Abstract
Background
Major depression (MD) and anxiety disorders such as panic disorder (PD), agoraphobia (AG) and social phobia (SP) are heritable and highly comorbid. However, the relative importance of genetic and environmental aetiology of the covariation between these disorders, particularly the relationship between PD and AG is less clear.
Methods
The present study measured MD, PD and AG in a population sample of 5440 twin pairs and 1245 single twins, about 45% of whom were also scored for SP. Prevalences, within individual comorbidity and twin odds ratios for comorbidity are reported. A behavioural genetic analysis of the four disorders using the classical twin design was conducted.
Results
Odds ratios for MD, PD, AG, and SP in twins of individuals diagnosed with one of the four disorders were increased. Heritability estimates under a threshold-liability model for MD, PD, AG, and SP respectively were 0.33 (CI:0.30–0.42), 0.38 (CI:0.24–0.55), 0.48 (CI:0.37–0.65) of, and 0.39 (CI:0.16–0.65), with no evidence for any variance explained by the common environment shared by twins. We find that a common genetic factor explains a moderate proportion of variance in these four disorders. The genetic correlation between PD and AG was 0.83.
Conclusion
MD, PD, AG, and SP strongly co-aggregate within families and common genetic factors explain a moderate proportion of variance in these four disorders. The high genetic correlation between PD and AG and the increased odds ratio for PD and AG in siblings of those with AG without PD suggests a common genetic aetiology for PD and AG.
Keywords: anxiety, depression, comorbidity, panic, agoraphobia, twin design
Introduction
Major Depression (MD) and Anxiety disorders such as Panic Disorder (PD), Agoraphobia (AG), and Social Phobia (SP) carry a substantial burden of impairment because of their disabling and chronic course. A recent survey of the burden of disease in Australia in terms of loss of life-years[1] ranked anxiety and depression as the first and third burdens of disease in women and men respectively, implying substantial financial and economical costs to society. Lifetime prevalence rates are reported to be between 1–25% for MD[2–4], 7–13% for SP[5;6], 1–3.5% for PD[7;8], and around 5.3% for AG[8].
Even though prevalence rates vary between studies, it has been consistently reported that women are approximately twice as likely as men to experience MD[1;4;9]. Similar sex differences have also been reported for most anxiety disorders in community-based and epidemiological studies[1;7;8;10]. PD without AG, for example, occurs twice as often in females as in males, while PD with AG is diagnosed up to three times as often in females as in males. Finally, AG without PD is diagnosed more often in females than males and the female to male ratio in social phobia has been shown to be approximately 3:2[5;11].
It is well-established that the symptoms of anxiety and depression commonly co-occur and that anxiety disorders exhibit high levels of lifetime comorbidity[12;13]. For example, more than half of all individuals with MD also develop an anxiety disorder during their lifetime[14;15]. About 50% of community members with a diagnosis of SP report a concomitant anxiety disorder such as PD, AG or generalized anxiety disorder and 20% meet lifetime criteria for MD[11;16;17]. Similarly, of the individuals diagnosed with PD 10–65% have comorbid MD and approximately one-third to one-half show agoraphobic avoidance behaviour[7;8]. Patients with a diagnosis of PD with AG were found to be at increased risk of developing MD as compared to patients diagnosed with PD only[7;18].
Co-morbidity between diseases may reflect overlapping diagnostic criteria, conditions that are different phases of the same disorder, or one disorder being a risk factor for the other disorder[19]. Family studies can provide insight into the shared aetiology of disorders and the availability of different types of family relationships allows dissection into relative contributions of shared genetic and environmental causes. Clinical and non-clinical family studies reveal evidence for familial aggregation in the aetiology of depression[20;21], SP[20;22;23], PD[22;24–27], and AG[23;24;26–28]. Twin studies also suggest that genetic influences play a significant role in the familial aggregation of depression and anxiety disorders[29–35]. For example, twin studies found heritability estimates of 30–60% for MD[36–38], 28–50% for SP[36;39;40], 30–40% for PD[31;34;35;41;42] and 30–40% for AG[31]. However, the genetic and environmental components of the comorbidity of anxiety disorders with one another and with major depression is less clear as studies have yielded inconsistent results[37]. While some studies found common genetic factors to be responsible for the relationship between these disorders[31;39;43–46], other controlled family studies reported unique genetic influences on the relation between anxiety disorders and major depression[26;47–49]. Nevertheless, taking into account only the twin studies, it seems that shared genetic risk factors largely account for this comorbidity, with genetic correlations ranging between 0.35–0.80[30–32;38;39]. Most of the few existing twin studies of these phenotypes and their comorbidity used relatively small sample sizes ranging from 81–6,744 twin individuals and report partly inconsistent findings. As such, the specific role of the genetic factors remains unclear[37].
The relationship between PD and AG is particularly controversial and the ongoing debate has been summarised by Hayward and Wilson[50]. Briefly, in the clinical setting agoraphobia is viewed as a complication of panic symptoms, formally recognised through the DSM-IV diagnostic classifications of PD without AG and PD with AG. The DSM-IV classification of AG without PD is not frequently observed in the clinical setting, despite high prevalence estimates (5.3%[8]) in population-based studies. This inconsistency between clinical and population based prevalence likely reflects self-management by those not experiencing panic attacks despite their agoraphobic fear. In contrast, the distress caused by panic attacks is more likely to lead to treatment seeking[10]. It has been argued that AG without a history of PD is part of a panic disorder syndrome[10] and has been shown to be predictive of onset of PD in a longitudinal study[51]. However, Wittchen et al.[24] have argued that AG exists as a clinically significant phobic condition independent of PD[8]. Evidence for a common genetic aetiology of AG without PD and PD would provide support for AG being part of a PD syndrome, but few family studies have been published and their interpretation is limited by small sample size, clinical setting and historical nosology [24]. In a community setting (young adults (N = 3021) and their parents), there was no evidence for increased prevalence of panic attacks, PD or AG in children of parents with AG without PD. By comparison, the odds (maximum 5.1) of all three conditions was increased in children of parents with PD (with and without AG) compared to children of unaffected parents[24] supporting the view that AG without PD is an etiologically independent disorder.
