Abstract
Hemorrhage and hemorrhagic shock instigate intestinal damage and inflammation. Multiple components of the innate immune response, including complement and neutrophil infiltration, are implicated in this pathology. To investigate the interaction of complement activation and other components of the innate immune response during hemorrhage, we treated mice post-hemorrhage with CR2-fH, a targeted inhibitor of the alternative complement pathway and assessed intestinal damage and inflammation 2 h after hemorrhage. In wildtype mice, CR2-fH attenuated hemorrhage-induced, mid-jejunal damage and inflammation as determined by decreased mucosal damage, macrophage infiltration, LTB4, IL-12p40, and TNF-α production. The critical nature of intestinal macrophage infiltration and activation in the response to hemorrhage was further determined using mice pre-treated with clodronate containing liposomes. The absence of either macrophages or IL-12p70 attenuated intestinal damage. These data suggest that complement activation and macrophage infiltration with IL-12p70 production are critical to hemorrhage induced mid-jejunal damage and inflammation.
Keywords: Inflammation, mouse, intestine, cytokines, complement inhibitor
INTRODUCTION
Hemorrhage decreases blood flow to the intestine and is followed by rapid intestinal damage and inflammation, which may initiate a systemic inflammatory response (1-2). The intestinal inflammatory response involves multiple components of the innate immune system including neutrophils, complement and cytokines (3-5). Hemorrhage-induced inflammation also includes activation of macrophages within the liver, spleen and lungs (6-8). However, the specific complement activation pathway and the early intestinal effects on macrophage infiltration and activation in response to hemorrhage are not well defined.
Comprised of more than thirty proteins in the blood, complement protects the body by removing pathogenic microorganisms. Initiated by multiple pathways, complement activation generates C3 opsonins, chemotactic peptides and the cytolytic terminal membrane attack complex. Antibody or lectin deposition on surface membranes initiates the classical and lectin pathways, respectively. The spontaneously activated, alternative pathway amplifies the classical and lectin pathways. However, excessive complement activation increases susceptibility to infections (9) and the amplification induced by the alternative pathway increases pathology in multiple disease states (10-11). Treatment with complement inhibitors, C5a receptor antagonist (3) or cobra venom factor (12), attenuated hemorrhage-induced intestinal damage without identifying the specific initiation pathway. It is likely that the alternative pathway either directly or by amplification contributes to hemorrhage-induced tissue damage. During excessive or inappropriate complement activation, natural inhibitors protect host cells and tissues. Factor H (fH) binds C3b, preventing or dissociating the alternative C3 convertase and the subsequent amplification loop. By inhibiting amplification of the classical and lectin pathways, fH is a logical therapeutic for excessive complement activation. In other disease models, complement receptor 2 (CR2) targeted fH significantly enhanced the effectiveness of fH by targeting the inhibitor to sites of complement activation (13).
Hemorrhage induces neutrophil and macrophage infiltration into the hypoxic intestine (12, 14). Multiple studies indicated that a neutrophil response with oxidative burst is critical to hemorrhage-induced damage (14-15). Hemorrhage also activates Kupffer cells in the liver and macrophages in the spleen (6-7) to release cytokines. A recent study indicated that intestinal tissues release the macrophage inflammatory cytokines, tumor necrosis factor –a (TNF), IL-6 and nitric oxide in response to hemorrhage (12). In addition, inhibition of IL-6 (16) or TNF (17-18) attenuated hemorrhage-induced inflammation. However, a role of macrophage-produced IL-12 in the intestinal response to hemorrhage is not well characterized. The proinflammatory IL-12p70 consists of 2 subunits, IL-12p40 and IL-12p35. IL-12p40 is also a subunit of IL-23, which stimulates Th17 cells. In addition, IL-12p40 homodimers directly antagonize both IL-12p70 and IL-23 (19). Therefore, hemorrhage-induced macrophage infiltration may control the inflammatory process through the production of specific IL-12 components.
We hypothesized that alternative complement activation interacts with macrophage secreted IL-12 and all three innate immune components are critical for hemorrhage-induced inflammation. We show that administering the targeted complement inhibitor, CR2-fH, to mice following hemorrhage significantly decreases intestinal damage and inflammation by reducing macrophage infiltration and IL-12p40 production. Furthermore, macrophage depletion with clodronate, IL-12p40 or IL-12p35 depletion attenuated injury and inflammation. In contrast, IL-23p19 depletion did not attenuate injury. Thus inhibition of the alternative complement activation pathway attenuates macrophage infiltration and IL-12p70 production during the hemorrhage-induced inflammatory response and provides multiple therapeutic targets within the innate immune response.
Materials and Methods
Mice
C57Bl/6J male mice (6-8 wks old) were bred and maintained in the Division of Biology at Kansas State University. IL-12p40-/- and IL-12p35-/- mice (N11F32) were obtained from Jackson Laboratories and rested 1 wk prior to use in experiments. All mice were housed in a 12-hour light-to-dark, temperature-controlled room and allowed food and water ad libitum. All research was approved by the Institutional Animal Care and Use Committee and conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations concerning animals.
