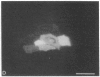
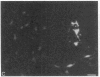
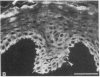
Abstract
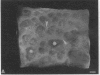
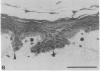
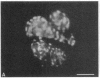
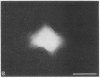
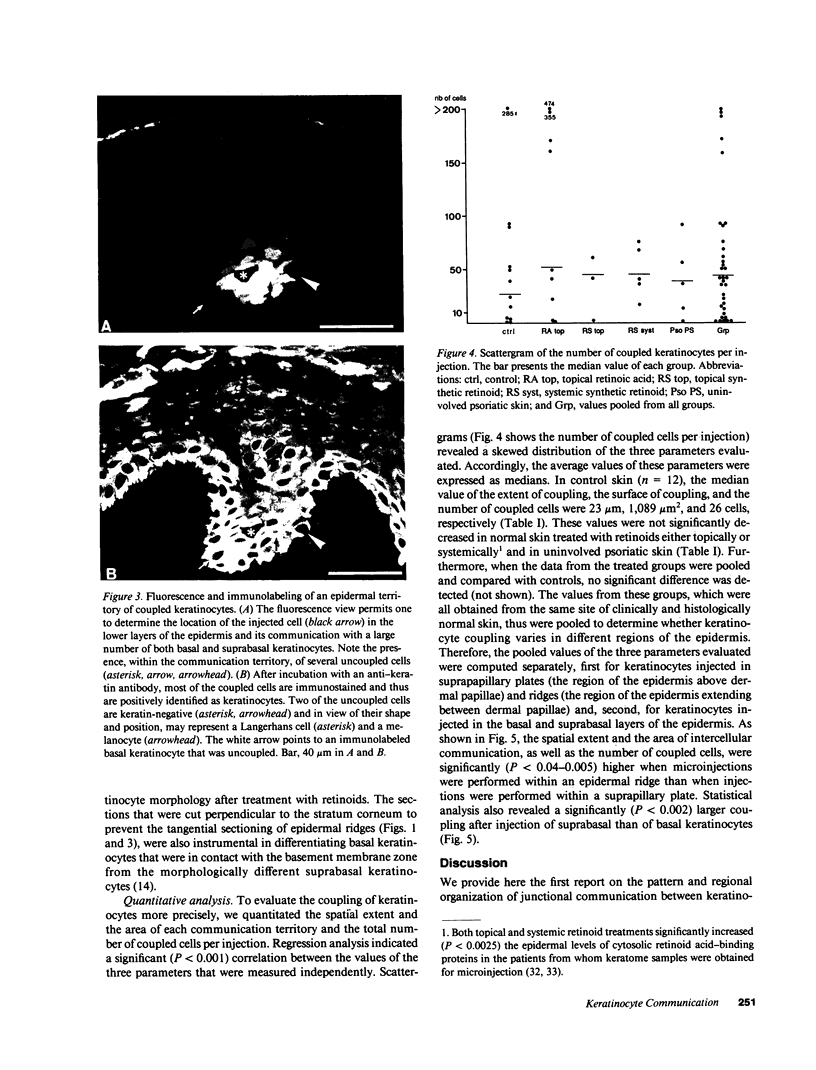
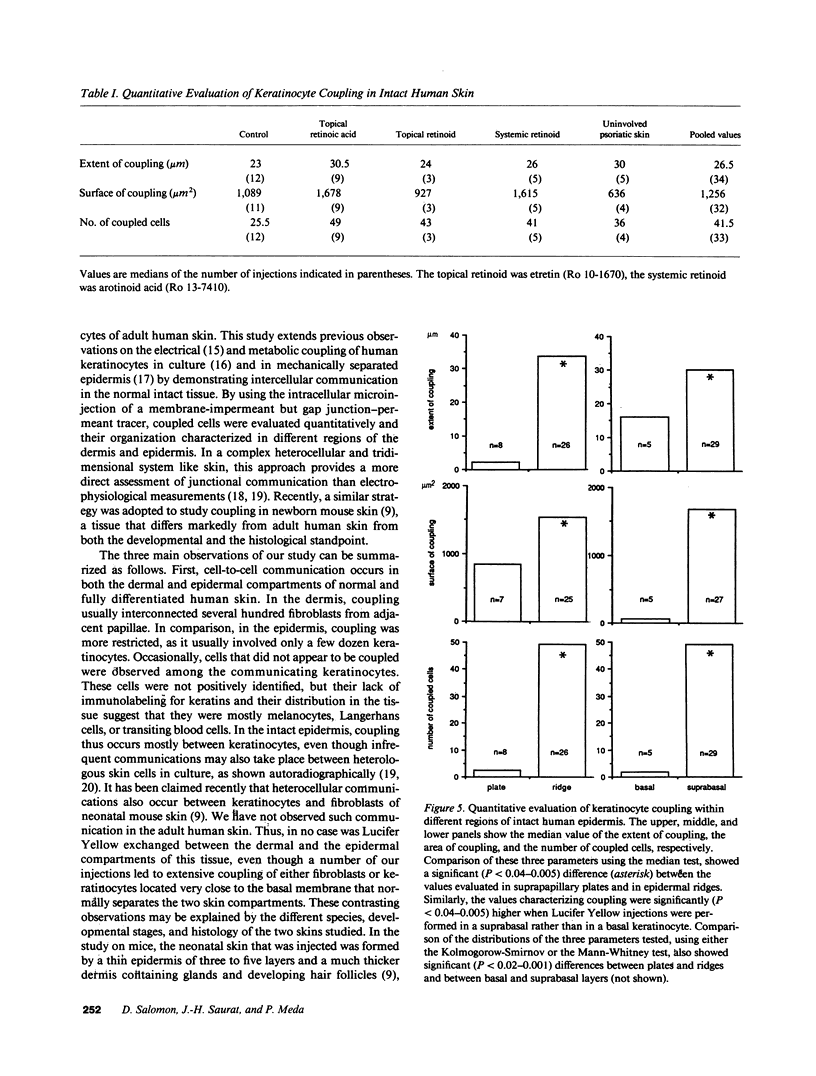
We have characterized cell-to-cell communication (coupling) within intact human skin by microinjecting single keratinocytes with a gap junction-permeant tracer (Lucifer Yellow). 25-50 keratinocytes from different layers of the epidermis were seen to be coupled after most injections (n = 31). A few noncommunicating cells were also microinjected (n = 3) or observed within large territories of coupled keratinocytes. Microinjections of dermal fibroblasts demonstrated an extensive coupling (greater than 100 fibroblasts); however, none of the keratinocyte (n = 34) or fibroblast (n = 3) injections revealed coupling between the epidermal and dermal compartments. Cell coupling was found to be more extensive in epidermal ridges than in suprapapillary plates and, in both regions, was less extensive after injection of the basal layer of the epidermis than after that of the suprabasal layers. This study shows that junctional cell-to-cell communications take place in normal and fully differentiated human tissue. The quantitative data gathered also indicate a regional heterogeneity of keratinocyte-to-keratinocyte communication within intact adult skin and the lack of effect of retinoids on this pattern.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caputo R., Gasparini G. Freeze-fracture replication technique of human skin. J Cutan Pathol. 1982 Aug;9(4):203–224. doi: 10.1111/j.1600-0560.1982.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Caputo R., Innocenti M., Gasparini G., Peluchetti D. Plasma membranes in psoriatic cells. A freeze-fracture study. J Invest Dermatol. 1978 Oct;71(4):245–249. doi: 10.1111/1523-1747.ep12515095. [DOI] [PubMed] [Google Scholar]
- Caputo R., Peluchetti D. The junctions of normal human epidermis. A freeze-fracture study. J Ultrastruct Res. 1977 Oct;61(1):44–61. doi: 10.1016/s0022-5320(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Cavoto F. V., Flaxman B. A. Communication between normal human epidermal cells in vitro. J Invest Dermatol. 1972 Nov;59(5):370–374. doi: 10.1111/1523-1747.ep12627491. [DOI] [PubMed] [Google Scholar]