The aim of the present study is to examine familial aggregation and comorbidity of MD, PD, AG, and SP using a large community-based sample of approximately 6,000 twin pairs. By reporting prevalence rates and odds-ratios for comorbidity as well as heritability estimates of MD, PD, AG and SP the present study aims to contribute to the knowledge about familial aggregation and heritability of these disorders. Further, we investigate the genetic and environmental aetiology of PD and AG and their comorbidity in order to clarify the diagnostic (in)dependence of AG.
Methods
Participants
The population-based sample was derived from the Australian Twin Registry, a volunteer register initiated in 1978. Data from two telephone-based surveys conducted between 1992–1993 and 1996–2000 were used for the present study. Both studies utilized the ‘Semi-Structured Assessment for the Genetics of Alcoholism’ (SSAGA)[52–54], adapted for use in Australia. The SSAGA is a highly reliable, semi-structured interview developed to assess physical, psychological, and social manifestations of alcoholism, medication and drug-use, and comorbid psychopathology such as depression and related disorders in adults and is based on previously validated research interviews (e.g., DIS, CIDI, HELPER, SAM, SADS, and SCID). Among other measures, it includes DSM-III-R diagnostic items (subsequently adapted to DSM-IV) for MD, PD, AG, and SP. The first survey (1992–1993) assessed 2729 complete twin pairs and 557 single twins ranging from 27–90 years of age (mean:44.0±12.4), while the second survey (1996–2000) assessed 2765 complete twin pairs and 688 single twins ranging from 23–39 years of age (mean:29.9±2.5). Both studies were approved by the Queensland Institute of Medical Research Human Research Ethics Committee. Interviewers received extensive training in administering the interview by phone and were blind to the results of a co-twin’s assessment. Further details are described elsewhere[55].
Nineteen participants were assessed in both surveys. Multiple entries for these individuals were removed from the merged dataset, and inconsistent diagnoses were treated as missing. The resulting dataset contained one record for each of 12214 individuals (5440 twin pairs), of which 83 individuals had missing values for any of the MD/PD/AG phenotypes. Of these, 5995 twin individuals (2721 twin pairs) were assessed for SP, as this phenotype was included in the first survey only. The zygosity of 32 individuals (11 complete pairs) was unknown. These individuals were only included in the calculation of the within-individual prevalences and comorbidities.
Measures
DSM-IV diagnoses of the anxiety disorders (PD (with or without AG), AG (with or without PD), and SP) and MD were derived using computer algorithms applied to individual questionnaire item responses. All participants were assessed for AG irrespective of their answers to the panic related questions. Furthermore, the respondents’ fear did not have to be specifically related to fear of panic attacks. In addition, the following four diagnostic categories were considered: PD with AG, PD without AG, AG without PD, and PD or AG.
Statistical Analysis
Prior to analysis we tested for participation bias and for differences in the distribution of scores by zygosity for each variable. Disorder prevalences and probability of comorbidity within individuals were calculated for each sex. Probandwise odds-ratios were calculated for monozygotic and dizygotic twins from logistic regression including sex and age as covariates, both within and between disorders.
For genetic modelling analyses, the liability to develop a disorder is assumed to be a continuous normal distribution with a threshold above which individuals develop the disorder[56]. This liability distribution receives contributions from both independent normally distributed genetic and environmental effects. The classical twin design allows partitioning of variance in and covariance between traits into that due to additive genetic (A) and environmental (shared within twin pairs, C, and non-shared, E) influences. This is possible as A, C, and E influences each predict different patterns of identical (monozygotic; MZ) and non-identical (dizygotic; DZ) twin pair correlations. The classical twin design assumes that the only difference between MZ and DZ twins is that MZ twins share all their genes and DZ twins share on average half their genes and that trait-relevant environments are similar for MZ and DZ twin pairs[57]. Normally, twin correlations can be explained by a combination of these three parameters (A, C, and E) and structural equation modelling can be used to determine the combination that best explains the observed data. Despite the large sample size, the number of twin pairs who both reported a disorder was low, owing to the low prevalences of some of the disorders; therefore, it was not possible to explore differences in variance components between sexes in the genetic modelling analysis.
Data were analysed using Maximum-Likelihood (ML) methods in Mx[58], utilising threshold modelling for ordinal data[59]. In this procedure, each twin group (MZ and DZ twins) has two thresholds (one for each twin) for each of the variables reflecting prevalence. Initially, a saturated model is fitted estimating all parameters, and then subsequently more restricted models are compared to the preceding model. Specific hypotheses regarding the significance of particular parameters can be tested statistically by comparing the goodness-of-fit of various models using the minus two times log-likelihood (−2LL) statistics (distributed as χ2).
Prior to genetic modeling, we tested for age and sex effects on the thresholds. A multivariate model was fitted to assess genetic and environmental factors that mediate the phenotypic covariation between depression and the three anxiety disorders. After estimating the relative magnitude of all parameters, sub-models were fit to test the significance of specific parameters and to determine the simplest model explaining the phenotypic variance of the four variables. Model reduction started with dropping the paths with the smallest parameter estimates. In order to confirm accuracy of the multivariate modelling results we also conducted univariate modelling.
Results
Preliminary analysis
No significant (p<.05) differences were found in the prevalence rates/thresholds within twin pairs or across zygosity groups for all variables. MD, PD, and AG showed significant age effects on thresholds, with the probability to report a past episode of PA and AG increasing with age while the probability of reporting MD decreased. MD also showed a sex effect, with females being more likely to report a depressive episode, so age and sex were retained as covariates in subsequent modeling. In order to control for participation bias, we checked whether the likelihood of being diagnosed with one of the disorders was different in singletons (co-twin not participating) compared to complete twin pairs[60;61]. There was no significant difference in diagnosis rates between full twin pairs and singletons, so participation rates appear to be independent of the psychological measures.