Hemorrhage Protocol
After anesthetizing with ketamine (16 mg/kg) and xylazine (8 mg/kg), a drop of 0.5% proparacaine hydrochloride ophthalmic solution was applied to the appropriate eye. Mice subjected to hemorrhage sustained removal of 25% total blood volume via the retro-orbital sinus as described previously (3, 20). Blood volume removed was determined by weight (grams) using the following equation (~25% = wt × 0.02) (3, 20) and completed within five minutes. Sham-treated mice were subjected to similar procedures with no blood removal. Body temperature of animals was maintained at 37° C using a water-circulating heating pad and all procedures were performed with the animals breathing spontaneously. Two hours after hemorrhage, mice were euthanized and sera and tissue collected. Mid-jejunal sections (2cm), approximately 10 cm distal to the gastroduodenal junction, were collected for histology and subsequent analyses. Additional mice underwent the same procedures as above with i.v. administration of CR2-fH (17.5uM) five minutes after bleeding. CR2-fH was produced as described previously (13). Some mice received neutralizing anti-IL-12p40 (R&D Systems) or anti-IL-23p19 (R&D Systems) mAb (mg/kg) i.p. at 1 hr prior to hemorrhage.
Histology & Injury Score
After fixation in 10% buffered formalin, paraffin embedded tissue sections were cut transversely (8μm) and H&E stained for scoring mucosal injury. Using a sixtiered scale as described previously (21), the average damage score of mid-jejunum intestine (75-150 villi) was determined after grading each villus from 0-6 with the following categories: Normal villi were assigned a score of zero; villi with tip distortion were assigned a score 1; a score of 2 was assigned when Guggenheims’ spaces were present; villi with slight disruption of the epithelial cells were assigned a score of 3; a score of 4 was assigned to villi with exposed but intact lamina propria; a score of 5 was assigned when the lamina propria was exuding; last, villi that displayed hemorrhage or were denuded were assigned a score of 6.
Ex vivo secretions
Ex vivo intestinal supernatants were generated as described previously (3, 21) and used to determine the secretions released in a 20 min period. Briefly, a 2 cm mid-jejunal section was minced, washed, resuspended in 37°C oxygenated Tyrode's buffer (Sigma-Aldrich) and incubated for 20 min at 37°C. Following incubation, the supernatants and tissues were collected and stored at -80°C until assayed. After overnight digestion in 0.1M NaOH at 37°C, protein content in the intestinal tissue was determined by BCA protein assay (Pierce). Cytokine concentrations in the intestinal supernatants were determined with a Milliplex MAP kit (Millipore) following the manufacturer's instructions and analyzed using xPONENT 3.1 and Analyst (Millipore). Since liposomes interfered with the Milliplex assay, IL-12p40 was also determined by ELISA (BioLegend). Leukotriene B4 (LTB4) concentrations in the intestinal supernatants were measured using a commercially available enzyme immunoassay kit (Cayman Chemicals). All concentrations in the intestinal supernatants were normalized to the total intestinal protein content and reported as pg/mg intestinal tissue. Sera C5a concentrations were determined by capture ELISA (BD Biosciences).
Immunohistochemistry
After Sham or hemorrhage treatment, a 2 cm mid-jejunal section was frozen in O.C.T. freezing medium and stored at -80°C until used. Intestinal cryosections, (8μm) were fixed in cold acetone and non-specific binding was blocked using 10% donkey serum in PBS. Tissues were stained for C3 deposition using a rat-anti-mouse C3 antibody (Hycult Biotechnologies) followed by an appropriate secondary antibody (Jackson Immunoresearch) or for F4/80 using directly conjugated rat-anti mouse F4/80 (eBioscience). Serial sections stained with isotype control antibodies were used as background. Slides were examined by a blinded observer by fluorescent microscopy using a Nikon 80i fluorescent microscope and images acquired using a CoolSnapCf camera (Photometrics) and MetaVue Imaging software (Molecular Devices).
Macrophage depletion
Infiltrating macrophages were depleted using clodronate liposomes as described previously (22-23). Briefly, mice were injected i.v. with 200 μl clodronate or PBS containing liposomes on days 0, 1, 3, 5 with hemorrhage occurring on day 5 or 6. Liposomes were produced using phosphatidylcholine (Lipoid GmbH, Ludwigshafen,Germany) and cholesterol (Sigma, St. Louis, MO). Cl2MDP (clodronate) was a gift of Roche Diagnostics GmbH, Mannheim, Germany.
Statistical Analysis
Data are presented as means ± SEM. Differences between treatment groups were considered significant if p ≤ 0.05 as determined by one-way ANOVA with a Newman Keuls post-hoc test.