- Chertow B. S., Baranetsky N. G., Sivitz W. I., Meda P., Webb M. D., Shih J. C. Cellular mechanisms of insulin release. Effects of retinoids on rat islet cell-to-cell adhesion, reaggregation, and insulin release. Diabetes. 1983 Jun;32(6):568–574. doi: 10.2337/diab.32.6.568. [DOI] [PubMed] [Google Scholar]
- Doran T. I., Vidrich A., Sun T. T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980 Nov;22(1 Pt 1):17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Friend D. S. Vitamin-A-induced mucous metaplasia. An in vitro system for modulating tight and gap junction differentiation. J Cell Biol. 1976 Feb;68(2):173–188. doi: 10.1083/jcb.68.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M., Grayson S., Caldwell T. M., McNutt N. S. Gap junction proliferation in retinoic acid-treated human basal cell carcinoma. Lab Invest. 1980 Apr;42(4):469–474. [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1980;74:101–139. [PubMed] [Google Scholar]
- Hertzberg E. L., Lawrence T. S., Gilula N. B. Gap junctional communication. Annu Rev Physiol. 1981;43:479–491. doi: 10.1146/annurev.ph.43.030181.002403. [DOI] [PubMed] [Google Scholar]
- Hunter G. K., Pitts J. D. Non-selective junctional communication between some different mammalian cell types in primary culture. J Cell Sci. 1981 Jun;49:163–175. doi: 10.1242/jcs.49.1.163. [DOI] [PubMed] [Google Scholar]
- Kam E., Melville L., Pitts J. D. Patterns of junctional communication in skin. J Invest Dermatol. 1986 Dec;87(6):748–753. doi: 10.1111/1523-1747.ep12456937. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science. 1982 Mar 5;215(4537):1239–1241. doi: 10.1126/science.7058342. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- McKay I. A., Taylor-Papadimitriou J. The non-selective junctional communication phenotype of normal and transformed human epidermal keratinocytes in vitro. Exp Cell Res. 1982 Sep;141(1):171–180. doi: 10.1016/0014-4827(82)90079-9. [DOI] [PubMed] [Google Scholar]