Within-twin prevalence and comorbidity
Lifetime prevalence and comorbidity of psychopathology within the twin individuals are presented in Table 1. The prevalences of the anxiety disorders ranged between ~ 1–3%. As expected, MD was the most common disorder in the sample with 19% of the male participants being affected during their lifetime and females were approximately 1.5 times as likely as males to be diagnosed with MD. Lifetime prevalence of MD for half of the present cohort have been reported previously by Bierut et al.[62]. Similar sex differences in prevalence rates were found for the anxiety disorders, with women being 1.5–2.0 times as likely as men to be diagnosed, with the exception of SP where the prevalence rates did not differ between sexes.
Table 1.
Prevalence for each disorder (in bold) on diagonal and the comorbidity within an individual of column diagnosis given a row diagnosis for males/females separately.
| PDa | AGa | PD or AGa | PD&AGa | PD no AGa | AG no PDa | MDa | SPb | |
|---|---|---|---|---|---|---|---|---|
| PD | 0.02/0.03 | 0.33/ 0.38 | NA | 0.31/0.38 | NA | NA | 0.51/0.65 | 0.20/0.14 |
| AG | 0.39/0.40 | 0.01/0.03 | NA | 0.36/0.39 | NA | NA | 0.52/0.68 | 0.38/0.18 |
| PD or AG | NA | NA | 0.02/0.05 | NA | NA | NA | 0.52/0.63 | 0.26/0.14 |
| PD&AG | NA | NA | NA | 0.01/ 0.01 | NA | NA | 0.52/0.83 | 0.41/0.26 |
| PD no AG | NA | NA | NA | NA | 0.01/0.02 | NA | 0.51/0.54 | 0.08/0.09 |
| AG no PD | NA | NA | NA | NA | NA | 0.01/ 0.02 | 0.54/0.58 | 0.39/0.12 |
| MD | 0.04/0.08 | 0.04/0.09 | 0.07/0.13 | 0.01/0.04 | 0.03/0.04 | 0.02/0.04 | 0.19/ 0.27 | 0.06/0.06 |
| SP | 0.23/0.28 | 0.41/0.37 | 0.46/0.49 | 0.18/0.15 | 0.05/0.12 | 0.23/0.19 | 0.42/0.59 | 0.02/0.02 |
Note. PD = Panic disorder; AG = Agoraphobia; PD&AG = Panic with agoraphobia; PD No AG = Panic without agoraphobia; AG no PD = Agoraphobia without panic; MD = Depression, SP = Social phobia. Standard errors ranged between 0.0007–0.006.
N = 5862 male twin individuals (1514 complete twin pairs and 1254 opposite-sex male twin individuals and 580 incomplete twin pairs) and 7352 female twin individuals (2683 complete twin pairs and 1254 opposite-sex female twin individuals and 732 incomplete twin pairs).
N = 2087 male twin individuals (637 complete twin pairs and 655 opposite-sex male twin individuals and 158 incomplete twin pairs) and 3908 female twin individuals (1480 complete twin pairs and 729 opposite-sex female twin individuals and 219 incomplete twin pairs).
The comorbidity probability for the various anxiety disorders with each other ranged between 0.20–0.41 for males and 0.14–0.40 for females. The probability of having an anxiety disorder when diagnosed with MD was very low (0.04–0.06 for males and 0.06–0.09 for females) as opposed to the chance of having MD when diagnosed with an anxiety disorder (0.42–0.52 for males and 0.59–0.69 for females).
Between-twin comorbidity
Table 2 presents the probandwise odds-ratios for diagnosis with a disorder if the co-twin has been diagnosed with the same, or a different disorder for MZ and DZ twins respectively. The odds-ratios ranged between 2.2 and 12.8 for MZ twins and this was slightly higher than the odds-ratios for DZ twins which ranged between 0 and 9.4. Despite the large sample size, the confidence intervals were relatively wide and often overlapping, indicating that the MZ and DZ odds ratios were mostly not significantly different.
Table 2.
Probandwise odds ratios for monozygotic and dizygotic twins of column diagnosis if twin has row diagnosis adjusted for sex and age.