RESULTS
CR2-fH attenuates hemorrhage-induced mucosal damage and inflammation
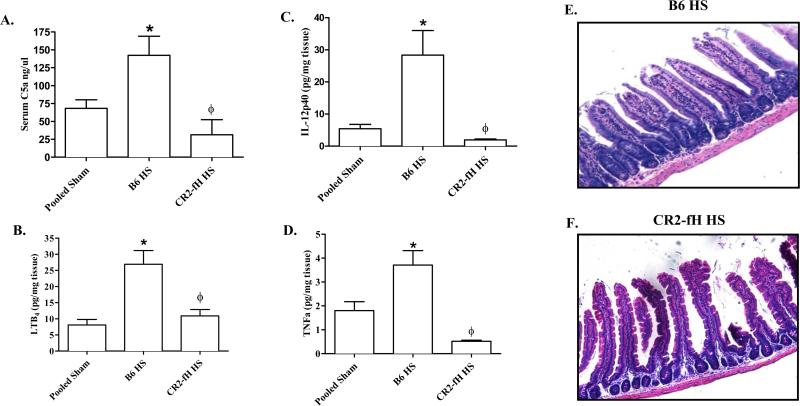
We verified the ability of CR2-fH to inhibit complement activation by measuring serum C5a in wildtype C57Bl/6 and CR2-fH treated mice. As no significant difference was observed between Shams from different treatment groups or mouse strains, these data are shown as pooled Shams in all graphs. Similar to previous studies (3), serum from hemorrhaged C57Bl/6 mice contained significantly more C5a than Sham-treated mice (Fig. 1A). In contrast, C5a was not significantly elevated in the serum of CR2-fH treated mice with or without hemorrhage (Fig. 1A), indicating that CR2-fH attenuated hemorrhage-induced complement activation. Importantly, administration of CR2-fH significantly attenuated intestinal damage in response to hemorrhage (Table 1 and Fig. 1E-F). CR2-fH treatment also attenuated the intestinal inflammatory response. Intestinal LTB4 production was significantly elevated in hemorrhage-treated mice only in the absence of CR2- fH treatment (Fig. 1B). In addition, at 2 h post hemorrhage, C57Bl/6 mice expressed significantly elevated levels of intestinal IL-12p40 and TNF-α, and CR2-fH treatment reduced these cytokine secretions to the level of Sham treated mice (Fig. 1C-D). These data indicate that in a fixed-volume mouse model of hemorrhage, inhibition of the alternative complement pathway attenuates the early LTB4 and cytokine response.
FIGURE 1. Targeted alternative pathway inhibitor attenuates hemorrhage induced complement activation, intestinal injury andinflammation.
C57Bl/6 (B6) mice subjected to Sham or hemorrhage (HS) in the presence or absence of CR2-fH. Serum C5a (A) and ex vivo intestinal LTB4 (B), IL-12p40 (C) and TNF-α (D) concentrations were determined. Representative photomicrographs (original magnification 100X) of H&E stained mid-jejunal tissue sections from HS-treated, C57Bl/6 mice with or without administration of CR2-fH (E-F). Each bar is the average ± SEM of 4-10 mice per group. (*) Indicates p ≤ 0.05 compared to pooled Sham treatment and (ϕ) indicates p ≤ 0.05 compared to C57Bl/6 HS mice.
Table 1.
Intestinal injury scores
| Treatment* | Injury Score ± SEM† |
|---|---|
| Pooled sham | 0.47 ± 0.05‡ |
| C57B1/6 HS | 1.99 ± 0.22 |
| C57B1/6 + CR2-fH HS | 0.58 ± 0.12‡ |
| IL-12p40–/– HS | 0.60 ± 0.11‡ |
| IL-12p35–/– HS | 0.62 ± 0.05‡ |
| C57Bl/6 + IL-12p40 Ab HS | 0.53 ± 0.08‡ |
| C57Bl/6 + IL-23p19 Ab HS | 1.99 ± 0.42 |
| PBS liposomes HS | 1.50 ± 0.16 |
| Clodronate liposomes HS | 0.53 ± 0.14§ |
Mice were subjected to sham or HS with or without additional treatment as indicated. After killing at 2 h after hemorrhage, midjejunal sections were removed, formalin fixed, hematoxylin-eosin stained, and scored for mucosal injury.
Data are expressed as mean injury score ± SEM from 4–10 animals per group.
P < 0.05 relative to B6 HS.
P < 0.05 relative to PBS liposomes HS.
