syndromes are different and important. Stroke. 2004;35(7):1780–1781. doi: 10.1161/01.STR.0000131929.98486.54. [DOI] [PubMed] [Google Scholar]
- 25.Varona JF, Guerra JM, Bermejo F, Molina JA, Gomez De La Cámara A. Causes of ischemic stroke in young adults, and evolution of the etiological diagnosis over the long term. European Neurology. 2007;57(4):212–218. doi: 10.1159/000099161. [DOI] [PubMed] [Google Scholar]
- 26.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32(12):2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 27.Leys D, Bandu L, Hénon H, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002;59(1):26–33. doi: 10.1212/wnl.59.1.26. [DOI] [PubMed] [Google Scholar]
- 28.Lyrer PA, Fluri F, Gostynski M, et al. Stroke severity, its correlates and impact on thrombolysis in a population-based study. European Neurology. 2009;62(4):231–236. doi: 10.1159/000232232. [DOI] [PubMed] [Google Scholar]