| Monozygotic twins | ||||||||
|---|---|---|---|---|---|---|---|---|
| PDa | AGa | PDorAGa | PD&AGa | PDNoAGa | AGnoPDa | MDa | SPb | |
| PD | 5.8 (3.5; 9.6) | |||||||
| AG | 5.1 (2.9; 8.9) | 10.3 (6.3; 16.8) | ||||||
| PDorAG | 5.9 (3.8; 9.1) | 8.1 (5.2; 12.4) | 7.7 (5.4; 11.0) | |||||
| PD&AG | 5.3 (2.3; 12.0) | 7.2 (3.3; 15.9) | 6.0 (3.0; 12.0) | 8.4 (2.9; 24.5) | ||||
| PDNoAG | 5.5 (3.0; 10.2) | 3.6 (1.7; 7.6) | 5.3 (3.1; 9.1) | 3.2 (1.0; 10.4) | 6.5 (3.2; 13.0) | |||
| AGnoPD | 5.0 (2.4; 10.4) | 11.1 (6.0; 20.4) | 9.1 (5.2; 15.7) | 6.0 (2.1; 17.3) | 4.0 (1.6; 10.3) | 12.8 (6.2; 26.6) | ||
| MD | 2.8 (2.0; 4.0) | 2.6 (1.8; 3.8) | 2.7 (2.0; 3.5) | 3.7 (2.1; 6.5) | 2.4 (1.6; 3.7) | 2.2 (1.4; 3.7) | 2.9 (2.5; 3.3) | |
| SP | 3.6 (1.5; 8.8) | 6.5 (3.1; 13.9) | 5.6 (2.9; 10.8) | 4.0 (0.9; 17.5) | 3.3 (1.1; 9.4) | 6.6 (2.7; 16.2) | 2.6 (1.5; 4.5) | 11.9 (3.7; 38.8) |
| Dizygotic twins | ||||||||
| PD | 3.6 (1.7; 6.6) | |||||||
| AG | 1.1 (0.4; 2.9) | 4.6 (2.5; 7.5) | ||||||
| PDorAG | 2.7 (1.4; 4.4) | 3.3 (1.9; 5.2) | 3.5 (2.2; 5.0) | |||||
| PD&AG | 0.9 (0.1; 6.3) | 1.6 (0.4; 6.5) | 1.6 (0.5; 5.1) | 0.0 (0.0; Inf) | ||||
| PDNoAG | 5.2 (2.4; 9.9) | 0.9 (0.2; 3.3) | 3.2 (1.5; 5.8) | 1.4 (0.2; 9.7) | 7.5 (3.2; 14.7) | |||
| AGnoPD | 1.4 (0.4; 4.0) | 6.7 (3.5; 11.3) | 4.6 (2.4; 7.5) | 2.6 (0.6; 10.5) | 0.7 (0.1; 4.5) | 9.4 (4.5; 16.5) | ||
| MD | 1.7 (1.2; 2.5) | 1.4 (1.0; 2.0) | 1.6 (1.3; 2.2) | 1.3 (0.7; 2.4) | 1.9 (1.3; 3.2) | 1.5 (1.0; 2.4) | 1.5 (1.3; 1.7) | |
| SP | 2.9 (1.1; 7.4) | 1.8 (0.6; 5.0) | 2.1 (0.9; 4.7) | 3.6 (0.8; 15.4) | 2.5 (0.7; 8.1) | 1.2 (0.3; 5.1) | 1.5 (0.8; 2.6) | 1.5 (0.2; 11.0) |
Note. PD = Panic disorder; AG = Agoraphobia; PDorAG = Panic or agoraphobia; PD&AG = Panic with agoraphobia; PDnoAG = Panic without agoraphobia; AGnoPD = Agoraphobia without panic; MD = Depression; SP = Social phobia.
N = 2495 monozygotic and 2945 dizygotic twin pairs.
N = 1337 monozygotic and 1384 dizygotic twin pairs.
Genetic modelling
A general multivariate ACE model was fitted to MD, PD, AG and SP, in which proportions of A, C and E were constrained to be equal in males and females. Estimates for individual traits were consistent between the univariate and multivariate analyses. As expected from the MZ and DZ odds ratios, the shared-environmental (C) effects were very small: 0.00, 0.11, 0.04 and 0.02 for MD, PD, AG and SP, respectively. All C influences could be dropped without a significant loss of model-fit (Δχ210=1.9, p>0.05) indicating that the most parsimonious explanation for the observed pattern of twin correlations was a general AE model. We could also remove the shared A factors between AG and SP and PD and SP in the Cholesky decomposition (Δχ21:1.4–2.7, p>0.05) but no other A or E factors (Δχ21:4.2–12.5, p<0.05). The full and the most parsimonious multivariate models are shown in Figure 1.
Figure 1.
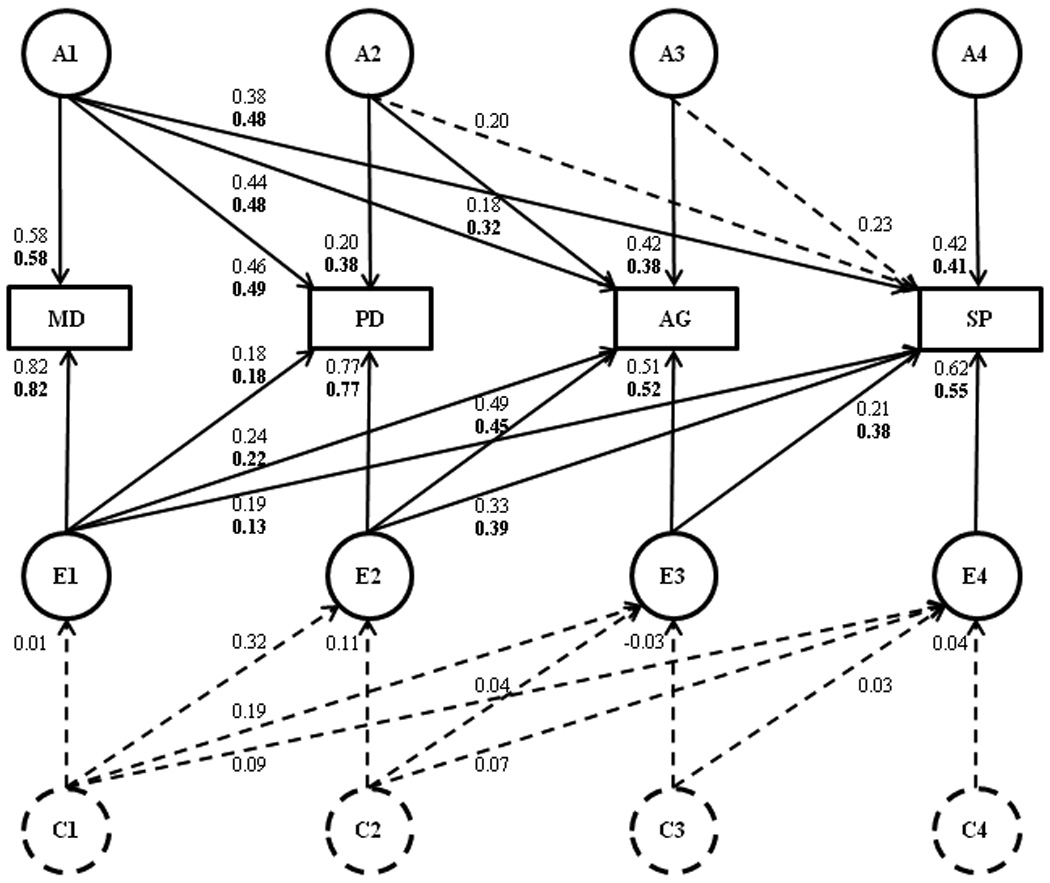
Full multivariate model in which a Cholesky decomposition is applied to the genetic and environmental variance-covariance matrices with standardized path coefficients showing the relationship between Depression (MD), Panic (PD), Agoraphobia (AG), and Social Phobia (SP). Path coefficients can be squared to get the percentage of variance accounted for. The Cholesky factors have been decomposed into additive genetic (A), shared (C) and non-shared environmental (E) influences. The most parsimonious model is shown with solid lines and path estimates in bold.