Macrophage depletion attenuates hemorrhage-induced intestinal damage and inflammation
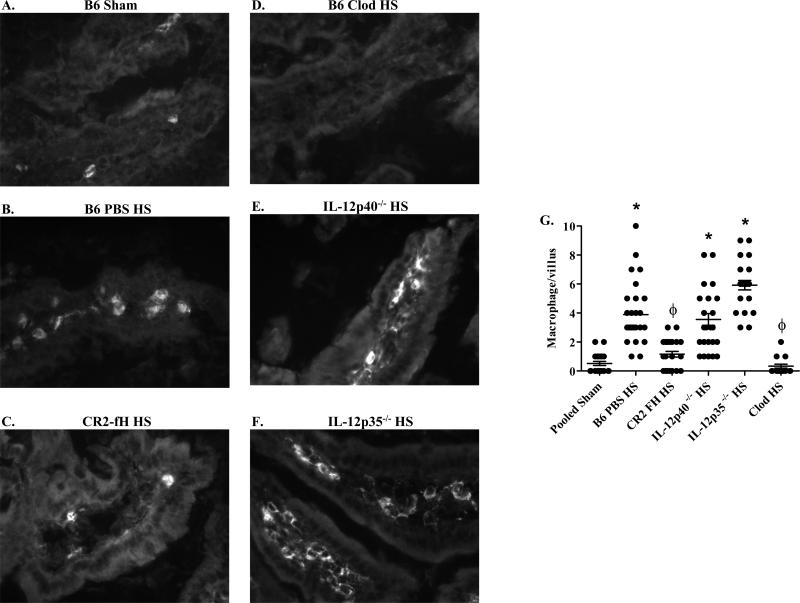
As the above cytokines may be produced by activated macrophages or epithelial cells, we hypothesized that hemorrhage induces an infiltration of F4/80+ macrophages in intestinal sections. Immunohistochemistry showed few, if any macrophages in the intestinal villi of Sham-treated mice (Fig. 2A, G). Intestinal villi from hemorrhage-treated mice contained significantly more F4/80+ cells (Fig. 2B, G). Similar to its effect on cytokine levels, CR2-fH significantly reduced the number of F4/80+ macrophages infiltrating the villi in response to hemorrhage (Fig. 2C, G).
FIGURE 2. Macrophages infiltrate into the intestinal tissue following hemorrhage.
Mid-jejunal tissue sections from C57Bl/6 (B6) (A-D), IL-12p40-/- (E) or IL-12p35-/- (F) mice after Sham (A) or hemorrhage (HS) (B-F) treated with liposomes containing PBS (B), CR2-fH (C) or liposomes containing clodronate (Clod) (D) were stained for F4/80. Photomicrographs original magnification of 400X (A-C) or 200X (D-F) are representative of three individual experiments with 5-7 photos per treatment group in each experiment. The number of macrophages per villus was quantitated from 3-4 mice per treatment group with 20-30 villi per mouse (G). (*) Indicates p ≤ 0.05 compared to respective pooled Sham treatment; (ϕ) indicates p ≤ 0.05 compared to C57Bl/6 HS-treated animals.
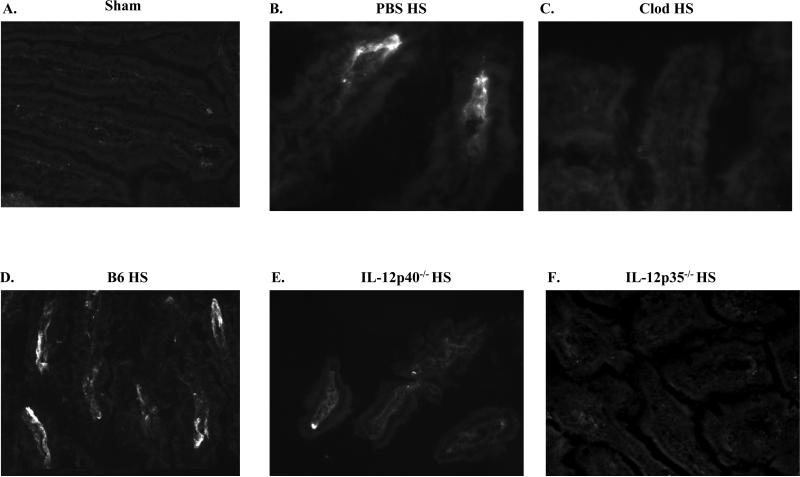
To determine if macrophages are required in hemorrhage-induced intestinal injury, we subjected mice to hemorrhage after depletion of macrophages with Cl2MDP containing liposomes. Immunohistochemistry confirmed macrophage depletion in Cl2MDP treated mice (Fig. 2D, G). As indicated in Table 1, macrophage depleted mice did not sustain hemorrhage-induced intestinal injury when compared to mice treated with PBS containing liposomes. Similar to untreated mice, in response to HS, mice treated with liposomes containing PBS produced significant quantities of LTB4 and IL-12p40 (3.2 ± 1.1 and 12 ± 1.11 pg/mg tissue, respectively). After treating with liposomes containing Cl2MDP, hemorrhage induced significantly lower concentrations of intestinal LTB4 and IL-12p40 (0.98 ± 0.13 and 4.6 ± 1.7 pg/mg tissue, respectively) compared to liposomes containing PBS. It was possible that macrophage depletion altered the mechanism of intestinal damage. Therefore, we examined C3 deposition in mice treated with liposomes containing PBS or Cl2MDP. Compared to Sham-treated mice, intestinal sections from mice pre-treated with PBS containing liposomes displayed more C3 deposition (Fig. 3A, B). In contrast, C3 deposition was not detected on the intestinal tissue of macrophage-depleted mice (Fig. 3C).
FIGURE 3. Macrophage or IL-12 depletion inhibits C3 deposition after hemorrhage.