- Meda P., Bruzzone R., Knodel S., Orci L. Blockage of cell-to-cell communication within pancreatic acini is associated with increased basal release of amylase. J Cell Biol. 1986 Aug;103(2):475–483. doi: 10.1083/jcb.103.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda P., Santos R. M., Atwater I. Direct identification of electrophysiologically monitored cells within intact mouse islets of Langerhans. Diabetes. 1986 Feb;35(2):232–236. doi: 10.2337/diab.35.2.232. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986 Jan 17;44(1):187–196. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- Miller T. M., Goodenough D. A. Gap junction structures after experimental alteration of junctional channel conductance. J Cell Biol. 1985 Nov;101(5 Pt 1):1741–1748. doi: 10.1083/jcb.101.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., O'Connor E. M., Etheridge C. L., Corson J. M. Optimal immunoreactivity of keratin proteins in formalin-fixed, paraffin-embedded tissue requires preliminary trypsinization. An immunoperoxidase study of various tumours using polyclonal and monoclonal antibodies. J Histochem Cytochem. 1985 May;33(5):465–473. doi: 10.1177/33.5.2580883. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Hamilton A. E., Kam E., Burk R. R., Murphy J. P. Retinoic acid inhibits junctional communication between animal cells. Carcinogenesis. 1986 Jun;7(6):1003–1010. doi: 10.1093/carcin/7.6.1003. [DOI] [PubMed] [Google Scholar]
- Pitts J., Kam E., Melville L., Watt F. M. Patterns of junctional communication in animal tissues. Ciba Found Symp. 1987;125:140–153. doi: 10.1002/9780470513408.ch9. [DOI] [PubMed] [Google Scholar]
- Siegenthaler G., Saurat J. H. Therapy with a synthetic retinoid--(Ro 10-1670) etretin--increases the cellular retinoic acid-binding protein in nonlesional psoriatic skin. J Invest Dermatol. 1986 Jul;87(1):122–124. doi: 10.1111/1523-1747.ep12523628. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Wälder L., Lützelschwab R. Effects of 12-O-tetradecanoylphorbol-13-acetate (TPA), retinoic acid and diazepam on intercellular communication in a monolayer of rat liver epithelial cells. Exp Cell Res. 1984 May;152(1):66–76. doi: 10.1016/0014-4827(84)90230-1. [DOI] [PubMed] [Google Scholar]
- van Heukelom J. S., Slaaf D. W., van der Leun J. C. Cell communication in the basal cells of the human epidermis. Biophys J. 1972 Oct;12(10):1266–1284. doi: 10.1016/S0006-3495(72)86161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]