Heritabilities, genetic and environmental correlations on the liability scale calculated under the best fitting model, together with phenotypic correlations between pairs of disorders are listed in Table 3. The genetic correlation between PD and AG is particularly high at 0.83. The lower environmental correlations between MD and the anxiety disorders partially result from the differences in prevalence rates. Sixty-three percent (i.e. 0.492/0.38*100) of the genetic variance in PD, 48% of the genetic variance in AG, and 59% of the genetic variance in SP was explained by genetic factors shared with MD. A further 21% of the genetic variance in AG was explained by genetic factors in common with PD. Three to 39% of the variance due to non-shared environmental influences of PD, AG and SP were explained by factors common with MD.
Table 3.
Heritabilities, genetic, environmental and phenotypic correlations on the liability scale under the most parsimonious model.
| MD | PD | AG | SP | |
|---|---|---|---|---|
| Heritabilities | 0.33 | 0.38 | 0.48 | 0.39 |
| 95% CIa | 0.30–0.42 | 0.24–0.55 | 0.37–0.65 | 0.16–0.65 |
| Genetic correlations | ||||
| MD | 0.79 | 0.70 | 0.76 | |
| PD | 0.83 | 0.60 | ||
| AG | 0.53 | |||
| Environmental correlations | ||||
| MD | 0.22 | 0.30 | 0.17 | |
| PD | 0.67 | 0.52 | ||
| AG | 0.71 | |||
| Phenotypic correlations | ||||
| MD | 0.42 | 0.45 | 0.37 | |
| PD | 0.73 | 0.54 | ||
| AG | 0.63 | |||
Note. PD = Panic disorder; AG = Agoraphobia; MD = Depression; SP = Social phobia.
95% confidence interval calculated in the univariate analyses.
Discussion
The present study aimed to investigate familial aggregation of MD, PD, AG, and SP as well as the genetic and environmental aetiology of the four disorders and their comorbidity. The sample prevalences were comparable to those found in general population studies for MD and PD, and were slightly lower than expected for AG and SP[7;8;63]. As expected, women were 1.5–2 times as likely to be diagnosed with MD or an anxiety disorder as men. For SP the absence of a sex difference for prevalence, may reflect the smaller sample size compared to the other variables.
The comorbidity between the anxiety disorders was moderate, ranging between 0.14 and 0.41. Although only 4–6% of males and 6–9% of the female participants with a diagnosis of MD also suffered from an anxiety disorder, 42–52% of males and 59–69% of females with an anxiety disorder were also diagnosed with MD, this asymmetry reflects the large differential in prevalence between MD and the anxiety disorders. These findings show that the anxiety disorders are highly comorbid with each other and with MD and are consistent with past studies in community and clinical samples[4;37].
The between-twin odds-ratios (Table 2) strongly point towards familial aggregation of MD, PD, AG, and SP and their comorbidity and indicate that genetic factors play a role in the variation and covariation of these disorders, which is in line with findings of past studies[21;22;24]. However, despite the large sample size confidence intervals in the present study were very wide due to the low prevalences of some disorders and therefore the differences in odds ratios between MZ and DZ twins, which were substantial, were not significant. Nonetheless, genetic modelling revealed that the most parsimonious model to explain the observed familial aggregation on the liability scale is one with moderate genetic influences on the variance of the MD, PD, AG, and SP, with heritabilities of 0.33 (CI:0.30–0.42), 0.38 (CI:0.24–0.55), 0.48 (CI:0.37–0.65), and 0.39 (CI:0.16–0.65) respectively, leaving the largest proportion of the variance in liability to be explained by non-shared environmental factors. Previous meta-analyses have consistently attributed 30–40% of the variance in liability to anxiety disorders and MD to additive genetic influences with little evidence for shared environmental contribution to any of these disorders and this is in agreement with the findings of the present study.
Genetic variance in PD, AG and SP shared with MD was 63%, 48% and 59% respectively, corresponding to genetic correlations of 0.79, 0.70 and 0.76 (Table 3). This indicates that overlapping etiological factors explain most of the comorbidity between MD and all three anxiety disorders and is consistent with previous studies suggesting genetic mediation of the phenotypic associations between MD and anxiety disorders[31;43–45;64–66]. Genetic correlations were lower between PD and SP (0.60) and between AG and SP (0.53) than for the other pairs of disorders. It has been shown previously that SP seems to share less genetic liability with AG than other phobic disorders share among each other[46;67]. The genetic correlation between PD and AG was very high at 0.83.
In an attempt to replicate the finding of Nocon et al.[24] who reported no familial component to the disorder of AG without PD, we considered the mutually exclusive diagnostic classes of PD without AG, PD with AG and AG without PD. Despite the large confidence intervals, the MZ and DZ odds ratios for AG without PD of 12.8 (CI:6.2–26.6) and 9.4 (CI:4.5–16.5) respectively suggest that there is a heritable component to this disorder. Moreover, the MZ and DZ odds ratios for AG without PD in twin siblings of those with PD with AG of 6.0 (CI:2.1–17.3) and 2.6 (CI:0.6–10.5) are suggestive that AG without PD is part of the PD spectrum (as suggested, amongst others, by Andrews and Slade[10]), rather than an etiologically distinct disorder as advocated in the related studies of Wittchen et al.[8] and Nocon et al.[24]. Unfortunately, the low prevalence rates did not allow for genetic bivariate modelling of PD and AG without PD, which would have given further information on the (in)dependence of AG and PD.