Mid-jejunal tissue sections from C57Bl/6 (B6) (A-D), IL-12p40-/- (E) or IL-12p35-/- (F) mice after Sham (A) or hemorrhage (HS) (B-F) treated with liposomes containing PBS (B) or liposomes containing clodronate (Clod) (C) were stained for C3 deposition. Photomicrographs (original magnification of 200X) are representative of three individual experiments with 5-7 photos per treatment group in each experiment).
IL-12p35 RNA expression increases in response to hemorrhage
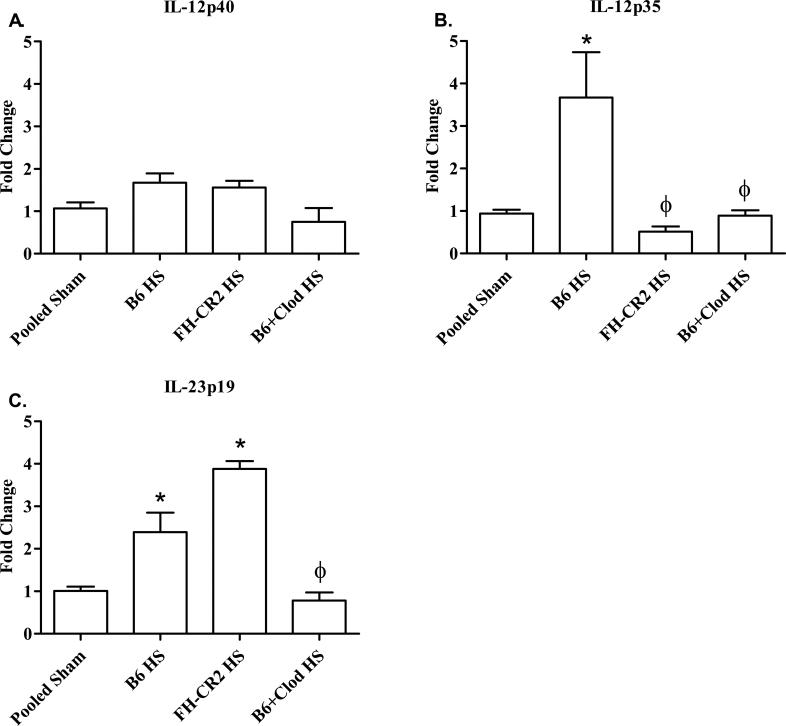
IL-12 family members, IL-12p70 and Il-23, play a role in other models of intestinal damage (24-27). Using real time PCR, we evaluated IL-12p40, IL-12p35 and IL-23p19 mRNA to determine the critical family members. As indicated in Figure 4A, IL-12p40 mRNA was maintained within all treatment groups. In contrast, IL-12p35 was significantly elevated in response to HS in wildtype mice and in wildtype mice after treatment with PBS containing liposomes (Fig. 4B). However, treatment with fH-CR2 or macrophage depletion eliminated the HS increase in IL-12p35 RNA (Fig. 4B). Although IL-23p19 RNA expression increased with HS, complement inhibition only altered Il-12p35 RNA expression not production of IL-23p19 RNA expression (Fig. 4C). Clodronate treatment eliminated hemorrhage-induced IL-12p40, IL-12p35 and IL-23p19 RNA expression (Fig. 4). These data suggest that HS induces macrophages to produce IL-12p70.
FIGURE 4. Complement inhibition or macrophage depletion attenuates hemorrhage induced IL-12p35 RNA.
C57Bl/6 (B6) mice were subjected to Sham or hemorrhage (HS) after inhibition of complement (FH-CR2) or macrophage depletion with liposomes containing Clodronate (Clod). Intestinal RNA transcripts from each treatment group were analyzed for IL-12p40, IL-12p35 and IL-23p19 by realtime RT-PCR. Each bar represents the average of 4-6 animals. (*) Indicates p ≤ 0.05 compared to pooled Sham treatment; (ϕ) indicates p ≤ 0.05 compared to C57Bl/6 HS-treated animals.
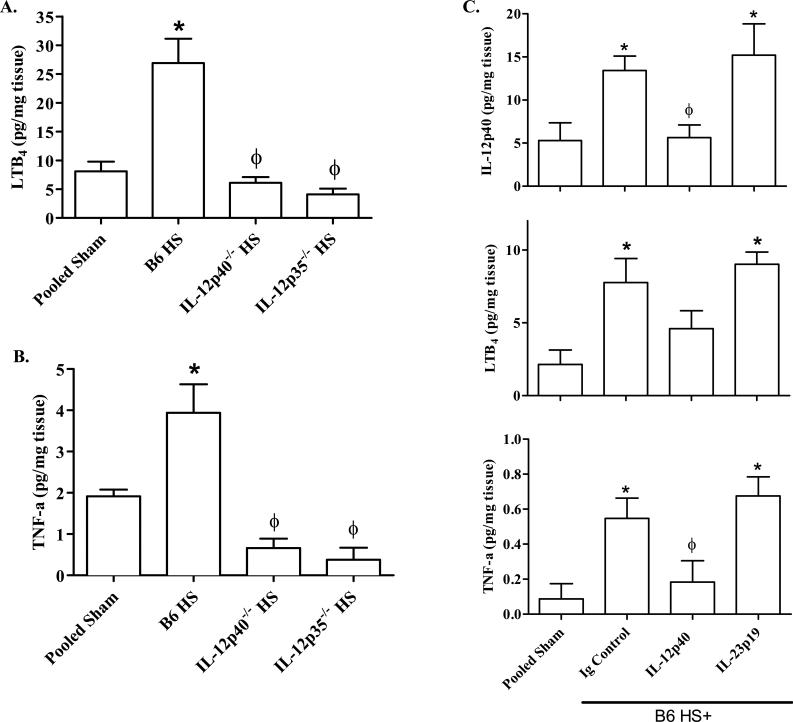
IL-12p70 is critical to hemorrhage-induced damage and inflammation
To establish if IL-12p70 or IL-23 play key roles in hemorrhage-induced intestinal injury and inflammation, we analyzed mucosal damage and complement activation in response to Sham or hemorrhage treatment in IL-12p40-/- or IL-12p35-/- mice. Hemorrhage induced significantly less mid-jejunal damage in IL-12p40-/- or IL-12p35-/- mice compared to C57Bl/6 mice (Table 1). The integrity of the villi and intestinal epithelium were maintained in both strains of IL-12 deficient mice at 2 h post-hemorrhage (Data not shown). In response to hemorrhage, intestinal sections from IL-12p40-/- mice contained significantly fewer C3 deposits compared to wildtype mice (Fig. 3D-E). Similar to Sham treated mice, no C3 deposits were observed on mid-jejunal tissues from hemorrhaged IL-12p35-/- mice. Similarly, the absence of IL-12p40 significantly attenuated hemorrhage-induced serum C5a compared to C57Bl/6 mice (138 ± 13 ng/ml and 194 ± 25 ng/ml, respectively). However, the hemorrhage-induced C5a levels in IL-12p40-/- mice were significantly higher than Sham treated mice (76 ± 6 ng/ml). Thus, IL-12p40-/- and Il-12p35-/-mice are protected from intestinal damage with lower levels of complement activation than C57Bl/6 mice. Despite increased macrophage infiltration after hemorrhage (Fig 2E-F), IL-12p40-/- and IL-12p35-/- mice produced significantly less intestinal LTB4 and TNF-α than wildtype mice (Fig. 5A-B).
FIGURE 5. IL-12p40 or IL-12p35 but not IL-23p19 deficiency attenuates inflammatory cytokine production following hemorrhage.
Ex vivo intestinal supernatants were used to determine LTB4 (A), and TNF-α (B) produced by C57Bl/6 (B6), IL-12p40-/- or IL-12p35-/- mice after Sham or hemorrhage (HS) treatment. One hour after injection of anti-IL-12p40, IL-23p19 or isotype (Ig) control antibodies, wildtype (B6) mice were subjected to Sham or hemorrhage (HS) treatment and intestinal cytokines determined (C). All concentrations were normalized to tissue protein content and expressed as pg per mg of intestinal tissue. Each bar represents the average of 4-10 animals. (*) Indicates p ≤ 0.05 compared to pooled Sham treatment; (ϕ) indicates p ≤ 0.05 compared to C57Bl/6 HS-treated animals.
To confirm that IL-12p70 and not IL-23 is a critical intestinal cytokine produced in response to HS, we administered either IL-12p40 or IL-23p19 neutralizing antibodies or isotype control antibodies to additional wildtype mice. As indicated in Table 1 and Fig. 5C, anti-mouse IL-12p40 mAb attenuated intestinal hemorrhage and inflammation similar to that of IL-12p40-/- or Il-12p35-/- mice. Mice receiving Ig control sustained mid-jejunal mucosal damage and inflammation similar to wildtype mice. In contrast, administration of even 100 times the neutralizing dose of anti-IL-23p19 specific antibodies did not attenuate intestinal damage, TNF or LTB4 production (Table 1 and Fig. 5C). Together these data suggest that macrophage production of IL-12p70 is critical for intestinal damage and inflammation.
Discussion
Although hypothesized to be the motor of systemic inflammation and subsequent mortality, intestinal damage and inflammation occurs rapidly (within 2 h) and repair occurs equally quickly (within 3-4 h) in the presence or absence of resuscitation (2, 12). Previous studies concluded that the hemorrhage-induced response requires complement activation without identifying the specific activation pathway (3, 20, 28-29). The present study indicates that inhibition of the alternative complement pathway with CR2-fH attenuates mid-jejunal mucosal damage and complement activation in a fixed-volume mouse model of hemorrhage. Complement inhibition also decreased the pro-inflammatory intestinal secretions TNF-α, IL-12p40 and LTB4. Furthermore, pre-treatment with Cl2MDP containing liposomes significantly reduced cytokine secretion and attenuated damage and inflammation, suggesting that hemorrhage induces macrophage secretion of TNF and IL-12. Finally, IL-12p40-/- and IL-12p35-/- sustained limited to no complement activation or hemorrhage-induced intestinal damage despite an intestinal macrophage infiltration. Together, these data suggest that the hemorrhage-induced inflammatory response requires complement activation, macrophage infiltration and IL-12 production.