Our results must be interpreted recognising the limitations of our study. Our structured telephone interviews were administered by trained lay interviewers who were closely supervised by a clinical psychologist. Some population based studies have been shown to misclassify those with specific phobias as AG resulting in an overestimation of AG, for example, prevalence of AG without PD was estimated to be 8.5% in a community sample, but this was later revised to 3.5% after clinical review[68]. However in this study prevalence of AG without PD was less than 2% suggesting that we did not over-diagnose. Despite our large study sample of 12125 individuals (including 5440 complete twin pairs) the low prevalence rates of each disorder necessarily mean large confidence interval for estimated parameters. In particular, the sample size for SP was much smaller than the sample for the other three measures as SP was only assessed in one of the two surveys included in the present study. The low prevalence rates also complicated the multivariate genetic modelling. Furthermore, it has been shown that a boundary problem may arise for multivariate genetic analysis, but it is not clear to date how much this affects the analysis with ordinal variables nor has a good solution to prevent this problem been suggested[69]. However, consistent estimates of heritability between the univariate and multivariate models provides some confidence in the validity of the estimates of genetic and environmental covariance. The overlapping confidence intervals for MZ and DZ odds ratios indicate that partitioning the variance into genetic and environmental components would be compromised. None-the-less, the best point estimates of the C components were very small relative to the A components justifying dropping C first.
In conclusion, a community sample of 5440 twin pairs showed that MD, PD, AG, and SP strongly co-aggregate within families and that common genetic factors explain a moderate to high proportion of variance in these four disorders with no evidence for influences of common environment. The high genetic correlation (0.83) between PD and AG and the increased odds ratio for PD and AG in siblings of those with AG without PD supports the theory of a common genetic aetiology for PD and AG.
Acknowledgement
This research was support by grants to NGM from the Australia National Health and Medical Research Council (NHMRC; 941177, 971232, 339450, 443011) and from NIH to ACH (AA07535, AA07728 & AA10249). We would like to thank David Smyth and Harry Beeby for computer support and James Scott for his helpful comments. Lastly, this research would not be possible without the willing co-operation of twins and their families who participate in the Australian Twin Registry studies. All participants provided informed consent under study protocols approved by the Queensland Institute of Medical Research and Washington University Human Research Ethics Committees.
References
- 1.Begg S, Vos T, Barker B, Stevenson C, et al. The burden of disease and injury in Australia 2003. Canberra: AIHW; 2007. p. 82. editor. [Google Scholar]
- 2.Landi M, Batini S, Faravelli L, Duma S, et al. Epidemiology of major depressive disorder. Quaderni Italiani di Psichiatria. 2008;27:47–54. [Google Scholar]
- 3.Kessler RC, Gruber M, Hettema JM, Hwang I, et al. Co-morbid major depression and generalized anxiety disorders in the National Comborbity Survey follow-up. Psychological Medicine. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) surveys (vol 12, pg 3, 2003) International Journal of Methods in Psychiatric Research. 2003;12:165–165. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furmark T. Social phobia: overview of community surveys. Acta Psychiatrica Scandinavica. 2002;105:84–93. doi: 10.1034/j.1600-0447.2002.1r103.x. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC. The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatrica Scandinavica. 2003;108:19–27. doi: 10.1034/j.1600-0447.108.s417.2.x. [DOI] [PubMed] [Google Scholar]
- 7.Association AP, editor. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington: 1994. [Google Scholar]
- 8.Wittchen HU, Nocon A, Beesdo K, Pine DS, et al. Agoraphobia and panic. Psychotherapy and Psychosomatics. 2008;77:147–157. doi: 10.1159/000116608. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm K, Mitchell P, Slade T, Brownhill S, et al. Prevalence and correlates of DSM-IV major depression in an Australian national survey. Journal of Affective Disorders. 2003;75:155–162. doi: 10.1016/s0165-0327(02)00040-x. [DOI] [PubMed] [Google Scholar]
- 10.Andrews G, Slade T. Agoraphobia without a history of panic disorder may be part of the panic disorder syndrome. Journal of Nervous and Mental Disease. 2002;190:624–630. doi: 10.1097/00005053-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lampe L, Slade T, Issakidis C, Andrews G. Social phobia in the Australian National Survey of Mental Health and Well-Being (NSMHWB) Psychological Medicine. 2003;33:637–646. doi: 10.1017/s0033291703007621. [DOI] [PubMed] [Google Scholar]
- 12.Maser JD, Cloninger CR. In: Comorbidity of Mood and Anxiety Disorders. Press AP, editor. Washington, DC: 1990. [Google Scholar]
- 13.Kessler RC. Epidemiology of Psychiatric Comorbididty. In: Sons JW, editor. Textbook in Psychiatric Epidemiology. New York: Inc.; 1995. pp. 179–198. [Google Scholar]
- 14.Regier DA, Rae DS, Narrow WE, Kaelber CT, et al. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. British Journal of Psychiatry. 1998;173:24–28. [PubMed] [Google Scholar]
- 15.Kessler RC, Nelson CB, McGonagle KA, Liu J, et al. Comorbidity of DSM-III-R major depressive disorder in the general population: Results from the US National Comorbidity Survey. British Journal of Psychiatry. 1996;168:17–30. [PubMed] [Google Scholar]