Previous studies showed that systemic blockade of central complement components, C3, C5 or C5a, attenuated hemorrhage-induced intestinal damage (3, 30-31). However, each of these systemic inhibitors increases the risk of sepsis by blocking multiple complement activation pathways. CR2-fH binds tissue-bound C3 activation products and provides local but not systemic complement inhibition (13, 32). In addition, when administered early after hemorrhage, CR2-fH effectively prevented hemorrhage-induced tissue damage and inflammation. These results are similar to the ability of CR2-fH to attenuate tissue damage in models of collagen induced arthritis, complement-dependent macular degeneration and direct intestinal ischemia/reperfusion (13, 32). Treatment with CR2-fH also significantly decreased TNF-α mRNA in arthritic joints (32), suggesting a decrease in macrophage cytokine production. We extended the anti-inflammatory properties of CR2-fH to include significantly reducing macrophage infiltration and associated cytokines in response to hemorrhage. Although the specific mechanism is not known, the alternative complement activation pathway is critical for hemorrhage-induced intestinal damage and inflammation.
Within the intestinal mucosa, blood monocytes infiltrate into inflamed tissues to replenish mucosal macrophages (33). In response to hemorrhage, macrophage infiltration increased significantly within 2 h post-hemorrhage. These data correlate with intestinal production of macrophage chemotactic factors which are produced in response to hemorrhage (20). When macrophages were depleted with liposomes containing Cl2MDP, intestinal damage, IL-12 and LTB4 production were significantly reduced. Similar to these results in the intestine, depletion of Kupffer cells by gadolinium chloride significantly reduced hemorrhage-induced liver damage (6). Thus, hemorrhage appears to induce an early excessive innate response by the macrophages.
Macrophage cytokine production mediates the recruitment of innate immune cells and coordinates activation of adaptive immune responses. Multiple studies indicate that TNF-α and IL-6 increase in the serum within 2 h post hemorrhage (6-7, 34). However IL-12 is more complex. IL-12p70 is involved in the differentiation of Th cells to the pro-inflammatory Th1 subset with macrophages and dendritic cells as the primary sources of this cytokine. IL-12p40 homodimers appear to have regulatory effects and, as a subunit of IL-23, IL-12p40 may induce IL-17 (35). With limited complement activation, hemorrhage of IL-12p40-/- or IL-12p35-/- mice resulted in significantly less tissue damage and inflammation. In addition, hemorrhage induced significant damage in mice treated with IL-23p19 neutralizing antibodies. Thus, it appears that IL-12p70 is the critical family member in hemorrhage-induced intestinal damage.
In response to hemorrhage, the alternative complement activation pathway induces a macrophage infiltrate into the intestine and IL-12 secretion. These data indicate that all three components of the innate immune response are required for hemorrhage-mediated mid-jejunal damage. Thus, therapeutics targeting local alternative complement activation not only prevent complement mediated damage, but also attenuate macrophage activation and cytokine production.
Acknowledgments
This work was supported by NIH Grants AI061691, P20 RR017686 and RR016475 from the Institutional Development Award (IDeA) Program of the NCRR, HHMI Undergraduate Science Educational Grant and Mark Chapman Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, Carles M, Howard M, Pittet JF. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming SD, Phillips LM, Lambris JD, Tsokos GC. Complement component C5a mediates hemorrhage-induced intestinal damage. J Surg Res. 2008;150:196–203. doi: 10.1016/j.jss.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frink M, Hsieh YC, Hsieh CH, Pape HC, Choudhry MA, Schwacha MG, Chaudry IH. Keratinocyte-derived chemokine plays a critical role in the induction of systemic inflammation and tissue damage after trauma-hemorrhage. Shock. 2007;28:576–581. doi: 10.1097/shk.0b013e31814b8e0d. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Li Y, Vodovotz Y, Billiar TR, Wilson MA. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am. J. Physiol. 2006;290:L738–L746. doi: 10.1152/ajplung.00280.2005. [DOI] [PubMed] [Google Scholar]
- 6.Hildebrand F, Hubbard WJ, Choudhry MA, Frink M, Pape HC, Kunkel SL, Chaudry IH. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am. J. Pathol. 2006;169:784–794. doi: 10.2353/ajpath.2006.060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrand F, Thobe BM, Hubbard WJ, Choudhry MA, Pape HC, Chaudry IH. Effects of 17beta-estradiol and flutamide on splenic macrophages and splenocytes after trauma-hemorrhage. Cytokine. 2006;36:107–114. doi: 10.1016/j.cyto.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Jarrar D, Kuebler JF, Rue LW, 3rd, Matalon S, Wang P, Bland KI, Chaudry IH. Alveolar macrophage activation after trauma-hemorrhage and sepsis is dependent on NF-kappaB and MAPK/ERK mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L799–805. doi: 10.1152/ajplung.00465.2001. [DOI] [PubMed] [Google Scholar]