- 16.Merikangas KR, Angst J. Comorbidity and social phobia - Evidence from clinical, epidemiologic, and genetic-studies. European Archives of Psychiatry and Clinical Neuroscience. 1995;244:297–303. doi: 10.1007/BF02190407. [DOI] [PubMed] [Google Scholar]
- 17.Lepine JP, Lellouch J. Classification and epidemiology of social phobia. European Archives of Psychiatry and Clinical Neuroscience. 1995;244:290–296. doi: 10.1007/BF02190406. [DOI] [PubMed] [Google Scholar]
- 18.Tsuang M, Domschke K, Jerskey BA, Lyons MJ. Agoraphobic behavior and panic attack: a study of male twins. Journal of Anxiety Disorders. 2004;18:799–807. doi: 10.1016/j.janxdis.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychological Medicine. 2005;35:611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- 20.Bellodi L, Battaglia M, Diaferia G, Draisci A, et al. Lifetime prevalence of depression and family history of patients with panic disorder and social phobia. European Psychiatry. 1993;8:147–152. [Google Scholar]
- 21.Biederman J, Monuteaux MC, Faraone SV, Hirshfeld-Becker DR, et al. Does referral bias impact findings in high-risk offspring for anxiety disorders? A controlled study of high-risk children of non-referred parents with panic disorder/agoraphobia and major depression. Journal of Affective Disorders. 2004;82:209–216. doi: 10.1016/j.jad.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Fyer AJ, Mannuzza S, Chapman TF, Lipsitz J, et al. Panic disorder and social phobia: Effects of comorbidity on familial transmission. Anxiety. 1996;2:173–178. doi: 10.1002/(SICI)1522-7154(1996)2:4<173::AID-ANXI3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Fyer AJ, Mannuzza S, Chapman TF, Martin LY, et al. Specificity in familial aggregation of phobic disorders. Archives of General Psychiatry. 1995;52:564–573. doi: 10.1001/archpsyc.1995.03950190046007. [DOI] [PubMed] [Google Scholar]
- 24.Nocon A, Wittchen HU, Beesdo K, Bruckl T, et al. Differential familial liability of panic disorder and agoraphobia. Depression and Anxiety. 2008;25:422–434. doi: 10.1002/da.20425. [DOI] [PubMed] [Google Scholar]
- 25.Maier W, Lichtermann D, Minges J, Oehrlein A, et al. A controlled family study in panic disorder. Pergamon-Elsevier Science Ltd.; 1993. pp. 79–87. [DOI] [PubMed] [Google Scholar]
- 26.Noyes R, Crowe RR, Harris EL, Hamra BJ, et al. Relationship between panic disorder and agoraphobia - A family study. Archives of General Psychiatry. 1986;43:227–232. doi: 10.1001/archpsyc.1986.01800030037004. [DOI] [PubMed] [Google Scholar]
- 27.Biederman J, Petty C, Faraone SV, Hirshfeld-Becker DR, et al. Parental predictors of pediatric panic disorder/agoraphobia: A controlled study in high-risk offspring. Depression and Anxiety. 2005;22:114–120. doi: 10.1002/da.20122. [DOI] [PubMed] [Google Scholar]
- 28.Crowe RR, Noyes R, Pauls DL, Slymen D. A family study of panic disorder. Archives of General Psychiatry. 1983;40:1065–1069. doi: 10.1001/archpsyc.1983.01790090027004. [DOI] [PubMed] [Google Scholar]
- 29.Carey G, Gottesman II. Twin and family studies of anxiety phobic and obsessive disorders. 1981 [Google Scholar]
- 30.Hettema JM, Prescott CA, Kendler KS. A population-based twin study of generalized anxiety disorder in men and women. Journal of Nervous and Mental Disease. 2001;189:413–420. doi: 10.1097/00005053-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Kendler KS, Neale MC, Kessler RC, Heath AC, et al. Panic disorder in women - A Population-based twin study. Psychological Medicine. 1993;23:397–406. doi: 10.1017/s003329170002849x. [DOI] [PubMed] [Google Scholar]
- 32.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS, Walters EE, Neale MC, Kessler RC, et al. The structure of the genetic and environmental risk-factros for 6 major psychiatric-disorders in women - Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Archives of General Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 34.Scherrer JF, True WR, Xian H, Lyons MJ, et al. Evidence for genetic influences common and specific to symptoms of generalized anxiety and panic. Journal of Affective Disorders. 2000;57:25–35. doi: 10.1016/s0165-0327(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 35.Perna G, Caldirola D, Arancio C, Bellodi L. Panic attacks: a twin study. Psychiatry Research. 1997;66:69–71. doi: 10.1016/s0165-1781(97)85177-3. [DOI] [PubMed] [Google Scholar]
- 36.Middeldorp CM, Birley AJ, Cath DC, Gillespie NA, et al. Familial clustering of major depression and anxiety disorders in Australian and Dutch twins and siblings. Twin Research and Human Genetics. 2005;8:609–615. doi: 10.1375/183242705774860123. [DOI] [PubMed] [Google Scholar]
- 37.Hettema JM. What is the genetic relationship between anxiety and depression? American Journal of Medical Genetics Part C-Seminars in Medical Genetics. 2008;148C:140–146. doi: 10.1002/ajmg.c.30171. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 39.Nelson EC, Grant JD, Bucholz KK, Glowinski A, et al. Social phobia in a population-based female adolescent twin sample: co-morbidity and associated suicide-related symptoms. Psychological Medicine. 2000;30:797–804. doi: 10.1017/s0033291799002275. [DOI] [PubMed] [Google Scholar]
- 40.Fyer AJ. Heritability of social anxiety - A brief review. Physicians Postgraduate Press; 1993. pp. 10–12. [PubMed] [Google Scholar]
- 41.Skre I, Onstad S, Torgersen S, Lygren S, et al. A twin study of DSM-III-R anxiety disorders. Acta Psychiatrica Scandinavica. 1993;88:85–92. doi: 10.1111/j.1600-0447.1993.tb03419.x. [DOI] [PubMed] [Google Scholar]
- 42.Torgersen S. Genetic-factors in anxiety disorders. Archives of General Psychiatry. 1983;40:1085–1089. doi: 10.1001/archpsyc.1983.01790090047007. [DOI] [PubMed] [Google Scholar]
- 43.Maier W, Minges J, Lichtermann D. The familial relationship between panic disorder and unipolar depresssion. Journal of Psychiatric Research. 1995;29:375–388. doi: 10.1016/0022-3956(95)00024-y. [DOI] [PubMed] [Google Scholar]
- 44.Coryell W, Endicott J, Andreasen NC, Keller MB, et al. Depression and panic attacks - The significance of overlap as reflected in follow-up and family study data. American Journal of Psychiatry. 1988;145:293–300. doi: 10.1176/ajp.145.3.293. [DOI] [PubMed] [Google Scholar]
- 45.Mendlewicz J, Papadimitriou G, Wilmotte J. Family study of panic disorder - Comparison with generalized anxiety disorder, major depression and normal subjects. Psychiatric Genetics. 1993;3:73–78. [Google Scholar]
- 46.Kendler KS, Myers J, Prescott CA, Neale MC. The genetic epidemiology of irrational fears and phobias in men. Archives of General Psychiatry. 2001;58:257–265. doi: 10.1001/archpsyc.58.3.257. [DOI] [PubMed] [Google Scholar]
- 47.Weissman MM, Wickramaratne P, Adams PB, Lish JD, et al. The relationship between panic disorder and major depression - A new family study. Archives of General Psychiatry. 1993;50:767–780. doi: 10.1001/archpsyc.1993.01820220017003. [DOI] [PubMed] [Google Scholar]
- 48.Horwath E, Weissman MM. In: Epidemiology of depression and anxiety disorders. Tsuang MT, Tohen M, Zahner GEP, editors. John Wiley and Sons, Inc.; John Wiley and Sons Ltd.; 1995. [Google Scholar]
- 49.Klein DN, Lewinsohn PM, Rohde P, Seeley JR, et al. Family study of co-morbidity between major depressive disorder and anxiety disorders. Psychological Medicine. 2003;33:703–714. doi: 10.1017/s0033291703007487. [DOI] [PubMed] [Google Scholar]
- 50.Hayward C, Wilson KA. Anxiety sensitivity - A missing piece to the agoraphobia-without-panic puzzle. Behavior Modification. 2007;31:162–173. doi: 10.1177/0145445506297015. [DOI] [PubMed] [Google Scholar]
- 51.Bienvenu OJ, Onyike CU, Stein MB, Chen LS, et al. Agoraphobia in adults: incidence and longitudinal relationship with panic. British Journal of Psychiatry. 2006;188:432–438. doi: 10.1192/bjp.bp.105.010827. [DOI] [PubMed] [Google Scholar]
- 52.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, et al. A validity study of the SSAGA - a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 53.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, et al. A New, Semistructured Psychiatric Interview for Use in Genetic-Linkage Studies - a Report on the Reliability of the Ssaga. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 54.Bucholz KK, Hesselbrock VM, Shayka JJ, Nurnberger JI, et al. Reliability of Individual Diagnostic Criterion Items for Psychoactive Substance Dependence and the Impact on Diagnosis. Journal of Studies on Alcohol. 1995;56:500–505. doi: 10.15288/jsa.1995.56.500. [DOI] [PubMed] [Google Scholar]
- 55.Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 56.Falconer DS, Mackay TFC. Introduction to quantitative genetics. Harlow UK: Longman Group Limited; 1996. [Google Scholar]
- 57.Kendler KS, Neale MC, Kessler RC, Heath AC, et al. A Test of the Equal-Environment Assumption in Twin Studies of Psychiatric-Illness. Behavior Genetics. 1993;23:21–27. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- 58.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modelling. Richmond: VA 23298: Department of Psychiatry; 2002. VCU Box 900126. [Google Scholar]
- 59.Neale MC, Maes HH. Methodology for Genetic Studies of Twins and Families. Dordrecht: Kluwer Academic Publishers B.V.; 2004. [Google Scholar]
- 60.Martin NG, Wilson SR. Bias in the Estimation of Heritability from Truncated Samples of Twins. Behavior Genetics. 1982;12:467–472. doi: 10.1007/BF01065638. [DOI] [PubMed] [Google Scholar]
- 61.Neale MC, Eaves LJ. Estimating and Controlling for the Effects of Volunteer Bias with Pairs of Relatives. Behavior Genetics. 1993;23:271–277. doi: 10.1007/BF01082466. [DOI] [PubMed] [Google Scholar]
- 62.Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, et al. Major depressive disorder in a community-based twin sample - Are there different genetic and environmental contributions for men and women? Archives of General Psychiatry. 1999;56:557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- 63.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 64.Kendler KS, Heath A, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population. The etiologic role of genetic and environmental factors. Arch Gen Psychiatry. 1986;43:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- 65.Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genetic Epidemiology. 1984;1:89–108. doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- 66.Kendler KS, Heath AC, Martin NG, Eaves LJ. Symptoms of anxiety and symptoms of depression - Same genes, different environments. Archives of General Psychiatry. 1987;44:451–457. doi: 10.1001/archpsyc.1987.01800170073010. [DOI] [PubMed] [Google Scholar]
- 67.Kendler KS, Neale MC, Kessler RC, Heath AC, et al. The genetic epidemiology of phobias in women - The interrelationship of agoraphobia, social phobia, situational phobia, and simple phobia. Archives of General Psychiatry. 1992;49:273–281. doi: 10.1001/archpsyc.1992.01820040025003. [DOI] [PubMed] [Google Scholar]
- 68.Wittchen HU, Reed V, Kessler RC. The relationship of agoraphobia and panic in a community sample of adolescents and young adults. Archives of General Psychiatry. 1998;55:1017–1024. doi: 10.1001/archpsyc.55.11.1017. [DOI] [PubMed] [Google Scholar]
- 69.Carey G. Cholesky problems. Behavior Genetics. 2005;35:653–665. doi: 10.1007/s10519-005-5355-9. [DOI] [PubMed] [Google Scholar]