- 9.Younger JG, Sasaki N, Waite MD, Murray HN, Saleh EF, Ravage ZA, Hirschl RB, Ward PA, Till GO. Detrimental effects of complement activation in hemorrhagic shock. J. Appl. Physiol. 2001;90:441–446. doi: 10.1152/jappl.2001.90.2.441. [DOI] [PubMed] [Google Scholar]
- 10.Holers VM, Thurman JM. The alternative pathway of complement in disease: opportunities for therapeutic targeting. Mol. Immunol. 2004;41:147–152. doi: 10.1016/j.molimm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 12.Hylton DJ, Phillips LM, Hoffman SM, Fleming SD. Hemorrhage-induced intestinal damage is complement independent in Helicobacter-hepaticus infected mice. Shock. 2010 doi: 10.1097/SHK.0b013e3181dc077e. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J. Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol. Immunol. 2007;44:2625–2630. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol. 2004;286:G225–233. doi: 10.1152/ajpgi.00447.2002. [DOI] [PubMed] [Google Scholar]
- 16.Mees ST, Toellner S, Marx K, Faendrich F, Kallen KJ, Schroeder J, Haier J, Kahlke V. Inhibition of interleukin-6-transsignaling via gp130-Fc in hemorrhagic shock and sepsis. J Surg Res. 2009;157:235–242. doi: 10.1016/j.jss.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Ertel W, Morrison MH, Ayala A, Chaudry IH. Biological significance of elevated TNF levels: in vivo administration of monoclonal antibody against tnf following haemorrhage shock increases the capacity of macrophages to release TNF while restoring immunoresponsiveness. Cytokine. 1994;6:624–632. doi: 10.1016/1043-4666(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 18.Ertel W, Morrison MH, Ayala A, Perrin MM, Chaudry IH. Anti-TNF monoclonal antibodies prevent haemorrhage-induced suppression of Kupffer cell antigen presentation and MHC class II antigen expression. Immunology. 1991;74:290–297. [PMC free article] [PubMed] [Google Scholar]
- 19.Holscher C. The power of combinatorial immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases. Med Microbiol Immunol. 2004;193:1–17. doi: 10.1007/s00430-003-0186-x. [DOI] [PubMed] [Google Scholar]
- 20.Rajnik M, Salkowski CA, Thomas KE, Li YY, Rollwagen FM, Vogel SN. Induction of early inflammatory gene expression in a murine model of nonresuscitated, fixed-volume hemorrhage. Shock. 2002;17:322–328. doi: 10.1097/00024382-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J. Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 22.Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239–1243. doi: 10.1053/jhep.1996.v23.pm0008621159. [DOI] [PubMed] [Google Scholar]
- 23.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 24.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr. Res. 2002;51:733–739. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Neta R, Stiefel SM, Ali N. In lethally irradiated mice interleukin-12 protects bone marrow but sensitizes intestinal tract to damage from ionizing radiation. Ann N Y Acad Sci. 1995;762:274–280. doi: 10.1111/j.1749-6632.1995.tb32332.x. discussion 280-271. [DOI] [PubMed] [Google Scholar]
- 26.Edgerton C, Crispin JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, Juang YT, Tsokos GC. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, Akagawa KS, Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Complement inhbition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782–792. doi: 10.1067/msy.1998.91489. [DOI] [PubMed] [Google Scholar]
- 29.Heller T, Hennecke M, Baumann U, Gessner JE, zu Vilsendorf AM, Baensch M, Boulay F, Kola A, Klos A, Bautsch W, Kohl J. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J. Immunol. 1999;163:985–994. [PubMed] [Google Scholar]
- 30.Horstick G, Kempf T, Lauterbach M, Bhakdi S, Kopacz L, Heimann A, Malzahn M, Horstick M, Meyer J, Kempski O. C1-esterase-inhibitor treatment at early reperfusion of hemorrhagic shock reduces mesentry leukocyte adhesion and rolling. Microcirculation. 2001;8:427–433. doi: 10.1038/sj/mn/7800115. [DOI] [PubMed] [Google Scholar]
- 31.Peckham RM, Handrigan MT, Bentley TB, Falabella MJ, Chrovian AD, Stahl GL, Tsokos GC. C5-blocking antibody reduces fluid requirements and improves responsiveness to fluid infusion in hemorrhagic shock managed with hypotensive resuscitation. J. Appl. Physiol. 2007;102:673–680. doi: 10.1152/japplphysiol.00917.2006. [DOI] [PubMed] [Google Scholar]
- 32.Banda NK, Levitt B, Glogowska MJ, Thurman JM, Takahashi K, Stahl GL, Tomlinson S, Arend WP, Holers VM. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J. Immunol. 2009;183:5928–5937. doi: 10.4049/jimmunol.0901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CH, Hsu JT, Hsieh YC, Frink M, Raju R, Hubbard WJ, Bland KI, Chaudry IH. Suppression of activation and costimulatory signaling in splenic CD4+ T cells after trauma-hemorrhage reduces T-cell function: a mechanism of post-traumatic immune suppression. Am J Pathol. 2009;175:1504–1514. doi: 10.2353/ajpath.2009.081174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]